To systematically study the technological advances in a particular sector, attributed to the utilization of the Internet, the term “Internet of Things” (IoT) was introduced. The present systematic review, aims to present and analyze the modern applications of the IoT within the surgical worlernet of Things” (IoT) was introduced. While not strictly defined, IoT describes a network of Internet-based connected things equipped with (embedded) sensing and actuating devices, with data production, processing, and consumption abilities. The utilization of the Internet and IoT in medical practice can take many shapes and forms. Ranging from the awe-inspiring telesurgical procedures to complex AI machine learning applications that aid in medical decision making [3], to a simple email containing a preoperative CT scan, the Internet of Surgical Things (IoST) is here to stay.
- surgical practice
- Internet of Surgical Things
- Internet of Things
- Surgery
- Telemonitoring
- Telesurgery
- Telemedicine
- Internet of Medical Things
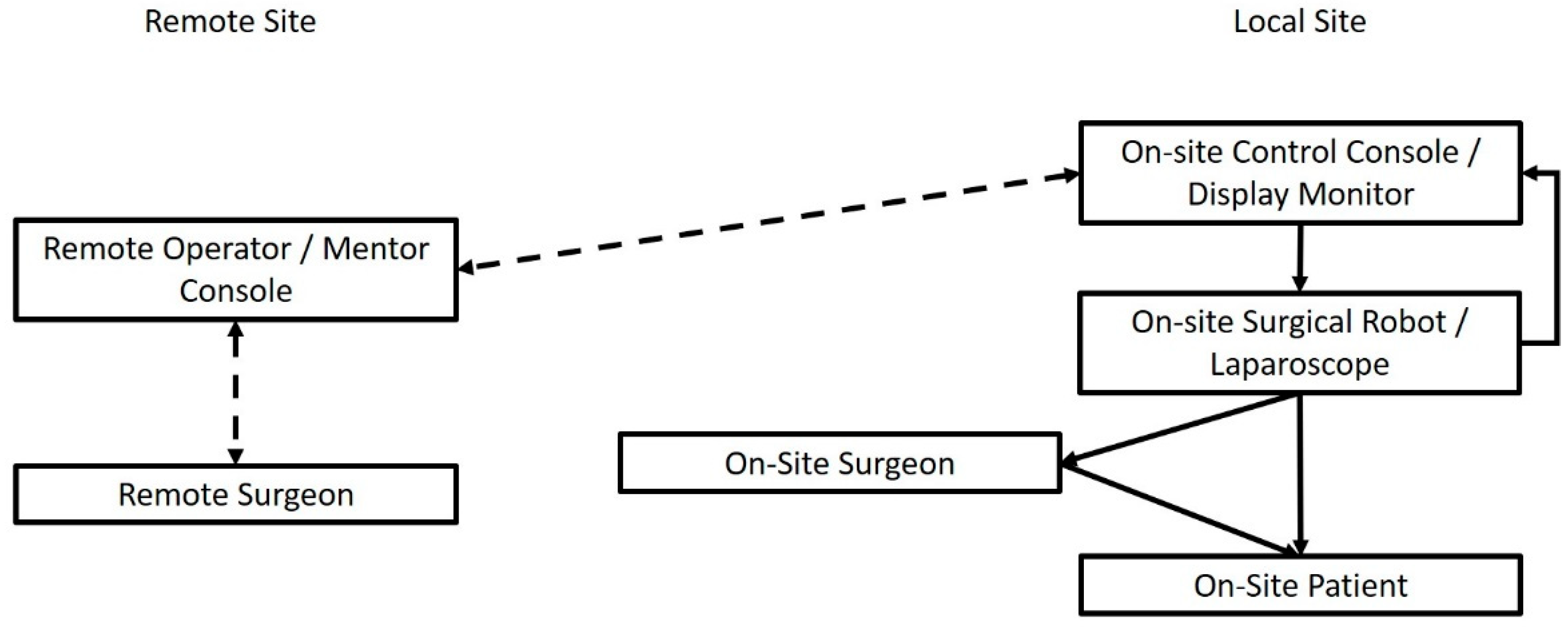
1. The Internet of Telesurgery and Surgical Telementoring
2. Image-Guided Surgery in the IoT Era
The basic principle of Image-Guided Surgery (IGS) is constituted by the utilization of a tracking device, alongside pre or even intra-operative imaging, to aid the surgeon in the spatial orientation during a surgical process. Authors of relevant publications have used IoT networks to incorporate patient imaging, as well as preoperative planning, into the operating room. The network-based sharing of data and the creation of a workflow through sequential data appraisals and data provision