Artificial intelligence (AI) is a branch of science and engineering that focuses on the computational understanding of intelligent behavior. Many human professions, including clinical diagnosis and prognosis, are greatly useful from AI. TAntimicrobial resistance (AMR) is among the rising incidence of antimicrobial resistance most critical challenges facing Pakistan and the rest of the world. The rising incidence of AMR has become a significant issue, and authorities must take measures to combat the overuse and incorrect use of antibiotics in order to combat rising resistance rates. The widespread use of antibiotics in clinical practice has not only resulted in drug resistance but has also increased the threat of superresistant bacteria emergence. As AMR rises, clinicians find it more difficult to treat many bacterial infections in a timely manner, and therapy becomes prohibitively costly for patients. To combat the rise in AMR rates, it is critical to implement an institutional antibiotic stewardship program that monitors correct antibiotic use, controls antibiotics, and generates antibiograms. Furthermore, these types of tools may aid in the treatment of patients in the event of a medical emergency in which a physician is unable to wait for bacterial culture results. AI’s applications in healthcare might be unlimited, reducing the time it takes to discover new antimicrobial drugs, improving diagnostic and treatment accuracy, and lowering expenses at the same time. The majority of suggested AI solutions for AMR are meant to supplement rather than replace a doctor’s prescription or opinion, but rather to serve as a valuable tool for making their work easier. When it comes to infectious diseases, AI has the potential to be a game-changer in the battle against antibiotic resistance. Finally, when selecting antibiotic therapy for infections, data from local antibiotic stewardship programs are critical to ensuring that these bacteria are treated quickly and effectively. Furthermore, organizations such as the World Health Organization (WHO) have underlined the necessity of selecting the appropriate antibiotic and treating for the shortest time feasible to minimize the spread of resistant and invasive resistant bacterial strains.
- AI
- AMR
- AST
1. Introduction
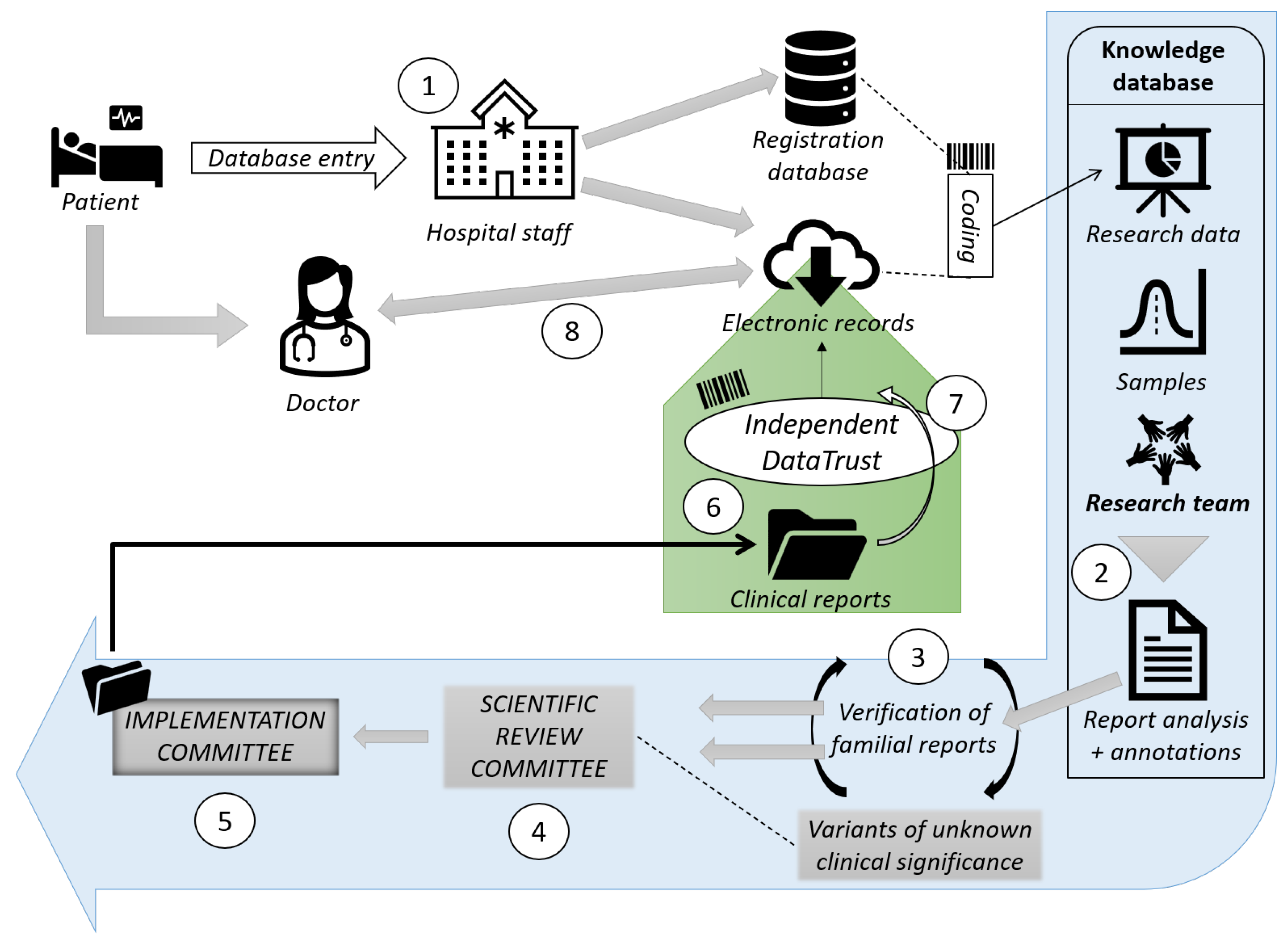
Antibiotic Resistance; the Current Scenario
2. Artificial Intelligence against Antibiotic Resistance
| AI Applications for AMR | Concepts | Advantages | Drawbacks |
|---|---|---|---|
| AI health industry and antibiotics | |||
| Antimicrobial peptides | A natural class of small host defense peptides, found in all classes of biological species. |
