Pyrazoles are known as versatile scaffolds in organic synthesis and medicinal chemistry, often used as starting materials for the preparation of more complex heterocyclic systems with relevance in the pharmaceutical field. Pyrazoles are also interesting compounds from a structural viewpoint, mainly because they exhibit tautomerism. This phenomenon may influence their reactivity, with possible impact on the synthetic strategies where pyrazoles take part, as well as on the biological activities of targets bearing a pyrazole moiety, since a change in structure translates into changes in properties. Investigations of the structure of pyrazoles that unravel the tautomeric and conformational preferences are therefore of upmost relevance. 3(5)-Aminopyrazoles are largely explored as precursors in the synthesis of condensed heterocyclic systems, namely pyrazolo[1,5-a]pyrimidines.
- pyrazoles
- tautomerism
- prototropy
- annular tautomerism
- reactivity
- 3(5)-aminopyrazoles
- heterocyclic synthesis
- pyrazolo[1
- 5-a]pyrimidines
1. Introduction
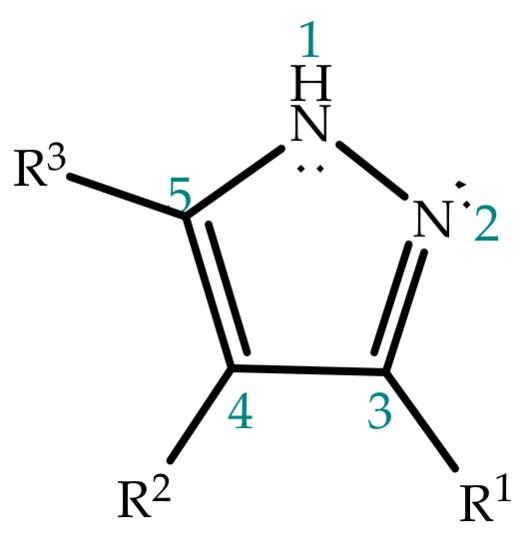
2. Pyrazole Properties
Pyrazole has a five-membered aromatic ring structure containing two vicinal nitrogen atoms, an acidic pyrrole-like nitrogen with a lone pair of electrons involved in aromaticity, a basic sp2-hybridized pyridine-like nitrogen and three carbon atoms (Figure 2) [34], and these combined features must be carefully taken into account in the context of reactivity. In the first place, given the nature of the nitrogen, N-unsubstituted pyrazoles hold amphoteric properties, acting as both acids and bases. While the acidic pyrrole-like NH group easily donates its proton, the basic pyridine-like