towards the next component makes IGS a prime example of an IoT application in surgery. An IGS system built around the IoT approach usually consists of input of preoperative imaging data of the surgical patient. These data are then used to make reconstructed models of the anatomical area of interest. Such models are then transmitted wirelessly to a modality of choice, ranging from augmented reality (AR) glasses to the viewing screen of a surgical robot [47][48][49][50][51][52]. A simplified schematic illustrating the workflow of these systems can be seen in Figure 2, encompassing the idea of the IoT concept defined as a network of inter-connected devices that process and exchange data.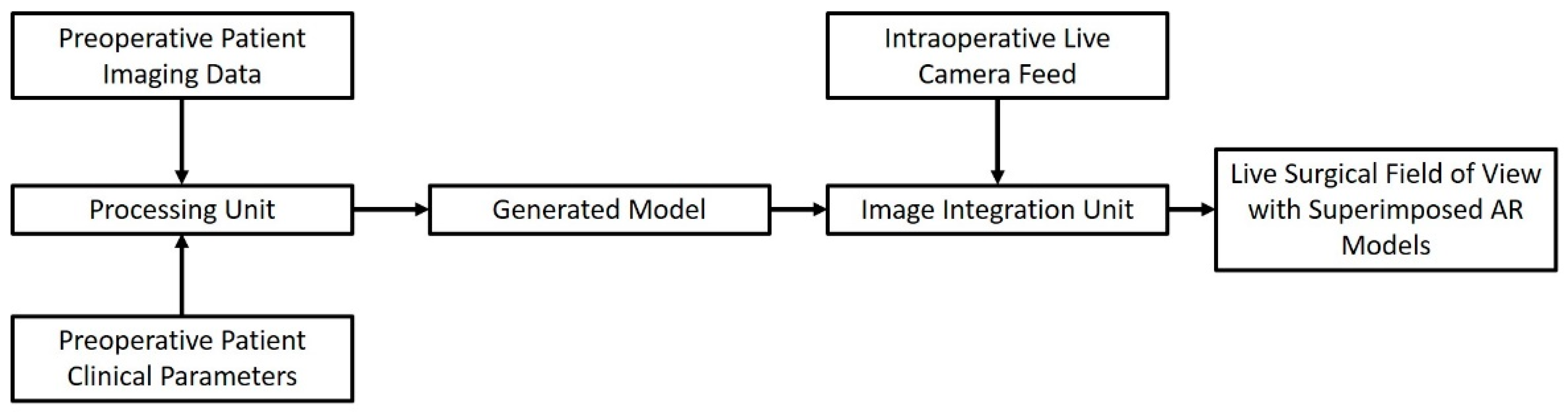
3. The Role of the IoT in Telemonitoring the Surgical Patient
Medical telemonitoring usually consists of a specialized “smart” device that captures target parameters and transmits them through a wireless Internet connection, either directly to the referring physician, or to a centralized repository from which they can be accessed (Figure 3). The role of the Internet here is more straightforward: instead of being the network substrate that interconnects a variety of operating stations, data repositories and data processing modalities, here, it is used as a unidirectional “data highway” that runs towards the physician. In contrast to previous advances, telemonitoring has been widely implemented in some healthcare systems.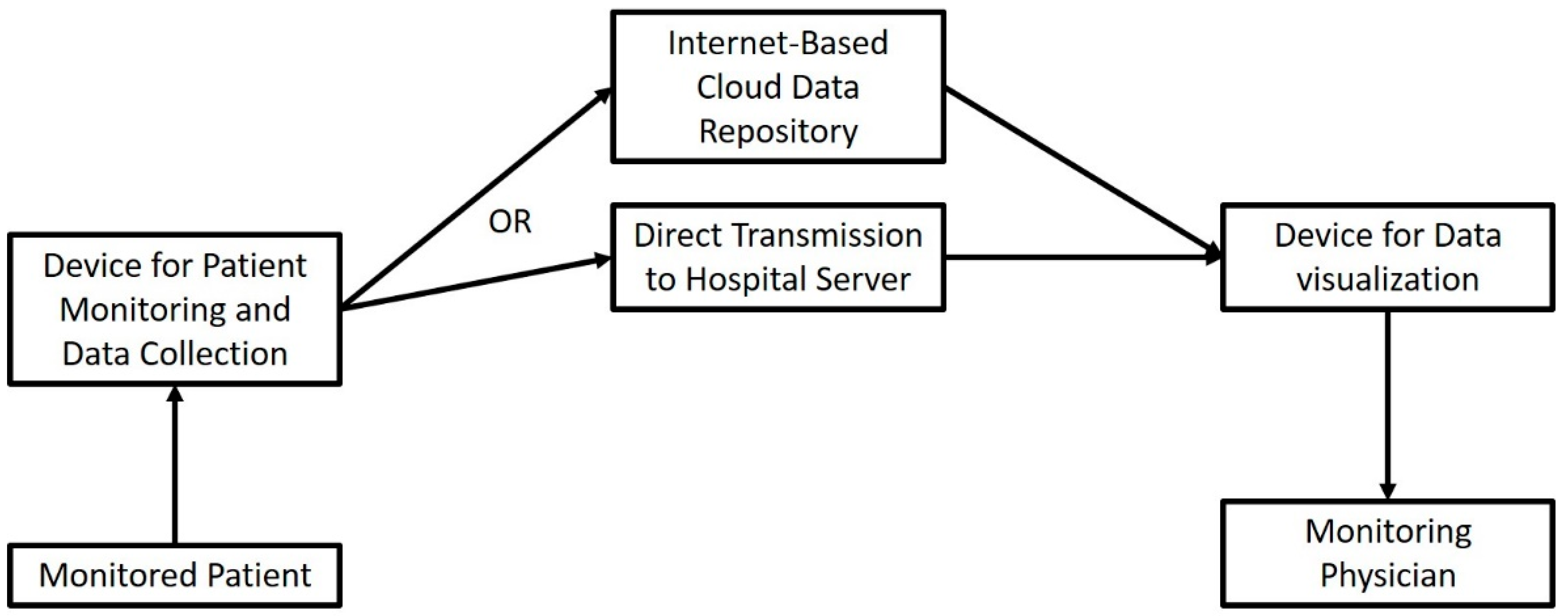
References
- Agrawal, R.; Mishra, S.K.; Mishra, A.; Chand, G.; Agarwal, G.; Agarwal, A.; Verma, A.K. Role of Telemedicine Technology in Endocrine Surgery Knowledge Sharing. Telemed. J. E-Health 2014, 20, 868–874.