|
|
| New antibiotics | Discovery of new and structurally different antibiotics from the ones already known using AI. |
|
|
| AI, infectious diseases, and pediatric practices | |||
| Appropriate antibiotic prescription | Appropriate therapy selection, dose, and correct administration route |
|
|
| Prediction of antibiotic resistance | ML techniques to predict early AMR or the probability of a microbial agent becoming resistant |
|
|
| The severity of infection prediction | Machine/deep learning tools for infectious pathology recognition and appropriate management |
|
|
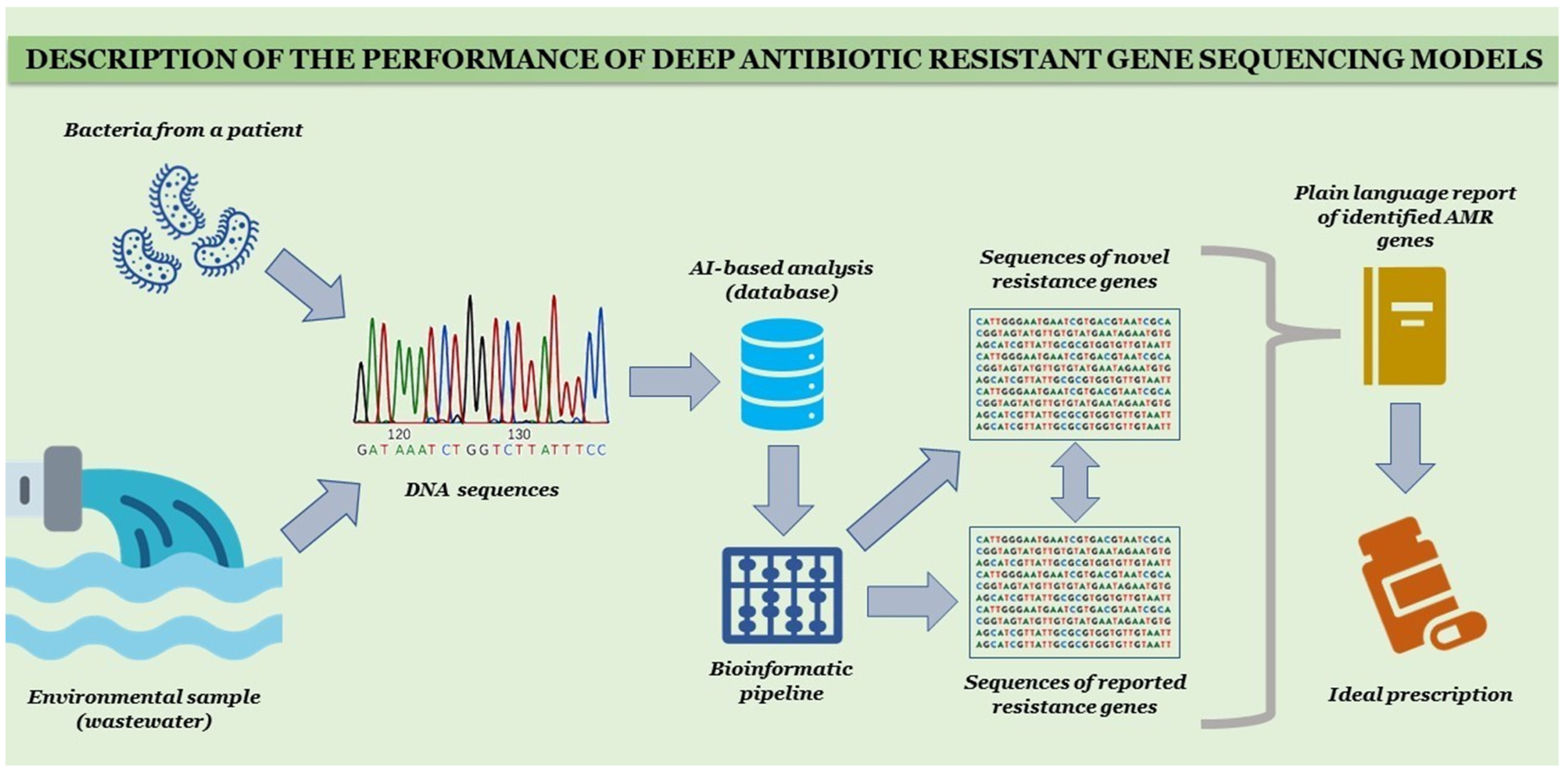
Assistance Strategies of Artificial Intelligence in Antimicrobial Resistance
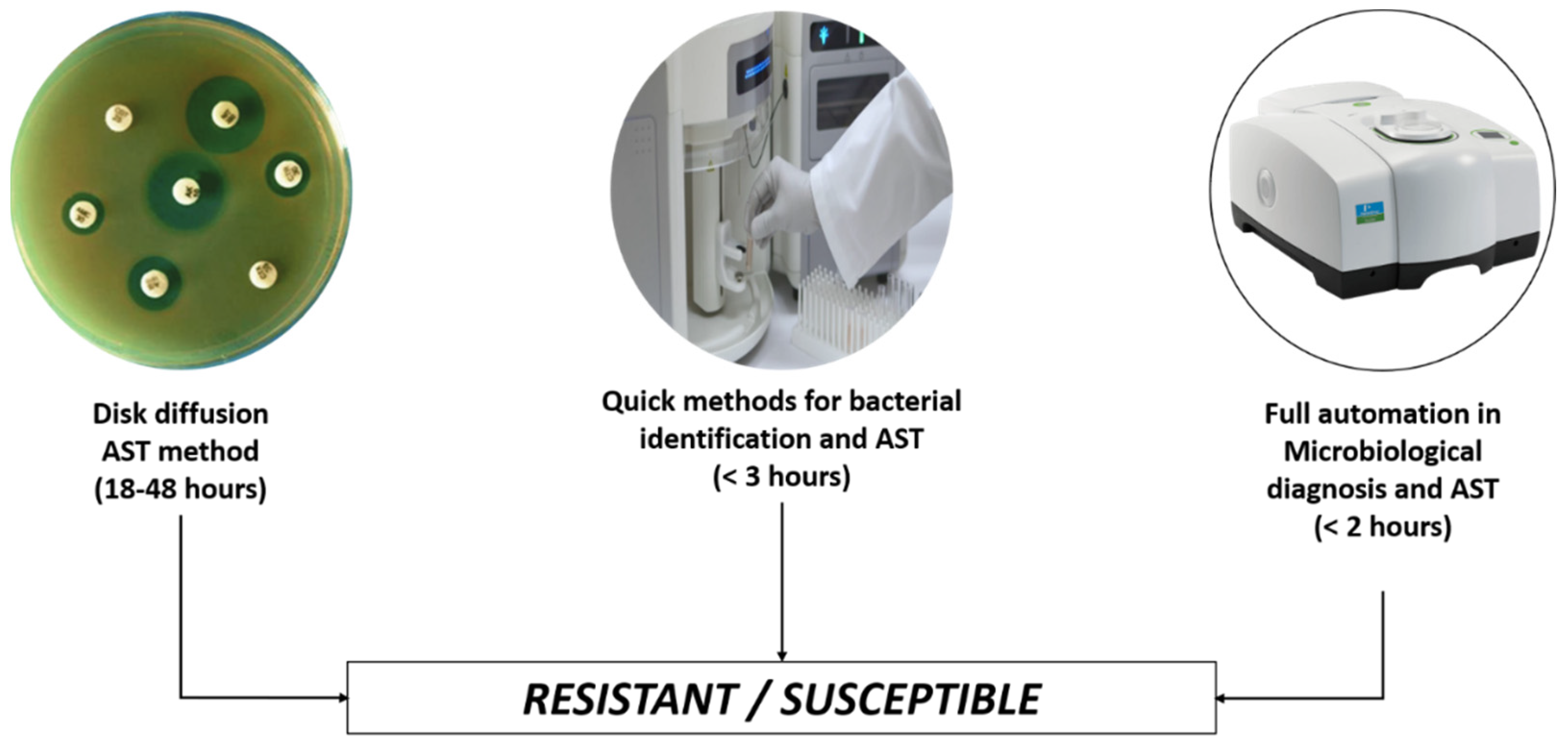
3. Artificial Intelligence vs. Antibiotic Stewardship Program
References
- Ahmed, I.; Rabbi, M.B.; Sultana, S. Antibiotic resistance in Bangladesh: A systematic review. Int. J. Infect. Dis. 2019, 80, 54–61.
- Bilal, H.; Khan, M.N.; Rehman, T.; Hameed, M.F.; Yang, X. Antibiotic resistance in Pakistan: A systematic review of past decade. BMC Infect. Dis. 2021, 21, 244.
- Bullens, M.; de Cerqueira Melo, A.; Raziq, S.; Lee, J.; Khalid, G.; Khan, S.; Zada, A.; Wailly, Y.; Zeshan, S.; Saad, N. Antibiotic resistance in patients with urinary tract infections in Pakistan. Public Health Action 2022, 12, 48–52.