nitrogen has the ability to accept protons even more readily, and hence, the basic character is generally prevalent. Nevertheless, substitutions on the ring can modulate these properties, as, for instance, electron donating groups were shown to increase the acidity of the pyrrole-like NH group [35][36][37]. Substituent effect and other modulators of proton transfer will be discussed in detail further in this work.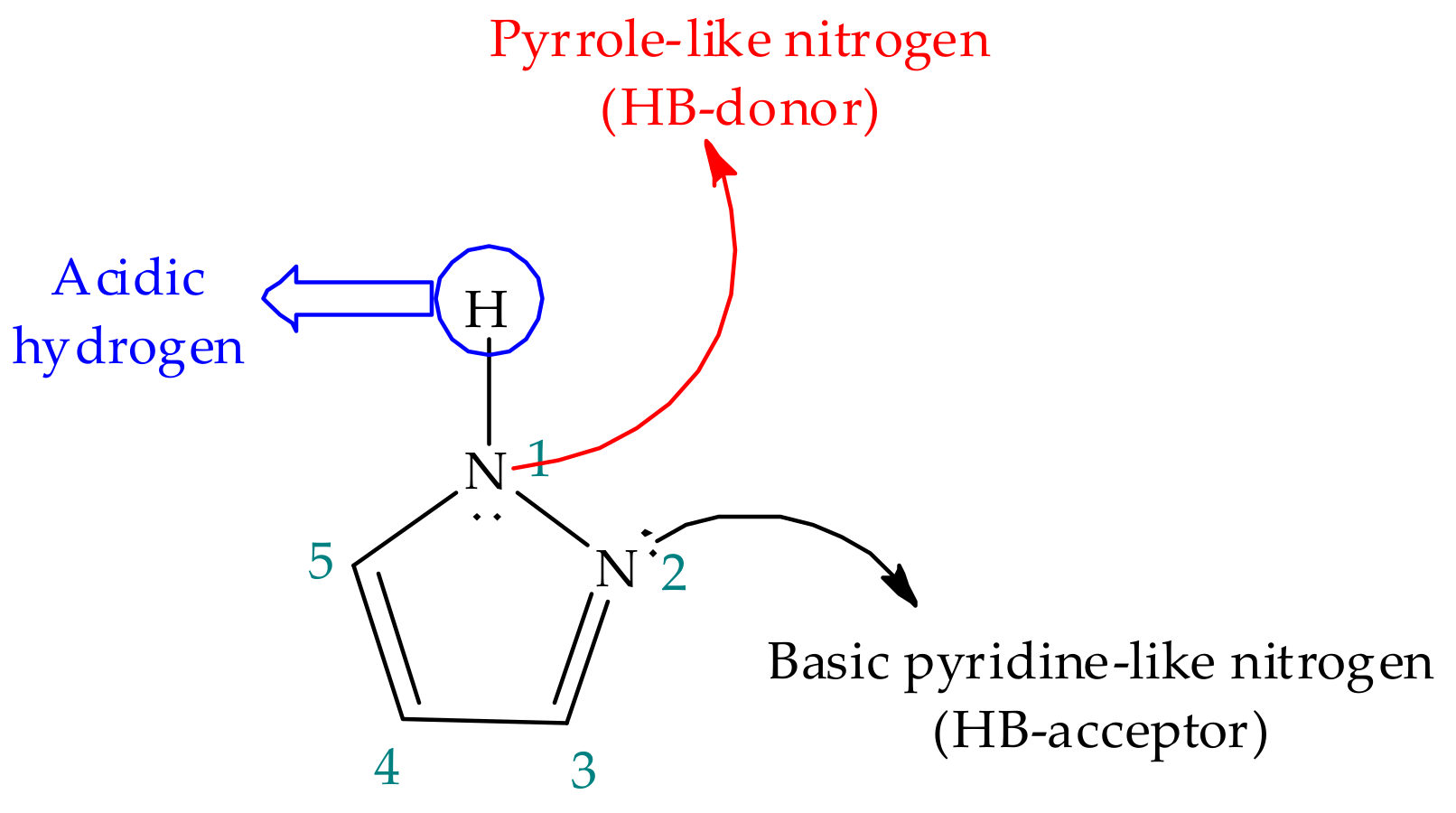
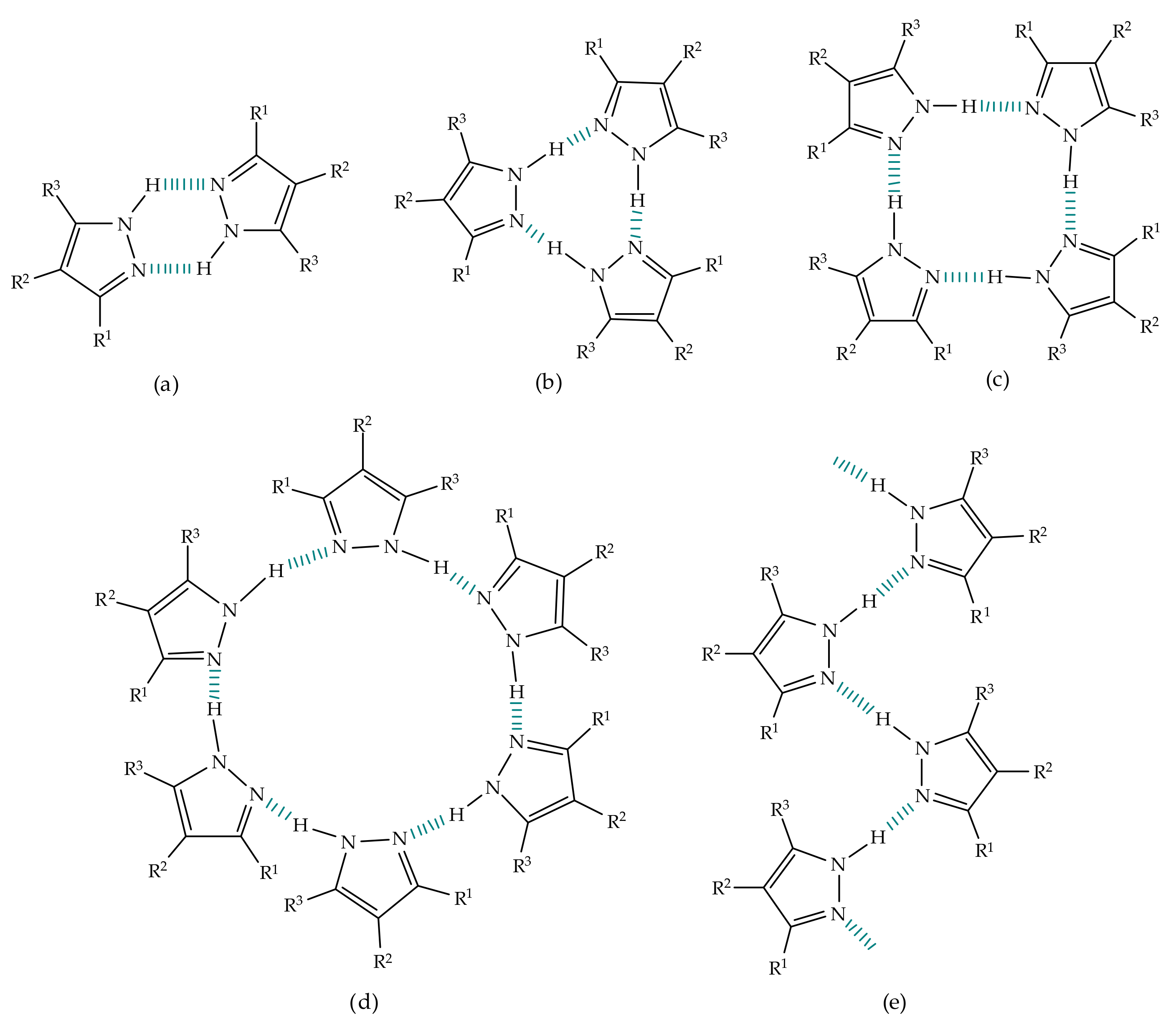
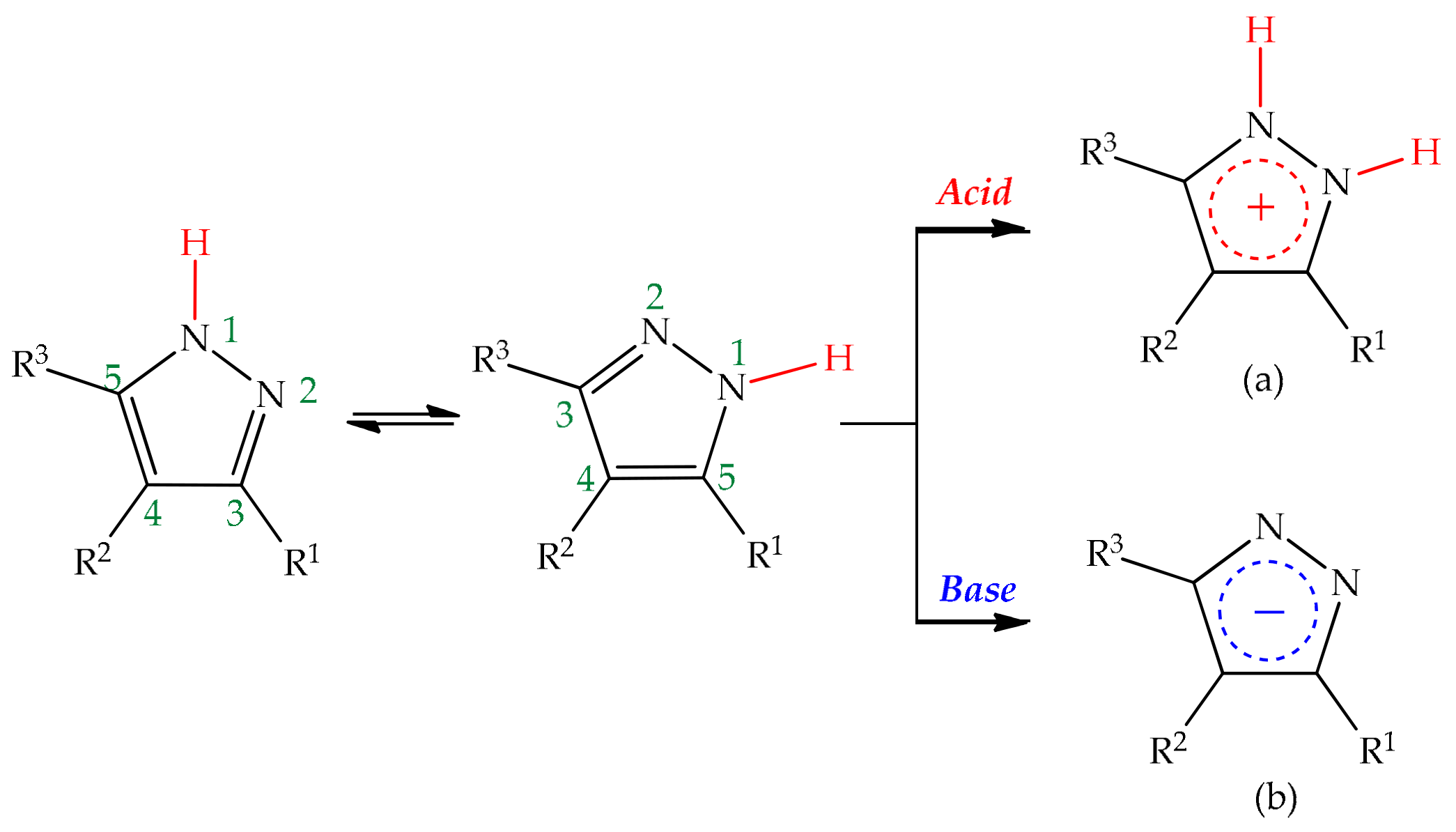
3. Tautomerism in Pyrazoles
Tautomerism is a key feature that stems from the aforementioned ability of pyrazoles to exchange protons. Ludwig Knorr, who discovered the mononuclear pyrazole motif as he stumbled upon a pyrazolone scaffold, during attempts to synthesize quinolones, was also one of the first chemists to contribute to the elucidation of the tautomerism phenomenon, back in the 19th century [1][45][46]. Classically, the concept of tautomerism is associated to a mobile equilibrium between two or more isomeric structures that interconvert easily, with energetic barriers for interconversion below circa 20 kcal/mol, i.e., the different forms may coexist in the same medium [47]. This phenomenon can manifest through several distinct forms, according to the type of exchange present. In heterocyclic systems, two criteria must be considered when discussing tautomerism: the structural aspect of the exchange and the nature of the exchanged element. Regarding the structural aspect, tautomerism is divided into four main types: annular tautomerism, side-chain tautomerism, ring-chain tautomerism and valence tautomerism. In the first type, as the name suggests, the