- Andersen, D.; Popescu, V.; Cabrera, M.E.; Shanghavi, A.; Mullis, B.; Marley, S.; Gomez, G.; Wachs, J.P. An Augmented Reality-Based Approach for Surgical Telementoring in Austere Environments. Mil. Med. 2017, 182, 310–315.
- Anderson, S.M.; Kapp, B.B.; Angell, J.M.; Abd, T.T.; Thompson, N.J.; Ritenour, C.W.M.; Issa, M.M. Remote Monitoring and Supervision of Urology Residents Utilizing Integrated Endourology Suites—A Prospective Study of Patients’ Opinions. J. Endourol. 2013, 27, 96–100.
- Andersen, D.; Popescu, V.; Cabrera, M.E.; Shanghavi, A.; Gomez, G.; Marley, S.; Mullis, B.; Wachs, J.P. Medical Telementoring Using an Augmented Reality Transparent Display. Surgery 2016, 159, 1646–1653.
- Artsen, A.M.; Burkett, L.S.; Duvvuri, U.; Bonidie, M. Surgeon Satisfaction and Outcomes of Tele-Proctoring for Robotic Gynecologic Surgery. J. Robot. Surg. 2022, 16, 563–568.
- Prince, S.W.; Kang, C.; Simonelli, J.; Lee, Y.H.; Gerber, M.J.; Lim, C.; Chu, K.; Dutson, E.P.; Tsao, T.C. A Robotic System for Telementoring and Training in Laparoscopic Surgery. Int. J. Med. Robot. 2020, 16, e2040.
- Patel, E.; Mascarenhas, A.; Subuhee, A.; Stirt, D.; Brady, I.; Perera, R.; Noël, J. Evaluating the Ability of Students to Learn and Utilize a Novel Telepresence Platform, Proximie. J. Robot. Surg. 2021, 2021, 1–7.
- Rojas-Muñoz, E.; Cabrera, M.E.; Lin, C.; Andersen, D.; Popescu, V.; Anderson, K.; Zarzaur, B.L.; Mullis, B.; Wachs, J.P. The System for Telementoring with Augmented Reality (STAR): A Head-Mounted Display to Improve Surgical Coaching and Confidence in Remote Areas. Surgery 2020, 167, 724–731.
