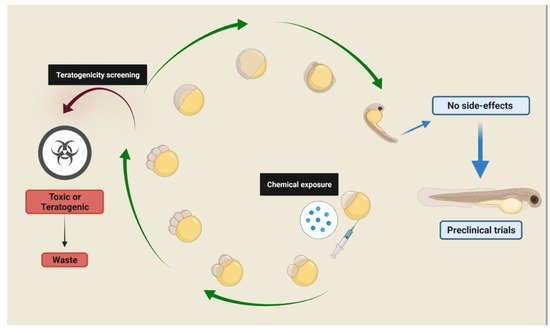
Drug screenings using the zebrafish embryo-larval stage fit the concept of replacement, reduction, and refinement (3Rs) in an effective way and can replace other vertebrates in studies involving all organic systems
[6]. Zebrafish independent feeding comprises several criteria such as a completely developed digestive tract, swimming ability to pursue the prey/food, and total yolk depletion, and these physiological events occur between 120 and 144 hpf
[7]. Concerning behavioral features, zebrafish are able to exhibit coordinate behavior in response to different stimuli and present major neuromodulatory systems by 96 hpf
[8][9][8,9]. Thus, according to Strahle et al. (2012), larvae below 120 hpf are considered an alternative model to animal research once they are classified as non-protected under the EU Directive of animal welfare (EU 2010/63/EU)
[6]. However, research using early-life stages of zebrafish still obeys the concept of 3Rs
[10].
The advantages of using an intact animal as a focus for screening are particularly evident for neurologic drug discovery, where the complexities of cell-cell interactions and endocrine signaling are challenging. In addition, behavior analysis can be combined with transgenic strains using methods to generate targeted genetic modification such as Clustered Regularly Interspaced Short Palindromic Repeats-associate protein 9 (CRISPR/Cas9), RNA interference (RNAi), zinc-finger nucleases (ZFNs), and antisense oligonucleotide morpholinos
[11].
Whereas cell-based assays provide limited information on the absorption, distribution, metabolism, excretion, and toxicity (ADME-Tox) of compounds, zebrafish screenings reveal insights into these pharmacological characteristics as zebrafish larvae have functional livers, kidneys, and blood–brain (BBB) and blood–retinal barriers (BRB)
[12][13][12,13], as well as drug-metabolizing enzymes and metabolic rates comparable to humans
[14]. To produce phenotypes in vivo in zebrafish assays, compounds must exhibit the ability to be absorbed, reach target tissue, and avoid rapid metabolism and excretion. This fact may explain the observation that several compounds that were discovered in zebrafish screenings were rapidly translated to mammalian models in vivo with minimal optimization of pharmacological properties.
2. Zebrafish Neurotransmitter Systems
The neuromodulatory circuits present in mammals can also be found in the larval zebrafish brain. Major neurotransmitter systems are conserved through vertebrates, and in zebrafish, glutamate, gamma-aminobutyric acid (GABA), acetylcholine, dopamine, serotonin (hydroxytryptamine or 5-HT), noradrenaline, and histamine systems are well described
[15][24]. Alterations in patterns of transmission are related to neurological disorders
[16][25].
The main excitatory neurotransmitter in vertebrates is glutamate, which regulates synaptic transmission and neuronal excitability. The 24 hpf zebrafish larvae present vesicular glutamate transporters (VGLUT2), and by 96 hpf zebrafish larvae express VGLUT 1 and VGLUT 2, besides glutamate metabotropic and ionotropic receptors in the olfactory bulb, optic tectum, hypothalamus, cerebellum, and retina
[17][26]. GABA is an inhibitory neurotransmitter expressed both in early-life stages and in adulthood
[18][19][27,28]. In zebrafish, GABA-ergic neurons appear in the olfactory bulb, subpallium, posterior preoptic area, the diencephalic basal plate, the central optic tectum, torus semicircularis, ventral mesencephalic tegmentum, valvula of the cerebellum and medulla oblongata
[20][29].
Zebrafish also present catecholamines as a major neurotransmitter. Noradrenaline (NA) acts in the autonomic nervous system and controls cognition, including learning and memory, as well as arousal and reward systems; the zebrafish noradrenergic system is very similar to mammals
[21][22][30,31]. In the same way, the histaminergic system is quite similar to mammalian and exerts an effect on memory, cognition, and circadian rhythm
[23][32]. Serotonin (5-HT) is a neurotransmitter present in the embryonic stage in the spinal cord and in the telencephalon, hindbrain, and the raphe region in zebrafish larvae and adults, with a clear correlation between life stages
[22][31].
Behavioral processes such as aggression, anxiety, cognition, and sleep are modulated by 5-HT. According to Ek et al. (2016), zebrafish have all the dopamine receptors, except dopamine receptor type 5 D5
[24][33]. The dopaminergic system is an important player in the regulation of locomotion of zebrafish larvae. Furthermore, zebrafish present correlated behavioral phenotypes to rats and humans, followed by dopaminergic system manipulation
[25][26][34,35].
Under stress conditions, zebrafish activate the hypothalamus-pituitary-interrenal (HPI) neuroendocrine axis, culminating in cortisol secretion, similar to humans. Cortisol binds to glucocorticoid receptors, regulating transcriptional responses related to glucose metabolism, ion regulation, immune system, and, ultimately, behavior
[27][36]. Cholinergic neurons appear at the embryonic stage and are amply distributed in the CNS of adults. The enzyme acetylcholinesterase (AChE) expression is initially found in 4 hpf embryos and increases by 210-folds in 144 hpf larvae
[28][37]. In zebrafish, similarly to humans, acetylcholine acts in cholinergic receptors, muscarinic and nicotinic
[29][30][38,39], modulating cognitive processes.