alterations occur within the ring system, between annular carbon and heteroatoms such as nitrogen and oxygen; in the second type, the side-chain elements participate in the interconversion involving the ring; the other two types are characterized by transformations leading to bond formation or rupture—while in ring-chain tautomerism, the migration of the species on the side chain results in ring closure, the valence tautomerism is not a result of migrations of any group or element but rather a rupture or formation of bonds carried by an energetic stimulus [47][48][49][50]. Regarding the type of element involved in the interconversion, tautomerism can be subdivided into prototropy, when the exchange is characterized by the displacement of a proton between two positions suffering alteration, elementotropy, when a heavier element participates in the transformation, frequently a metal atom (metallotropy), and aniontropy and cationtropy, where the two isomers differ only on the position of an anion or cation, respectively [42][49][51][52]. Tautomeric equilibria do not happen solely within one molecule, they can be both intra and intermolecular phenomena. To set straight which kind of process is ongoing, one must take into account the accordance with the thermodynamic and kinetic principles of tautomerism as well as the substitution patterns and the environment conditions to which the compounds are submitted, such as the solvent medium, if present, since solvents usually catalyze isomeric transformations, as the presence and ratio of the tautomers strongly depend on these factors [53]. In other words, many internal and external factors influence tautomeric equilibria, with the