- Rojas-Muñoz, E.; Cabrera, M.E.; Andersen, D.; Popescu, V.; Marley, S.; Mullis, B.; Zarzaur, B.; Wachs, J. Surgical Telementoring without Encumbrance: A Comparative Study of See-through Augmented Reality-Based Approaches. Ann. Surg. 2019, 270, 384–389.
- Rojas-Munõz, E.; Cabrera, M.E.; Lin, C.; Sánchez-Tamayo, N.; Andersen, D.; Popescu, V.; Anderson, K.; Zarzaur, B.; Mullis, B.; Wachs, J.P. Telementoring in Leg Fasciotomies via Mixed-Reality: Clinical Evaluation of the STAR Platform. Mil. Med. 2020, 185, 513–520.
- Rojas-Muñoz, E.; Lin, C.; Sanchez-Tamayo, N.; Cabrera, M.E.; Andersen, D.; Popescu, V.; Barragan, J.A.; Zarzaur, B.; Murphy, P.; Anderson, K.; et al. Evaluation of an Augmented Reality Platform for Austere Surgical Telementoring: A Randomized Controlled Crossover Study in Cricothyroidotomies. NPJ Digit. Med. 2020, 3, 75.
- Safir, I.J.; Shrewsberry, A.B.; Issa, I.M.; Ogan, K.; Ritenour, C.W.M.; Sullivan, J.; Issa, M.M. Impact of Remote Monitoring and Supervision on Resident Training Using New ACGME Milestone Criteria. Can. J. Urol. 2015, 22, 7959–7964.
- Schlachta, C.M.; Lefebvre, K.L.; Sorsdahl, A.K.; Jayaraman, S. Mentoring and Telementoring Leads to Effective Incorporation of Laparoscopic Colon Surgery. Surg. Endosc. 2010, 24, 841–844.
- Talbot, M.; Harvey, E.J.; Berry, G.K.; Reindl, R.; Tien, H.; Stinner, D.J.; Slobogean, G. A Pilot Study of Surgical Telementoring for Leg Fasciotomy. BMJ Mil. Health 2018, 164, 83–86.
- Trujillo Loli, Y.; D’Carlo Trejo Huamán, M.; Campos Medina, S. Telementoring of In-Home Real-Time Laparoscopy Using Whatsapp Messenger: An Innovative Teaching Tool during the COVID-19 Pandemic. A Cohort Study. Ann. Med. Surg. 2021, 62, 481–484.
- Andersen, D.S.; Cabrera, M.E.; Rojas-Muñoz, E.J.; Popescu, V.S.; Gonzalez, G.T.; Mullis, B.; Marley, S.; Zarzaur, B.L.; Wachs, J.P. Augmented Reality Future Step Visualization for Robust Surgical Telementoring. Simul. Healthc. 2019, 14, 59–66.
- Dawe, P.; Kirkpatrick, A.; Talbot, M.; Beckett, A.; Garraway, N.; Wong, H.; Hameed, S.M. Telementored Damage-Control and Emergency Trauma Surgery: A Feasibility Study Using Live-Tissue Models. Am. J. Surg. 2018, 215, 927–929.
- DeKastle, R. Telesurgery: Providing Remote Surgical Observations for Students. AORN J. 2009, 90, 93–101.
- Din, N.; Chan, C.C.; Cohen, E.; Iovieno, A.; Dahan, A.; Rootman, D.S.; Litvin, G. Remote Surgeon Virtual Presence: A Novel Telementoring Method for Live Surgical Training. Cornea 2022, 41, 385–389.
- Hinata, N.; Miyake, H.; Kurahashi, T.; Ando, M.; Furukawa, J.; Ishimura, T.; Tanaka, K.; Fujisawa, M. Novel Telementoring System for Robot-Assisted Radical Prostatectomy: Impact on the Learning Curve. Urology 2014, 83, 1088–1092.
- Altieri, M.S.; Carmichael, H.; Jones, E.; Robinson, T.; Pryor, A.; Madani, A. Educational Value of Telementoring for a Simulation-Based Fundamental Use of Surgical EnergyTM (FUSE) Curriculum: A Randomized Controlled Trial in Surgical Trainees. Surg. Endosc. 2020, 34, 3650–3655.
- Glenn, I.C.; Bruns, N.E.; Hayek, D.; Hughes, T.; Ponsky, T.A. Rural Surgeons Would Embrace Surgical Telementoring for Help with Difficult Cases and Acquisition of New Skills. Surg. Endosc. 2017, 31, 1264–1268.
