Sickle leg ulcers (SLU) are malleoli lesions with exuberant hemolytic pathophysiology. The microRNAs are potential genetic biomarkers for several pathologies. Thereby, we aimed to assess the expression of circulating miR-199a-5p, miR-144, and miR-126 in association with hemolytic biomarkers in SLU. This cross-sectional study included 69 patients with sickle cell disease, 52 patients without SLU (SLU-) and 17 patients with active SLU or previous history (SLU+). The results demonstrated elevated expression of circulating miR-199a-5p and miR-144 in SLU+ patients while miR-126 expression was reduced. Circulating miR-199a-5p and miR-144 were associated with hemolytic biomarkers such as LDH, indirect bilirubin, AST, GGT, iron, ferritin, RBC, hemoglobin, and NOm, in
addition to association with impaired clinical profile of SLU. Furthermore, in silico analyses indicated interactions of miR-199a-5p with HIF1A, Ets-1, and TGFB2 genes, which are associated with vasculopathy and reduced NO. In contrast, miR-126 was associated with an attenuating clinical profile of SLU, in addition to not characterizing hemolysis. In summary, this study demonstrates, for the first time, that hemolytic mechanism in SLU can be characterized by circulating miR-199a-5p and miR-144. The circulating miR-126 may play a protective role in SLU. Thus, these microRNAs can support to establish prognosis and therapeutic strategy in SLU was assessed.
- sickle cell disease
- sickle leg ulcer
- microRNA
- hemolysis
- biomarkers
1. Introduction
2. Circulating miRNAs Expression in SCD Patients and between HbSS and HbSC Genotype with and without SLU
Regarding SLU, there was higher miR-199a-5p (Figure 1a) and miR-144 (Figure 1b) expression, as well as lower miR-126 (Figure 1c) expression in SLU+ patients (p < 0.05).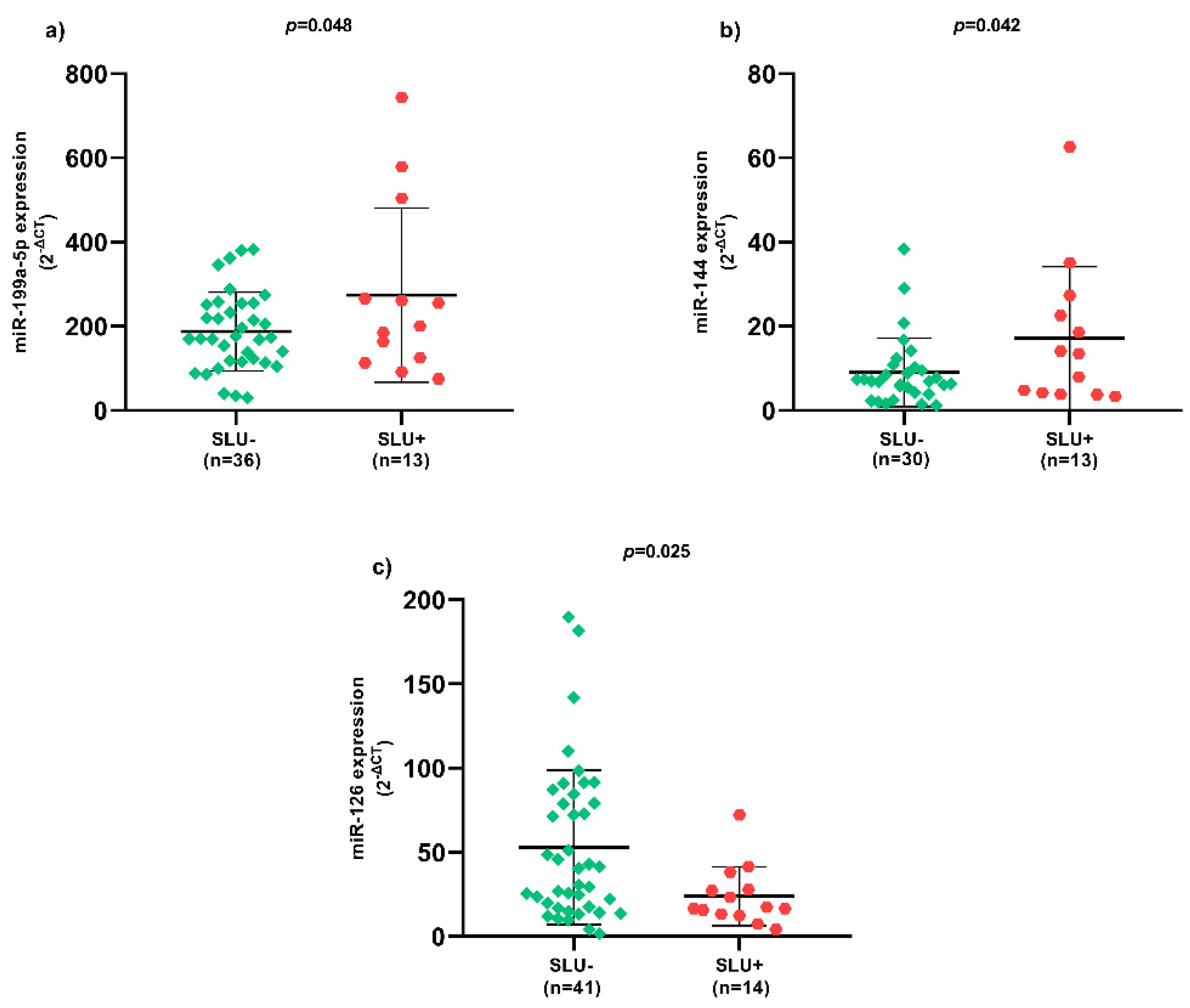
3. Correlation Coefficients between Hemolytic Biomarkers and Circulating miRNAs Expression in SLU+ Patients