dielectric constant of the medium and the temperature also intervening as active modulators of these transformations [47]. It was shown by Alkorta et al. that the proton exchange in pyrazoles is an intermolecular process rather than an intramolecular one, since the energy barrier determined for the intramolecular process displays values surrounding 50 kcal/mol, while the observed values for the intermolecular counterpart do not surpass the range of 10–14 kcal/mol [54]. The information available indicates that protons migrate either with the help of third parties, such as solvent molecules, as for example water molecules were shown to reduce the activation energies when attached to pyrazole, both in the gas-phase and in liquid environment [44], or of other pyrazole molecules, in self-assembled complexes.4. Pyrazoles in Organic Synthesis
Pyrazoles are electron-rich heterocyclic systems which can be readily employed in organic synthesis, given their versatile chemistry. Within the pyrazole scaffold, besides the acidic and basic properties referred in the previous chapters, three positions display nucleophilic nature (N1, N2, C4) and two electrophilic ones (C3, C5), represented in Figure 4 as blue and red rectangles, respectively. This way, pyrazole functionalization may be sought at any ring position, with nucleophiles adding preferentially to C3 and C5 positions, whereas electrophile addition occurs preferentially at C4 position and/or to any of the nitrogen atoms, depending on the reaction conditions. One way to efficiently introduce substituents at annular carbons is through directed cross-coupling reactions, such as Suzuki-couplings [55], in which case C4-halogenations are usually necessary as prior steps to boronic acid or ester cross-coupling at the indicated position [56].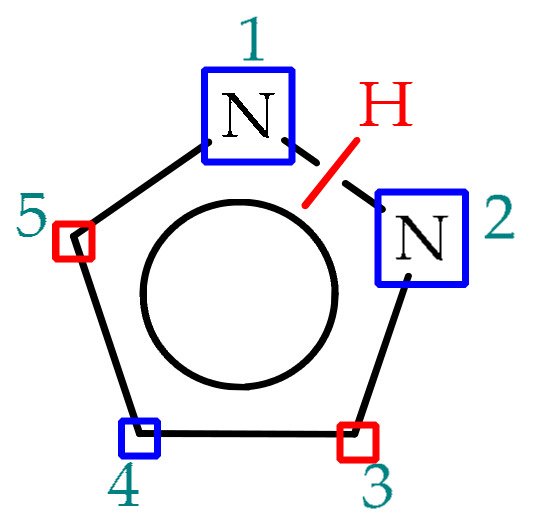