- Lenihan, J.; Brower, M. Web-Connected Surgery: Using the Internet for Teaching and Proctoring of Live Robotic Surgeries. J. Robot. Surg. 2012, 6, 47–52.
- Moore, A.M.; Carter, N.H.; Wagner, J.P.; Filipi, C.J.; Chen, D.C. Web-Based Video Assessments of Operative Performance for Remote Telementoring. Surg. Technol. Int. 2017, 25, 25–30.
- Tel, A.; Bortuzzo, F.; Pascolo, P.; Costa, F.; Sembronio, S.; Bresadola, V.; Baldi, D.; Robiony, M. Maxillofacial Surgery 5.0: A New Paradigm in Telemedicine for Distance Surgery, Remote Assistance, and Webinars. Minerva Stomatol. 2020, 69, 191–202.
- Shin, D.H.; Dalag, L.; Azhar, R.A.; Santomauro, M.; Satkunasivam, R.; Metcalfe, C.; Dunn, M.; Berger, A.; Djaladat, H.; Nguyen, M.; et al. A Novel Interface for the Telementoring of Robotic Surgery. BJU Int. 2015, 116, 302–308.
- Kirkpatrick, A.W.; McKee, J.L.; Netzer, I.; McBeth, P.B.; D’Amours, S.; Kock, V.; Dobron, A.; Ball, C.G.; Glassberg, E. Transoceanic Telementoring of Tube Thoracostomy Insertion: A Randomized Controlled Trial of Telementored Versus Unmentored Insertion of Tube Thoracostomy by Military Medical Technicians. Telemed. E-Health 2019, 25, 730–739.
- Liu, P.; Li, C.; Xiao, C.; Zhang, Z.; Ma, J.; Gao, J.; Shao, P.; Valerio, I.; Pawlik, T.M.; Ding, C.; et al. A Wearable Augmented Reality Navigation System for Surgical Telementoring Based on Microsoft HoloLens. Ann. Biomed. Eng. 2021, 49, 287–298.
- Lacy, A.M.; Bravo, R.; Otero-Piñeiro, A.M.; Pena, R.; De Lacy, F.B.; Menchaca, R.; Balibrea, J.M. 5G-Assisted Telementored Surgery. Br. J. Surg. 2019, 106, 1576–1579.
- Netzer, I.; Kirkpatrick, A.W.; Nissan, M.; McKee, J.L.; McBeth, P.; Dobron, A.; Glassberg, E. Rubrum Coelis: The Contribution of Real-Time Telementoring in Acute Trauma Scenarios-A Randomized Controlled Trial. Telemed. E-Health 2019, 25, 1108–1114.
- Greenberg, J.A.; Schwarz, E.; Paige, J.; Dort, J.; Bachman, S. At-Home Hands-on Surgical Training during COVID19: Proof of Concept Using a Virtual Telementoring Platform. Surg. Endosc. 2021, 35, 1963–1969.
- Forgione, A.; Kislov, V.; Guraya, S.Y.; Kasakevich, E.; Pugliese, R. Safe Introduction of Laparoscopic Colorectal Surgery Even in Remote Areas of the World: The Value of a Comprehensive Telementoring Training Program. J. Laparoendosc. Adv. Surg. Tech. 2015, 25, 37–42.
- Chu, G.; Yang, X.; Luo, L.; Feng, W.; Jiao, W.; Zhang, X.; Wang, Y.; Yang, Z.; Wang, B.; Li, J.; et al. Improved Robot-Assisted Laparoscopic Telesurgery: Feasibility of Network Converged Communication. Br. J. Surg. 2021, 108, e377–e379.
- Wirz, R.; Torres, L.G.; Swaney, P.J.; Gilbert, H.; Alterovitz, R.; Webster, R.J.; Weaver, K.D.; Russell, P.T. An Experimental Feasibility Study on Robotic Endonasal Telesurgery. Neurosurgery 2015, 76, 479–484.
- Zheng, J.; Wang, Y.; Zhang, J.; Guo, W.; Yang, X.; Luo, L.; Jiao, W.; Hu, X.; Yu, Z.; Wang, C.; et al. 5G Ultra-Remote Robot-Assisted Laparoscopic Surgery in China. Surg. Endosc. 2020, 34, 5172–5180.
- Park, J.W.; Lee, D.H.; Kim, Y.W.; Lee, B.H.; Jo, Y.H. Lapabot: A Compact Telesurgical Robot System for Minimally Invasive Surgery: Part II. Telesurgery Evaluation. Minim. Invasive Ther. Allied Technol. 2012, 21, 195–200.