- Hormozi, S.F.; Vasei, N.; Aminianfar, M.; Darvishi, M.; Saeedi, A.A. Antibiotic resistance in patients suffering from nosocomial infections in Besat Hospital. Eur. J. Transl. Myol. 2018, 28, 7594.
- Susmita, R.C.; Zubayed, A.; Krishna, R.; Abdullah, A.N.; Rashid, M.H.; Kamol, C.M. Emerging threats of antibiotic resistance in Salmonella typhi and Salmonella paratyphi A among enteric fever cases of Dhaka, Bangladesh. Afr. J. Bacteriol. Res. 2022, 14, 8–15.
- Martino, F.; Tijet, N.; Melano, R.; Petroni, A.; Heinz, E.; De Belder, D.; Faccone, D.; Rapoport, M.; Biondi, E.; Rodrigo, V. Isolation of five Enterobacteriaceae species harbouring bla NDM-1 and mcr-1 plasmids from a single paediatric patient. PLoS ONE 2019, 14, e0221960.
- Gemert, T.V. On the Influence of Dataset Characteristics on Classifier Performance. Bachelor’s Thesis, Utrecht University, Utrecht, The Netherlands, 2017.
- Ahmed, N.; Zeshan, B.; Naveed, M.; Afzal, M.; Mohamed, M. Antibiotic resistance profile in relation to virulence genes fimH, hlyA and usp of uropathogenic E. coli isolates in Lahore, Pakistan. Trop. Biomed. 2019, 36, 559–568.
- Lv, J.; Deng, S.; Zhang, L. A review of artificial intelligence applications for antimicrobial resistance. Biosaf. Health 2021, 3, 22–31.