5. 3(5)-Aminopyrazole
5.1. Tautomerism in 3(5)-Aminopyrazole
3(5)-Aminopyrazoles bear an amine substituent at the positions 3 or 5 of the pyrazole ring, as a result of prototropic annular tautomerism. In theory, the frame of 3(5)-aminopyrazole is also susceptible to side-chain tautomerism, given the presence of an amine side-chain prone to participate in proton exchange. Therefore, four tautomeric structures should be expected for 3(5)-aminopyrazoles, as depicted in Scheme 2. However, studies indicate that the imino forms are not formed, and therefore only an annular rearrangement between positions 1 and 2 is predicted for 3(5)-aminopyrazoles (structures (a) and (d) of Scheme 2), as happens for pyrazole [50][62].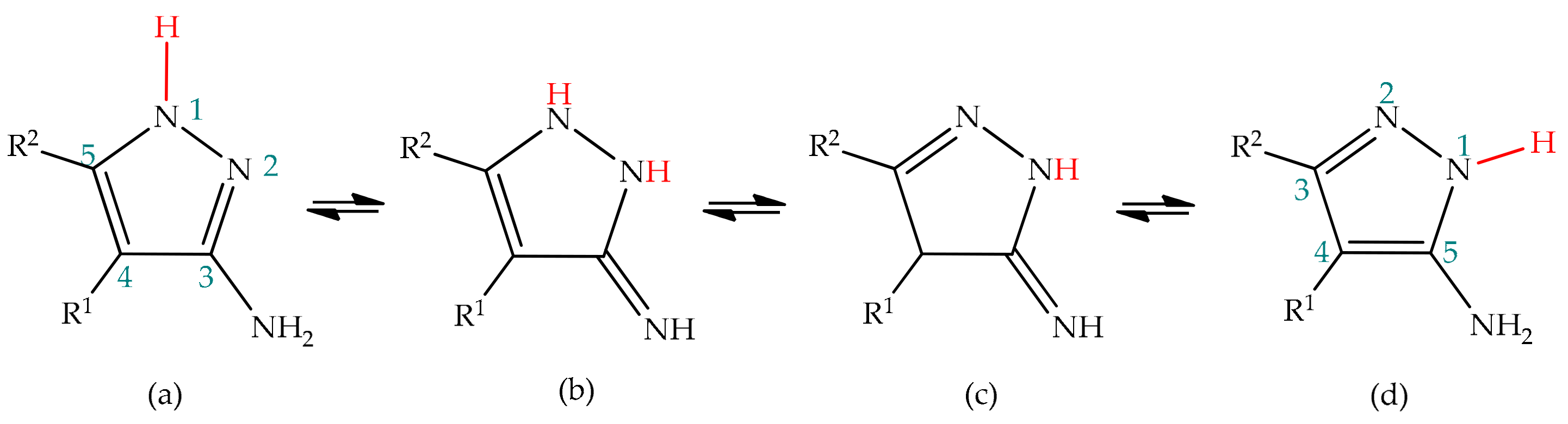
5.2. 3(5)-Aminopyrazole in Heterocyclic Synthesis
Synthesis of 3(5)-aminopyrazoles is usually carried out by incorporating the NH2 group in the synthons rather than functionalization of the pre-built pyrazole, which corresponds most commonly to condensation reactions of 1,3-dielectrophilic nitriles and hydrazines, since this pathway represents the most efficient and straightforward strategy (Scheme 3) [71].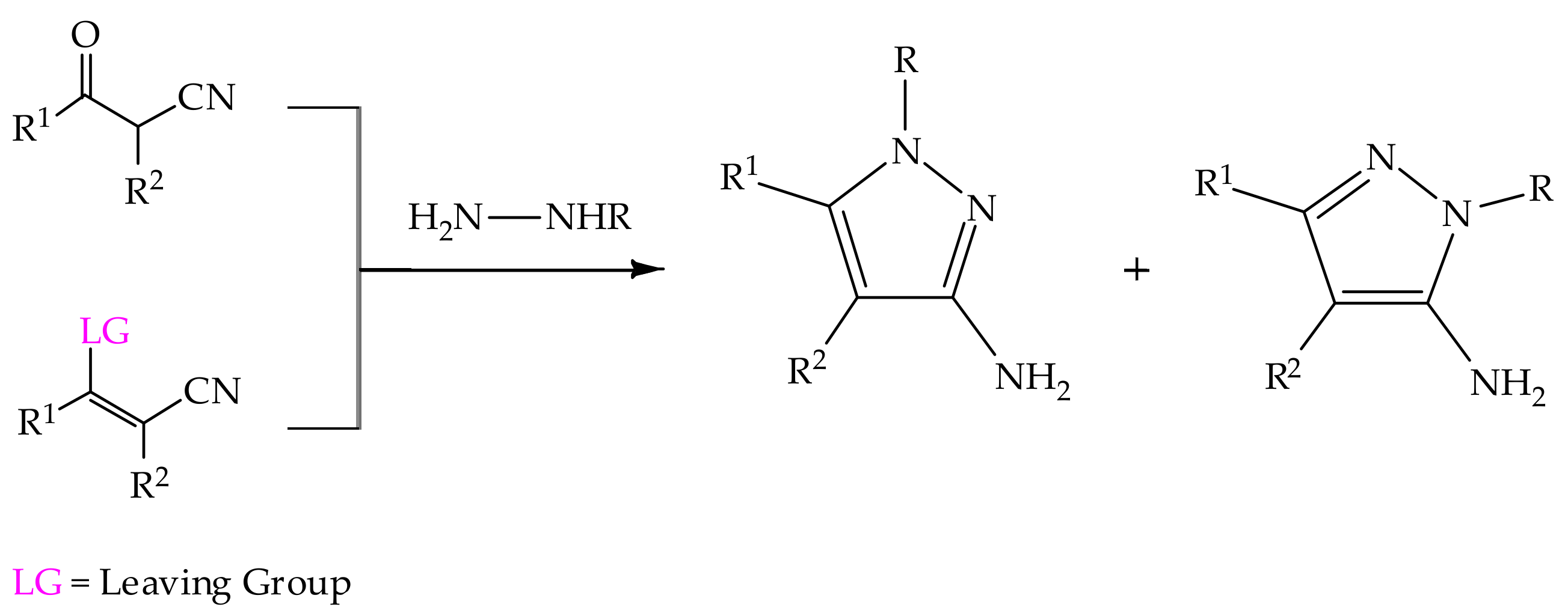
References
- Schofield, K.; Grimmett, M.R.; Grimmett, M.R.; Keene, B.R. Heteroaromatic Nitrogen Compounds: The Azoles; Cambridge University Press: Cambridge, UK, 1976.
- Andes, D.R.; Dismukes, W.E. Azoles. In Essentials of Clinical Mycology, 2nd ed.; Kauffman, C.A., Pappas, P.G., Sobel, J.D., Dismukes, W.E., Eds.; Springer: New York, NY, USA, 2011; pp. 61–93.