- Acemoglu, A.; Peretti, G.; Trimarchi, M.; Hysenbelli, J.; Krieglstein, J.; Geraldes, A.; Deshpande, N.; Ceysens, P.M.V.; Caldwell, D.G.; Delsanto, M.; et al. Operating From a Distance: Robotic Vocal Cord 5G Telesurgery on a Cadaver. Ann. Intern. Med. 2020, 173, 940–941.
- Tian, W.; Fan, M.; Zeng, C.; Liu, Y.; He, D.; Zhang, Q. Telerobotic Spinal Surgery Based on 5G Network: The First 12 Cases. Neurospine 2020, 17, 114–120.
- Morohashi, H.; Hakamada, K.; Kanno, T.; Kawashima, K.; Akasaka, H.; Ebihara, Y.; Oki, E.; Hirano, S.; Mori, M. Social Implementation of a Remote Surgery System in Japan: A Field Experiment Using a Newly Developed Surgical Robot via a Commercial Network. Surg. Today 2021, 52, 705–714.
- Huang, E.Y.; Knight, S.; Guetter, C.R.; Davis, C.H.; Moller, M.; Slama, E.; Crandall, M. Telemedicine and telementoring in the surgical specialties: A narrative review. Am. J. Surg. 2019, 218, 760–766.
- Fuertes-Guiró, F.; Vitali-Erion, E.; Rodriguez-Franco, A. A Program of Telementoring in Laparoscopic Bariatric Surgery. Minim. Invasive Ther. Allied Technol. 2016, 25, 8–14.
- Sachdeva, N.; Klopukh, M.; Clair, R.S.; Hahn, W.E. Using conditional generative adversarial networks to reduce the effects of latency in robotic telesurgery. J. Robot. Surg. 2021, 11, 635–641.
- Shabir, D.; Abdurahiman, N.; Padhan, J.; Trinh, M.; Balakrishnan, S.; Kurer, M.; Ali, O.; Al-Ansari, A.; Yaacoub, E.; Deng, Z.; et al. Towards Development of a Telementoring Framework for Minimally Invasive Surgeries. Int. J. Med. Robot. 2021, 17, e2305.
- Nguyen, N.T.; Okrainec, A.; Anvari, M.; Smith, B.; Meireles, O.; Gee, D.; Moran-Atkin, E.; Baram-Clothier, E.; Camacho, D.R. Sleeve gastrectomy telementoring: A SAGES multi-institutional quality improvement initiative. Surg. Endosc. 2018, 32, 682–687.
- Snyderman, C.H.; Gardner, P.A.; Lanisnik, B.; Ravnik, J. Surgical telementoring: A new model for surgical training. Laryngoscope 2016, 126, 1334–1338.
- Kirkpatrick, A.W.; Tien, H.; LaPorta, A.T.; Lavell, K.; Keillor, J.; Beatty, H.E.W.L.; McKee, J.L.; Brien, S.; Robert, D.J.; Wong, J.; et al. The marriage of surgical simulation and telementoring for damage-control surgical training of operational first responders: A pilot study. J. Trauma Acute Care Surg. 2015, 79, 741–747.
- Li, G.; Su, H.; Cole, G.A.; Shang, W.; Harrington, K.; Camilo, A.; Pilitsis, J.G.; Fischer, G.S. Robotic System for MRI-Guided Stereotactic Neurosurgery. IEEE Trans. Biomed. Eng. 2015, 62, 1077–1088.
- Louis, R.G.; Steinberg, G.K.; Duma, C.; Britz, G.; Mehta, V.; Pace, J.; Selman, W.; Jean, W.C. Early Experience with Virtual and Synchronized Augmented Reality Platform for Preoperative Planning and Intraoperative Navigation: A Case Series. Oper. Neurosurg. 2021, 21, 189–196.
- Ivan, M.E.; Eichberg, D.G.; Di, L.; Shah, A.H.; Luther, E.M.; Lu, V.M.; Komotar, R.J.; Urakov, T.M. Augmented Reality Head-Mounted Display–Based Incision Planning in Cranial Neurosurgery: A Prospective Pilot Study. Neurosurg. Focus 2021, 51, E3.
- De Momi, E.; Ferrigno, G.; Bosoni, G.; Bassanini, P.; Blasi, P.; Casaceli, G.; Fuschillo, D.; Castana, L.; Cossu, M.; Lo Russo, G.; et al. A Method for the Assessment of Time-Varying Brain Shift during Navigated Epilepsy Surgery. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 473–481.