- Lau, H.J.; Lim, C.H.; Foo, S.C.; Tan, H.S. The role of artificial intelligence in the battle against antimicrobial-resistant bacteria. Curr. Genet. 2021, 67, 421–429.
- Raisch, S.; Krakowski, S. Artificial intelligence and management: The automation–augmentation paradox. Acad. Manag. Rev. 2021, 46, 192–210.
- Song, L.; Gildea, D.; Zhang, Y.; Wang, Z.; Su, J. Semantic neural machine translation using AMR. Trans. Assoc. Comput. Linguist. 2019, 7, 19–31.
- Ahmed, N.; Khan, M.; Saleem, W.; Karobari, M.I.; Mohamed, R.N.; Heboyan, A.; Rabaan, A.A.; Mutair, A.A.; Alhumaid, S.; Alsadiq, S.A. Evaluation of bi-lateral co-infections and antibiotic resistance rates among COVID-19 patients. Antibiotics 2022, 11, 276.
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 137.
- Sattar, H.; Toleman, M.; Nahid, F.; Zahra, R. Co-existence of bla NDM-1 and bla KPC-2 in clinical isolates of Klebsiella pneumoniae from Pakistan. J. Chemother. 2016, 28, 346–349.
- Williams, M.A.; Wyner, S.N. Antimicrobial Resistance: Facing the Rise of a Global Threat; American Public Health Association: Washington, DC, USA, 2019.
- Hadjadj, L.; Syed, M.A.; Bushra, J.; Abbasi, S.A.; Rolain, J.-M. Emergence of Vancomycin-resistant Enterococcus faecium ST 80 in Pakistan. Surg. Infect. 2019, 20, 524–525.
- Dantas, G.; Sommer, M.O.; Oluwasegun, R.D.; Church, G.M. Bacteria subsisting on antibiotics. Science 2008, 320, 100–103.
- Sohail, M.; Khurshid, M.; Saleem, H.G.M.; Javed, H.; Khan, A.A. Characteristics and antibiotic resistance of urinary tract pathogens isolated from Punjab, Pakistan. Jundishapur J. Microbiol. 2015, 8, e19272.
- Fasih, N.; Zafar, A.; Khan, E.; Jabeen, K.; Hasan, R. Clonal dissemination of vanA positive Enterococcus species in tertiary care hospitals in Karachi, Pakistan. J. Pak. Med. Assoc. 2010, 60, 805.
- Miller, J.R.; Dunham, S.; Mochalkin, I.; Banotai, C.; Bowman, M.; Buist, S.; Dunkle, B.; Hanna, D.; Harwood, H.J.; Huband, M.D. A class of selective antibacterials derived from a protein kinase inhibitor pharmacophore. Proc. Natl. Acad. Sci. USA 2009, 106, 1737–1742.
- Arango-Argoty, G.; Garner, E.; Pruden, A.; Heath, L.S.; Vikesland, P.; Zhang, L. DeepARG: A deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome 2018, 6, 23.
- Cánovas-Segura, B.; Campos, M.; Morales, A.; Juarez, J.M.; Palacios, F. Development of a clinical decision support system for antibiotic management in a hospital environment. Prog. Artif. Intell. 2016, 5, 181–197.
- Ahmed, N.; Ali, Z.; Riaz, M.; Zeshan, B.; Wattoo, J.I.; Aslam, M.N. Evaluation of antibiotic resistance and virulence genes among clinical isolates of Pseudomonas aeruginosa from cancer patients. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1333.
- Zafar, A.; Hasan, R.; Nizami, S.Q.; von Seidlein, L.; Soofi, S.; Ahsan, T.; Chandio, S.; Habib, A.; Bhutto, N.; Siddiqui, F.J. Frequency of isolation of various subtypes and antimicrobial resistance of Shigella from urban slums of Karachi, Pakistan. Int. J. Infect. Dis. 2009, 13, 668–672.
- Nava Lara, R.A.; Aguilera-Mendoza, L.; Brizuela, C.A.; Peña, A.; Del Rio, G. Heterologous machine learning for the identification of antimicrobial activity in Human-Targeted drugs. Molecules 2019, 24, 1258.