- Kumar, S.S.; Kavitha, H.P. Synthesis and Biological Applications of Triazole Derivatives—A Review. Mini. Rev. Org. Chem. 2013, 10, 40–65.
- Wei, C.X.; Bian, M.; Gong, G.H. Tetrazolium compounds: Synthesis and applications in medicine. Molecules 2015, 20, 5528–5553.
- Bai, S.Q.; Young, D.J.; Hor, T.S.A. Nitrogen-rich azoles as ligand spacers in coordination polymers. Chem. Asian J. 2011, 6, 292–304.
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. Reactivity of Five-membered Rings with Two or More Heteroatoms. In Handbook of Heterocyclic Chemistry, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 473–604.
- Behr, L.C.; Fusco, R.; Jarboe, C.H. Pyrazoles, Pyrazolines, Pyrazolidines, Indazoles and Condensed Rings; John Wiley & Sons: New York, NY, USA, 1967; p. 150.
- Kumar, V.; Kaur, K.; Gupta, G.K.; Sharma, A.K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753.
- Vicentini, C.B.; Mares, D.; Tartari, A.; Manfrini, M.; Forlani, G. Synthesis of Pyrazole Derivatives and Their Evaluation as Photosynthetic Electron Transport Inhibitors. J. Agric. Food Chem. 2004, 52, 1898–1906.
- El-Mekabaty, A. Utility of 5-amino-1-phenyl-1H-pyrazole-4-carboxamide in heterocyclic synthesis. Synth. Commun. 2014, 44, 875–896.
- Kumari, S.; Paliwal, S.; Chauhan, R. Synthesis of pyrazole derivatives possessing anticancer activity: Current status. Synth. Commun. 2014, 44, 1521–1578.
- Katritzky, A.R. Advances in Heterocyclic Chemistry 76; Academic Press: San Diego, CA, USA, 2000.
- Shaabani, A.; Nazeri, M.T.; Afshari, R. 5-Amino-pyrazoles: Potent reagents in organic and medicinal synthesis. Mol. Divers. 2019, 23, 751–807.
- Elmaati, T.M.A.; El-Taweel, F.M. New Trends In The Chemistry Of 5-Aminopyrazoles. J. Heterocycl. Chem. 2004, 41, 109–134.
- Elnagdi, M.H.; Elmoghayar, M.R.H.; Elgemeie, G.E.H. Chemistry of Pyrazolopyrimidines. Adv. Heterocycl. Chem. 1987, 41, 319–376.
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; EL-Gawad, H.H.A.; Shehab, M.A.M.; El-Khalafawy, A. Recent synthetic methodologies for pyrazolopyrimidine. Synth. Commun. 2019, 49, 1750–1776.
- Cherukupalli, S.; Karpoormath, R.; Chandrasekaran, B.; Hampannavar, G.A.; Thapliyal, N.; Palakollu, V.N. An insight on synthetic and medicinal aspects of pyrazolo pyrimidine scaffold. Eur. J. Med. Chem. 2017, 126, 298–352.
- Mohamed, M.S.; Mahmoud, A.M. Believes versus evidence-based regio-orientation in the structure assignment of pyrazolo pyrimidines. J. Appl. Pharm. Sci. 2019, 9, 126–144.
- Brown, A.W. Recent Developments in the Chemistry of Pyrazoles, 1st ed.; Elsevier Inc.: San Diego, CA, USA, 2018; Volume 126.
- Anwar, H.F.; Elnagdi, M.H. Recent developments in aminopyrazole chemistry. ARKIVOC 2009, 2009, 198–250.
- Mert, S.; Kasimogullari, R.; Ok, S. A Short Review on Pyrazole Derivatives and their Applications. J. Postdr. Res. 2014, 2, 64–72.
- Kalaria, P.N.; Karad, S.C.; Raval, D.K. A review on diverse heterocyclic compounds as the privileged scaffolds in antimalarial drug discovery. Eur. J. Med. Chem. 2018, 158, 917–936.
- Kumar, H.; Saini, D.; Jain, S.; Jain, N. Pyrazole scaffold: A remarkable tool in the development of anticancer agents. Eur. J. Med. Chem. 2013, 70, 248–258.
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; Santos, M.S.d.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903.
- Makino, K.; Kim, H.S.; Kurasawa, Y. Synthesis of pyrazoles and condensed pyrazoles. J. Heterocycl. Chem. 1999, 36, 321–332.
- Schmidt, A.; Dreger, A. Recent Advances in the Chemistry of Pyrazoles. Properties, Biological Activities, and Syntheses. Curr. Org. Chem. 2011, 15, 1423–1463.
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M. Synthesis and pharmacological activities of Pyrazole derivatives: A review. Molecules 2018, 23, 134.
- Alam, M.J.; Alam, O.; Alam, P.; Naim, M.J. A Review on Pyrazole chemical entity and Biological Activity. Int. J. Pharma Sci. Res. 2015, 6, 1433–1442.
- Kumar, K.A.; Jayaroopa, P. Pyrazoles: Synthetic strategies and their pharmaceutical applications-an overview. Int. J. PharmTech Res. 2013, 5, 1473–1486.
- Chauhan, A.; Sharma, P.K.; Kaushik, N. Pyrazole: A versatile moiety. Int. J. ChemTech Res. 2011, 3, 11–17.