- Kiarostami, P.; Dennler, C.; Roner, S.; Sutter, R.; Fürnstahl, P.; Farshad, M.; Rahm, S.; Zingg, P.O. Augmented Reality-Guided Periacetabular Osteotomy—Proof of Concept. J. Orthop. Surg. Res. 2020, 15, 540.
- Padilla, J.B.; Arango, R.; García, H.F.; Cardona, H.D.V.; Orozco, Á.A.; Álvarez, M.A.; Guijarro, E. NEURONAV: A Tool for Image-Guided Surgery—Application to Parkinson’s Disease. In International Symposium on Visual Computing; Springer: Cham, Switzerland, 2015; Volume 9474, pp. 349–358.
- Watanabe, E.; Satoh, M.; Konno, T.; Hirai, M.; Yamaguchi, T. The Trans-Visible Navigator: A See-Through Neuronavigation System Using Augmented Reality. World Neurosurg. 2016, 87, 399–405.
- Yoon, J.W.; Chen, R.E.; ReFaey, K.; Diaz, R.J.; Reimer, R.; Komotar, R.J.; Quinones-Hinojosa, A.; Brown, B.L.; Wharen, R.E. Technical Feasibility and Safety of Image-Guided Parieto-Occipital Ventricular Catheter Placement with the Assistance of a Wearable Head-up Display. Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13, e1836.
- Fan, X.; Roberts, D.W.; Schaewe, T.J.; Ji, S.; Holton, L.H.; Simon, D.A.; Paulsen, K.D. Intraoperative Image Updating for Brain Shift Following Dural Opening. J. Neurosurg. 2017, 126, 1924–1933.
- Eftekhar, B. A Smartphone App to Assist Scalp Localization of Superficial Supratentorial Lesions--Technical Note. World Neurosurg. 2016, 85, 359–363.
- Hu, L.; Wang, M.; Song, Z. A Convenient Method of Video See-through Augmented Reality Based on Image-Guided Surgery System. In Proceedings of the 2013 Seventh International Conference on Internet Computing for Engineering and Science, Shanghai, China, 20–22 September 2013; Volume 2013, pp. 100–103.
- Guo, Z.; Dong, Z.; Lee, K.H.; Cheung, C.L.; Fu, H.C.; Ho, J.D.L.; He, H.; Poon, W.S.; Chan, D.T.M.; Kwok, K.W. Compact Design of a Hydraulic Driving Robot for Intraoperative MRI-Guided Bilateral Stereotactic Neurosurgery. IEEE Robot. Autom. Lett. 2018, 3, 2515–2522.
- Ushimaru, Y.; Takahashi, T.; Souma, Y.; Yanagimoto, Y.; Nagase, H.; Tanaka, K.; Miyazaki, Y.; Makino, T.; Kurokawa, Y.; Yamasaki, M.; et al. Innovation in Surgery/Operating Room Driven by Internet of Things on Medical Devices. Surg. Endosc. 2019, 33, 3469–3477.
- Cos, H.; Li, D.W.; Williams, G.; Chininis, J.; Dai, R.X.; Zhang, J.W.; Srivastava, R.; Raper, L.; Sanford, D.; Hawkins, W.; et al. Predicting Outcomes in Patients Undergoing Pancreatectomy Using Wearable Technology and Machine Learning: Prospective Cohort Study. J. Med. Internet Res. 2021, 23, 11.
- Wildemeersch, D.; D’Hondt, M.; Bernaerts, L.; Mertens, P.; Saldien, V.; Hendriks, J.M.; Walcarius, A.S.; Sterkens, L.; Hans, G.H. Implementation of an Enhanced Recovery Pathway for Minimally Invasive Pectus Surgery: A Population-Based Cohort Study Evaluating Short- and Long-Term Outcomes Using E-Health Technology. JMIR Perioper. Med. 2018, 1, e10996.
- Msayib, Y.; Gaydecki, P.; Callaghan, M.; Dale, N.; Ismail, S. An Intelligent Remote Monitoring System for Total Knee Arthroplasty Patients. J. Med. Syst. 2017, 41, 90.
- Peysson, L.; Gomez, C.; Giovannetti, P.; Coltey, B.; Dufeu, N.; Bregeon, E.; Gaubert, J.; Dutau, H.; Thomas, P.; Reynaud-Gaubert, M. Internet-Based Telemonitoring System of Daily Home Spirometry in Lung Transplant Recipients. J. Heart Lung Transplant. 2015, 34, S141.