The similarity in the zebrafish neuroendocrine repertoire added to easy genetic and pharmacological modulation allows the reproduction of complex behavioral models that mirror those of human neurological disorders such as Alzheimer’s disease
[31][32][40,41], Parkinson’s disease
[33][34][42,43], depression and anxiety
[19][35][28,44], epilepsy
[36][37][38][45,46,47], and amyotrophic lateral sclerosis—ALS
[39][40][48,49]. In this way, the use of behavioral models in zebrafish larvae supports the study and development of drugs for the CNS.
3. Neurological Functions and Behavior Models towards Pre-Clinical Assays
Zebrafish are diurnal and can accomplish behavioral tasks under a normal light setting. Tests with zebrafish can be performed quickly, with large numbers of compounds in parallel in contained testing arenas, through the integration of infrared cameras with programmable stimuli control. Moreover, in experimental conditions, all behavioral phenomena can be quantified by high-level automated tools
[41][42][50,51]. In addition to generating results as satisfactory as those obtained in experiments with adults, it provides a reduction in the time of experiments and in the size of the apparatus for carrying them out, which makes studies with zebrafish larvae more efficient.
Neurological functions such as spatial and social learning, memory, anxiety, and social or sickness behaviors driven by neurotransmitters can be exploited in zebrafish larvae behavior models. Behavioral phenotypes detected in both larval and adult stages of zebrafish can be separated by social behavior (exploratory and locomotor abilities) and sickness behavior, which is characterized by lethargy, anxiety, reduced physiological function such as locomotor activity, and exploratory and social interaction
[43][52]. Escape swimming in response to touch and sound is a reflexive response, despite great complexity.
Behavioral phenomena such as memory or processing and spatial learning take place in the lateral pallium of the telencephalic area of zebrafish, and fear response is associated with the habenula, like mammalian hippocampus and amygdala, respectively
[44][45][46][53,54,55]. Among the main types of learning and memory models in zebrafish larvae, it is possible to mention habituation, characterized by an animal’s response to repeated stimuli; sensitization, based on painful or noxious stimuli; conditioning, which consists of associating a neutral stimulus with a reinforcing stimulus; and social learning, based on the animal’s preference to shoal formation
[21][30].
Furthermore, zebrafish explore novel objects or environments with more emphasis than the known ones
[47][48][56,57], and according to Santacà, Dadda, Petrazzini, and Bisazza (2021), zebrafish can distinguish novel or known objects with different sizes, shapes, and colors
[49][58]. Besides this visual discrimination learning, zebrafish show a robust cognitive repertoire such as avoidance learning, spatial learning, and reinforcement-based learning
[50][59]. Environmental novelty induces a robust behavioral response in zebrafish, in both larvae and adults
[46][51][52][55,60,61]. As in other vertebrates, the zebrafish response to novelty is consistent with an anxiety-like behavior; since the same neurotransmitters and neuroendocrine system are also present
[20][53][54][29,62,63].
In a stressful situation, which evokes the activation of the HPI axis and the action of the corticotropin-releasing hormone (CRH) cascade, culminating with cortisol release, zebrafish response is consistent with an anxiety-like behavior
[51][52][55][60,61,64]. In behavioral assays, anxiety states are reflected by reduced exploration, and this behavior is clearly demonstrated by zebrafish, along with freezing episodes and erratic movement
[56][57][58][65,66,67] (
Table 1).
Table 1.
Selected studies using zebrafish larvae as experimental model in behavior paradigms.
|
BEHAVIORAL TEST
|
ENDPOINTS
|
REFERENCE
|
|
LIGHT-DARK TEST
|
Total distance traveled
|
[59][68]
|
|
VISUAL MOTOR RESPONSE
|
Velocity, total distance moved, and mobility time
|
[60][69]
|
|
LOCOMOTOR ACTIVITY
|
Velocity, total distance moved, and mobility time
|
[61][70]
|
|
LOCOMOTOR ACTIVITY
|
Total distance traveled
|
[62][71]
|
|
ACOUSTIC STARTLE RESPONSE
|
Head angle
|
[63][72]
|
|
VISUAL MOTOR RESPONSE
|
Total distance traveled
|
[64][73]
|
|
VISUAL MOTOR RESPONSE
|
Average distance traveled
|
[65][74]
|
|
VISUAL MOTOR RESPONSE
|
Burst swim
|
[66][75]
|
|
VISUAL MOTOR RESPONSE
|
Total distance traveled
|
[67][76]
|
|
VIBRATIONAL STARTLE RESPONSE
|
Total distance traveled
|
[67][76]
|
|
LOCOMOTOR ACTIVITY
|
Total distance traveled, mean speed, turn angle
|
[68][77]
|
|
THIGMOTAXIS
|
Entries in outer area
|
[68][77]
|
|
LIGHT-DARK TEST
|
Total distance traveled
|
[69][78]
|
|
THIGMOTAXIS
|
Distance traveled in outer area
|
[69][78]
|
|
THIGMOTAXIS
|
Percentage of distance moved in outer zone
|
[70][79]
|
|
VISUAL MOTOR RESPONSE
|
Total distance traveled
|
[71][80]
|
|
THIGMOTAXIS
|
Distance traveled/time spent in each zone
|
[71][80]
|
|
THIGMOTAXIS
|
Percentage of distance moved in outer zone
|
[72][81]
|
|
LOCOMOTOR ACTIVITY
|
Average distance traveled
|
[72][81]
|
|
PHOTOMOTOR RESPONSE
|
Movements/5 min
|
[73][82]
|
|
LOCOMOTOR ACTIVITY
|
Total distance traveled
|
[73][82]
|
|
VISUAL MOTOR RESPONSE
|
Total distance traveled
|
[74][83]
|