- Bekhit, A.A.; Hymete, A.; Bekhit, A.E.A.; Damtew, A.; Aboul-Enein, H.Y. Pyrazoles as Promising Scaffold for the Synthesis of Anti-Inflammatory and/or Antimicrobial Agent: A Review. Mini Rev. Med. Chem. 2012, 10, 1014–1033.
- Nimbalkar, S.; Hote, S.V. Pyrazole Derivatives and their Synthesis—A review. Int. J. Recent Innov. Trends Comput. Commun. 2015, 3, 61–65.
- Fustero, S.; María, S.; Barrio, P.; Sim, A. From 2000 to Mid-2010 : A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034.
- Giller, S.A.; Mazheika, I.B.; Grandberg, I.I. Electron density distribution in heterocyclic systems with two vicinal nitrogen atoms. II. Pyrazole dipole moments. Khimiya Geterotsiklicheskikh Soedin. 1965, 1, 103–106.
- Chermahini, A.N.; Teimouri, A.; Beni, A.S.; Dordahan, F. Theoretical studies on the effect of substituent in the proton transfer reaction of 4-substituted pyrazoles. Comput. Theor. Chem. 2013, 1008, 67–73.
- Catalán, J.; Sánchez-Cabezudo, M.; de Paz, J.L.G.; Elguero, J.; Taft, R.W.; Anvia, F. The tautomerism of 1,2,3-triazole, 3(5)-methylpyrazole and their cations. J. Comput. Chem. 1989, 10, 426–433.
- Catalan, J.; Abboud, J.L.M.; Elguero, J. Basicity and Acidity of Azoles. Adv. Heterocycl. Chem. 1987, 41, 187–274.
- Castaneda, J.P.; Denisov, G.S.; Kucherov, S.Y.; Schreiber, V.M.; Shurukhina, A.V. Infrared and ab initio studies of hydrogen bonding and proton transfer in the complexes formed by pyrazoles. J. Mol. Struct. 2003, 660, 25–40.
- Claramunt, R.M.; López, C.; María, M.D.S.; Sanz, D.; Elguero, J. The use of NMR spectroscopy to study tautomerism. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 169–206.
- Pérez, J.; Riera, L. Pyrazole complexes and supramolecular chemistry. Eur. J. Inorg. Chem. 2009, 33, 4913–4925.
- Claramunt, R.M.; Cornago, P.; Torres, V.; Pinilla, E.; Torres, M.R.; Samat, A.; Lokshin, V.; Valés, M.; Elguero, J. The structure of pyrazoles in the solid state: A combined CPMAS, NMR, and crystallographic study. J. Org. Chem. 2006, 71, 6881–6891.
- Alkorta, I.; Goya, P.; Elguero, J.; Singh, S.P. A simple approach to the tautomerism of aromatic heterocycles. Natl. Acad. Sci. Lett. 2007, 30, 139–159.
- Chermahini, A.N.; Teimouri, A. Theoretical studies on proton transfer reaction of 3(5)-substituted pyrazoles. J. Chem. Sci. 2014, 126, 273–281.
- Oziminski, W.P. The kinetics of water-assisted tautomeric 1,2-proton transfer in azoles: A computational approach. Struct. Chem. 2016, 27, 1845–1854.
- Knorr, L. Ueber Abkömmlinge der Phenolform des 1-Phenyl-3-methyl-5-pyrazolons. Eur. J. Inorg. Chem. 1895, 28, 706–714.
- Elattar, K.M.; Fadda, A.A. Chemistry of antipyrine. Synth. Commun. 2016, 46, 1567–1594.
- Katritzky, A.R.; Hall, C.D.; El-Gendy, B.E.D.M.; Draghici, B. Tautomerism in drug discovery. J. Comput. Aided. Mol. Des. 2010, 24, 475–484.
- Taylor, P.J.; van der Zwan, G.; Antonov, L. Tautomerism: Introduction, History, and Recent Developments in Experimental and Theoretical Methods. In Tautomerism: Methods and Theories, 1st ed.; Antonov, L., Ed.; Wiley-VCH: Weinheim, Germany, 2014; pp. 1–24.
- Raczyńska, E.D.; Kosińska, W.; Ośmiałowski, B.; Gawinecki, R. Tautomeric equilibria in relation to Pi-electron delocalization. Chem. Rev. 2005, 105, 3561–3612.
- Minkin, V.I.; Garnovskii, A.D.; Elguero, J.; Katritzky, A.R.; Denisko, O.V. Tautomerism of Heterocycles: Five-Membered Rings with Two or More Heteroatoms. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 159–323.
- Minkin, V.I.; Olekhnovich, L.P.; Zhdanov, Y.A. Molecular Design of Tautomeric Compounds; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1981; Volume 14.
- Elguero, J.; Katritzky, A.R.; Denisko, O.V. Prototropic Tautomerism of Heterocycles: Heteroaromatic Tautomerism—General Overview and Methodology. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 2–84.
- Martin, Y.C. Let’s not forget tautomers. J. Comput. Aided. Mol. Des. 2009, 23, 693–704.