- van Belkum, A.; Burnham, C.-A.D.; Rossen, J.W.; Mallard, F.; Rochas, O.; Dunne, W.M. Innovative and rapid antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2020, 18, 299–311.
- Gajdács, M.; Paulik, E.; Szabó, A. Knowledge, attitude and practice of community pharmacists regarding antibiotic use and infectious diseases: A cross-sectional survey in Hungary (KAPPhA-HU). Antibiotics 2020, 9, 41.
- Yelin, I.; Snitser, O.; Novich, G.; Katz, R.; Tal, O.; Parizade, M.; Chodick, G.; Koren, G.; Shalev, V.; Kishony, R. Personal clinical history predicts antibiotic resistance of urinary tract infections. Nat. Med. 2019, 25, 1143–1152.
- Hameed, F.; Khan, M.A.; Muhammad, H.; Sarwar, T.; Bilal, H.; Rehman, T.U. Plasmid-mediated mcr-1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: First report from Pakistan. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190237.
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.J. The structure and diversity of human, animal and environmental resistomes. Microbiome 2016, 4, 1–15.
- Ahmed, N.; Khalid, H.; Mushtaq, M.; Basha, S.; Rabaan, A.A.; Garout, M.; Halwani, M.A.; Al Mutair, A.; Alhumaid, S.; Al Alawi, Z.; et al. The Molecular Characterization of Virulence Determinants and Antibiotic Resistance Patterns in Human Bacterial Uropathogens. Antibiotics 2022, 11, 516.
- Zahra, N.; Zeshan, B.; Qadri, M.M.A.; Ishaq, M.; Afzal, M.; Ahmed, N. Phenotypic and Genotypic Evaluation of Antibiotic Resistance of Acinetobacter baumannii Bacteria Isolated from Surgical Intensive Care Unit Patients in Pakistan. Jundishapur J. Microbiol. 2021, 14, 104922.
- Zeshan, B.; Karobari, M.I.; Afzal, N.; Siddiq, A.; Basha, S.; Basheer, S.N.; Peeran, S.W.; Mustafa, M.; Daud, N.H.A.; Ahmed, N. The usage of antibiotics by COVID-19 patients with comorbidities: The risk of increased antimicrobial resistance. Antibiotics 2021, 11, 35.
- Saleem, Z.; Godman, B.; Azhar, F.; Kalungia, A.C.; Fadare, J.; Opanga, S.; Markovic-Pekovic, V.; Hoxha, I.; Saeed, A.; Al-Gethamy, M. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): A narrative review and the implications. Expert Rev. Anti-Infect. Ther. 2022, 20, 71–93.
- Oluwafemi, R.; Olawale, I.; Alagbe, J. Recent trends in the utilization of medicinal plants as growth promoters in poultry nutrition—A review. Res. Agric. Vet. Sci. 2020, 4, 5–11.
- Rodríguez-González, A.; Zanin, M.; Menasalvas-Ruiz, E. Public health and epidemiology informatics: Can artificial intelligence help future global challenges? An overview of antimicrobial resistance and impact of climate change in disease epidemiology. Yearb. Med. Inform. 2019, 28, 224–231.
- Feretzakis, G.; Sakagianni, A.; Loupelis, E.; Kalles, D.; Skarmoutsou, N.; Martsoukou, M.; Christopoulos, C.; Lada, M.; Petropoulou, S.; Velentza, A. Machine Learning for Antibiotic Resistance Prediction: A Prototype Using Off-the-Shelf Techniques and Entry-Level Data to Guide Empiric Antimicrobial Therapy. Healthc. Inform. Res. 2021, 27, 214–221.
- Cartelle Gestal, M.; Dedloff, M.R.; Torres-Sangiao, E. Computational health engineering applied to model infectious diseases and antimicrobial resistance spread. Appl. Sci. 2019, 9, 2486.
- Anahtar, M.N.; Yang, J.H.; Kanjilal, S. Applications of machine learning to the problem of antimicrobial resistance: An emerging model for translational research. J. Clin. Microbiol. 2021, 59, e01260-20.
- Agnello, S.; Brand, M.; Chellat, M.F.; Gazzola, S.; Riedl, R. A structural view on medicinal chemistry strategies against drug resistance. Angew. Chem. Int. Ed. 2019, 58, 3300–3345.