- Jonker, L.T.; Lahr, M.M.H.; Festen, S.; Oonk, M.H.M.; de Bock, G.H.; van Leeuwen, B.L. Perioperative Telemonitoring of Older Adults with Cancer: Can We Connect Them All? J. Geriatr. Oncol. 2020, 11, 1244–1249.
- Cornelis, N.; Buys, R.; Dewit, T.; Benoit, D.; Claes, J.; Fourneau, I.; Cornelissen, V. Satisfaction and Acceptability of Telemonitored Home-Based Exercise in Patients with Intermittent Claudication: Pragmatic Observational Pilot Study. JMIR Rehabil. Assist. Technol. 2021, 8, e18739.
- Mullen-Fortino, M.; Rising, K.L.; Duckworth, J.; Gwynn, V.; Sites, F.D.; Hollander, J.E. Presurgical Assessment Using Telemedicine Technology: Impact on Efficiency, Effectiveness, and Patient Experience of Care. Telemed. E-Health 2019, 25, 137–142.
- Vilallonga, R.; Lecube, A.; Fort, J.M.; Boleko, M.A.; Hidalgo, M.; Armengol, M. Internet of Things and Bariatric Surgery Follow-up: Comparative Study of Standard and IoT Follow-Up. Minim. Invasive Ther. Allied Technol. 2013, 22, 304–311.
- Luo, M.; Yuan, R.; Sun, Z.; Li, T.; Xie, Q. A web-based computer aided system for liver surgery planning: Initial implementation on RayPlus. In Proceedings of the Medical Imaging 2016: Image-Guided Procedures, Robotic Interventions, and Modeling, San Diego, CA, USA, 18 March 2016; pp. 504–509.
- Yanni, R.M.T.; El-Bakry, H.M.; Riad, A.; El-Khamisy, N. Internet of Things for Surgery Process Using Raspberry Pi. Int. J. Online Biomed. Eng. 2020, 16, 96–115.
- Caggianese, G.; Calabrese, M.; Gallo, L.; Sannino, G.; Vecchione, C. Cardiac surgery rehabilitation system (CSRS) for a personalized support to patients. In Proceedings of the International IEEE Conference on Signal-Image Technologies and Internet-Based System, Jaipur, India, 4–7 December 2017; pp. 83–90.
- Wang, R.; Wang, S.; Duan, N.; Wang, Q. From Patient-Controlled Analgesia to Artificial Intelligence-Assisted Patient-Controlled Analgesia: Practices and Perspectives. Front. Med. 2020, 7, 145.
- McGillion, M.; Ouellette, C.; Good, A.; Bird, M.; Henry, S.; Clyne, W.; Turner, A.; Ritvo, P.; Ritvo, S.; Dvirnik, N.; et al. Postoperative Remote Automated Monitoring and Virtual Hospital-to-Home Care System Following Cardiac and Major Vascular Surgery: User Testing Study. J. Med. Internet Res. 2020, 22, e15548.
- Colomina, J.; Drudis, R.; Torra, M.; Pallisó, F.; Massip, M.; Vargiu, E.; Nadal, N.; Funentes, A.; Bravo, M.O.; Miralles, F.; et al. Implementing mHealth-Enabled Integrated Care for Complex Chronic Patients With Osteoarthritis Undergoing Primary Hip or Knee Arthroplasty: Prospective, Two-Arm, Parallel Trial. J. Med. Internet Res. 2021, 23, e28320.
- Holmes, M.; Nieto, M.P.; Song, H.; Tonkin, E.; Grant, S.; Flach, P. Modelling Patient Behaviour Using IoT Sensor Data: A Case Study to Evaluate Techniques for Modelling Domestic Behaviour in Recovery from Total Hip Replacement Surgery. J. Healthc. Inform. Res. 2020, 4, 238–260.
- Kong, H.; Chen, J. Medical Monitoring and Management System of Mobile Thyroid Surgery Based on Internet of Things and Cloud Computing. Wirel. Commun. Mob. Comput. 2021, 2021, 7065910.
- Rouholiman, D.; Gamble, J.G.; Dobrota, S.D.; Encisco, E.M.; Shah, A.G.; Grajales, F.J., III; Chu, L.F. Improving Health-Related Quality of Life of Patients With an Ostomy Using a Novel Digital Wearable Device: Protocol for a Pilot Study. JMIR Res. Protoc. 2018, 7, e7470.
- Kim, S.H.; Shin, H.S.; Lee, S.H. ‘Internet of Things’ Real-Time Free Flap Monitoring. J. Craniofac. Surg. 2018, 29, e22–e25.