- Alkorta, I.; Elguero, J. 1,2-Proton shifts in pyrazole and related systems: A computational study of -sigmatropic migrations of hydrogen and related phenomena. J. Chem. Soc. Perkin Trans. 1998, 2, 2497–2504.
- Hall, D.G. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine; WILEY-VCH: Weinheim, Germany, 2005.
- McLaughlin, M.; Marcantonio, K.; Chen, G.Y.; Davies, I.W. A simple, modular method for the synthesis of 3,4,5-trisubstituted pyrazoles. J. Org. Chem. 2008, 73, 4309–4312.
- Huang, A.; Wo, K.; Lee, S.Y.C.; Kneitschel, N.; Chang, J.; Zhu, K.; Mello, T.; Bancroft, L.; Norman, N.J.; Zheng, S.L. Regioselective Synthesis, NMR, and Crystallographic Analysis of N1-Substituted Pyrazoles. J. Org. Chem. 2017, 82, 8864–8872.
- Kost, A.N.; Grandberg, I.I. Progress in Pyrazole Chemistry. Adv. Heterocycl. Chem. 1966, 6, 347–429.
- Halcrow, M.A. Pyrazoles and pyrazolides—Flexible synthons in self-assembly. J. Chem. Soc. Dalt. Trans. 2009, 9226, 2059–2073.
- Aggarwal, R.; Kumar, S. 5-Aminopyrazole as precursor in design and synthesis of fused pyrazoloazines. Beilstein J. Org. Chem. 2018, 14, 203–242.
- Kumar, A.; Paliwal, D.; Saini, D.; Thakur, A.; Aggarwal, S.; Kaushik, D. A comprehensive review on synthetic approach for antimalarial agents. Eur. J. Med. Chem. 2014, 85, 147–178.
- Puello, J.Q. Structure and tautomerism of 3(5)-amino-5(3)-arylpyrazoles in the solid state and in solution: An X-ray and NMR study. Tetrahedron 1997, 53, 10783–10802.
- Kusakiewicz-Dawid, A.; Porada, M.; Dziuk, B.; Siodlak, D. Annular Tautomerism of 3(5)-Disubstituted-1H-pyrazoles with Ester and Amide Groups. Molecules 2019, 24, 2632.
- Begtrup, M.; Boyer, G.; Cabildo, P.; Cativiela, C.; Claramunt, R.M.; Elguero, J.; García, J.I.; Toiron, C.; Vedsø, P. 13C NMR of pyrazoles. Magn. Reson. Chem. 1993, 31, 107–168.
- Gonzalez, E.; Faure, R.; Vincent, E.-J.; Espada, M.; Elguero, J. Etude en Résonance Magnétique Nucléaire du Carbone-13 de Quelques Aminopyrazoles. Sur le Problème de la Détermination des Constantes d’Equilibre Tautomère. Org. Magn. Reson. 1979, 12, 587–592.
- Dorn, H. Tautomerie und Nomenklatur der ‘Pyrazolone’ und Amino-pyrazole. J. Für Prakt. Chem. 1973, 315, 382–418.
- Lim, F.P.L.; Tan, K.C.; Luna, G.; Tiekink, E.R.T.; Dolzhenko, A.V. A new practical synthesis of 3-amino-substituted 5-aminopyrazoles and their tautomerism. Tetrahedron 2019, 75, 2314–2321.
- Larina, L.I. Tautomerism and Structure of Azoles: Nuclear Magnetic Resonance Spectroscopy. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Elsevier Ltd.: San Diego, CA, USA, 2018; Volume 126, pp. 233–321.
- Catalán, J.; Menéndez, M.; Laynez, J.; Claramunt, R.M.; Bruix, M.; De Mendoza, J.; Elguero, J. Basicity of azoles. Basicity of C-Aminopyrazoles in Relation to Tautomeric and Protonation Studies. J. Heterocycl. Chem. 1985, 22, 997–1000.
- Marín-Luna, M.; Alkorta, I.; Elguero, J. A theoretical study of the gas phase (proton affinity) and aqueous (pKa) basicity of a series of 150 pyrazoles. New J. Chem. 2015, 39, 2861–2871.
- Fichez, J.; Busca, P.; Prestat, G. Recent advances in aminopyrazoles synthesis and functionalization. Targets Heterocycl. Syst. 2017, 21, 322–347.
- El-Sattar, N.E.A.A.; Badawy, E.H.K.; Abdel-Mottaleb, M.S.A. Synthesis of Some Pyrimidine, Pyrazole, and Pyridine Derivatives and Their Reactivity Descriptors. J. Chem. 2018, 1–11.
- Trujillo, C.; Sánchez-Sanz, G.; Alkorta, I.; Elguero, J. Computational Study of Proton Transfer in Tautomers of 3- and 5-Hydroxypyrazole Assisted by Water. ChemPhysChem 2015, 16, 2140–2150.
- Kruszyk, M.; Jessing, M.; Kristensen, J.L.; Jørgensen, M. Computational Methods to Predict the Regioselectivity of Electrophilic Aromatic Substitution Reactions of Heteroaromatic Systems. J. Org. Chem. 2016, 81, 5128–5134.
- Jarończyk, M.; Dobrowolski, J.C.; Mazurek, A.P. Theoretical studies on tautomerism and IR spectra of pyrazole derivatives. J. Mol. Struct. THEOCHEM 2004, 673, 17–28.