miR-199a-5p expression was positively correlated with NOm, LDH, AST, GGT, iron, and ferritin levels, and negatively correlated with red blood cell (RBC) count and hemoglobin levels (p < 0.05) (Figure 2a). Moreover, there were positive correlations between miR-144 expression and NOm, LDH, indirect bilirubin, AST, GGT, iron, and ferritin levels, as well as negative correlations with RBC count and hemoglobin levels (p < 0.05) (Figure 2b). Regarding miR-126 expression, there were negative correlations with indirect bilirubin, iron and ferritin levels (p < 0.05) (Figure 2c).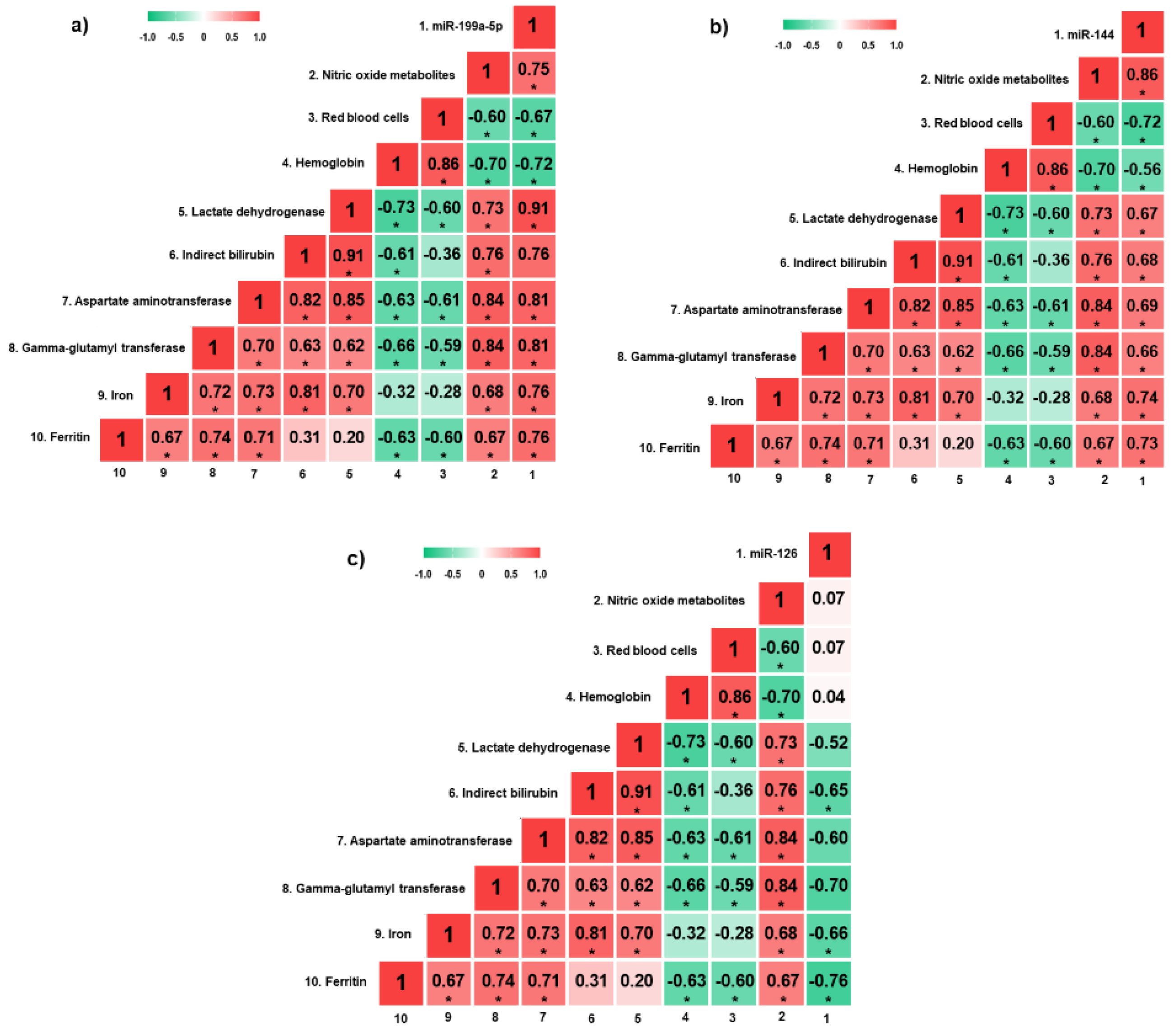
4. Circulating miRNAs Expression in Active SLU
Patients whose SLU wound bed was constituted by unviable tissues, such as necrotic and sloughy tissues, presented higher miR-144 expression (Figure 3a) and lower miR-126 expression (Figure 3b) (p < 0.05). Patients with more than one SLU simultaneously presented higher miR-144 (Figure 3c) and miR-199a-5p (Figure 3d) expression (p < 0.05). In addition, SLU recurrence between 4 to 7 episodes presented higher miR-199a-5p expression (Figure 3e) and lower miR-126 expression (Figure 3f) (p < 0.05). SLU with sick edges were associated with higher miR-199a-5p (Figure 3g) and miR-144 (Figure 3h) expression (p < 0.05).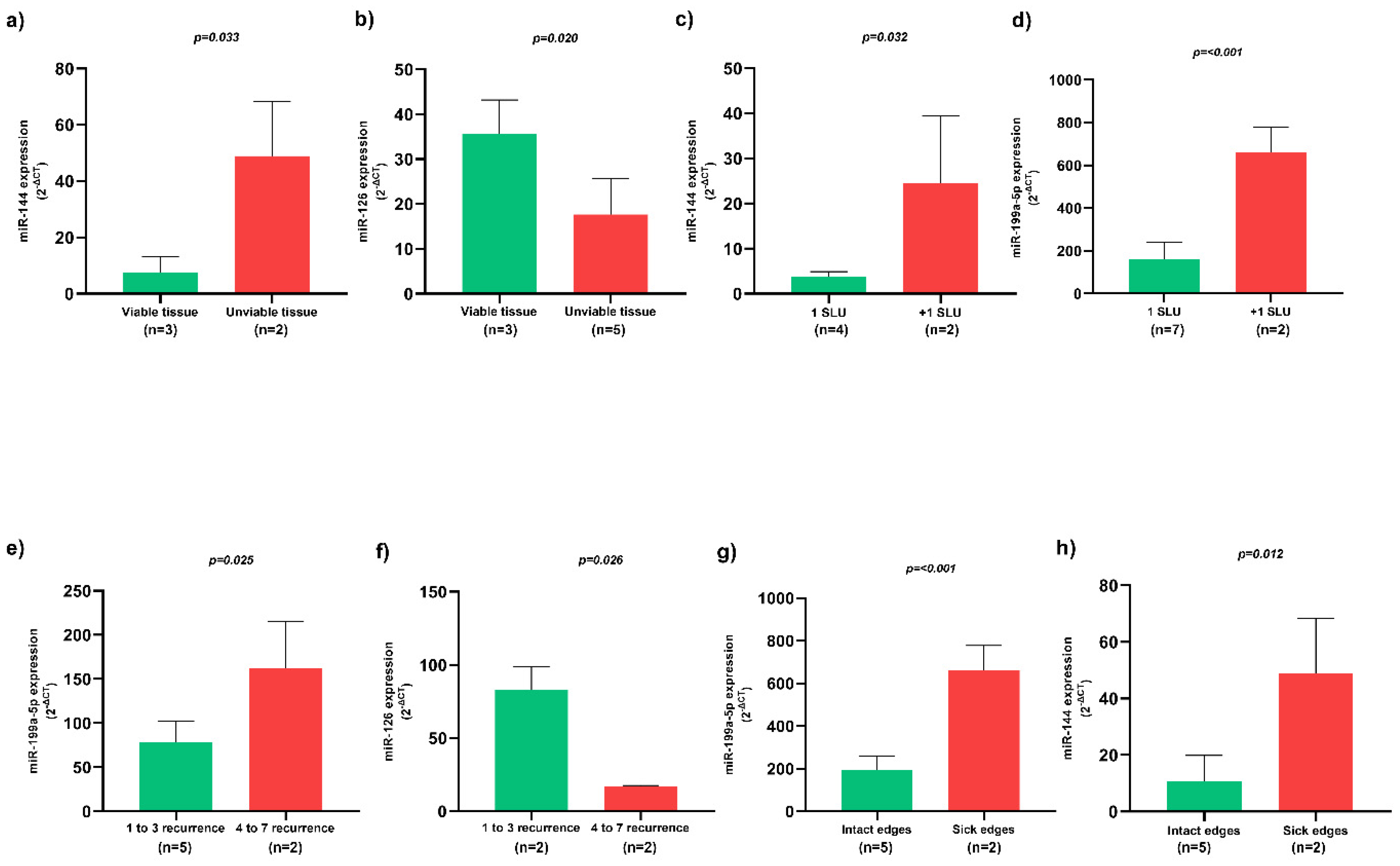
5. Association of HU with Circulating miRNAs Expression in Patients with and without SLU
Two MLR models were performed with HU as dependent variable. In SLU- patients, miR-199a-5p and miR-126 expression were independently associated with HU (R2 = 0.486; p < 0.05), while in SLU+ patients only miR-126 expression presented independent association (R2 = 0.779; p < 0.05) (Table 1).| Independent Variables | Dependent Variable | p-Value | β | R2 | p-Value of the Model | |
|---|---|---|---|---|---|---|
| SLU- patients N = 43 |
Circulating miR-199a-5p | Hydroxyurea | 0.007 | 0.695 | 0.486 | 0.022 |
| Circulating miR-144 | 0.096 | 0.468 | ||||
| Circulating miR-126 | 0.009 | −0.834 | ||||
| SLU+ patients N = 15 |
Circulating miR-199a-5p | 0.485 | 0.191 | 0.779 | 0.043 | |
| Circulating miR-144 | 0.997 | −0.001 | ||||
| Circulating miR-126 | 0.011 | −0.885 |
SLU-, patients without sickle leg ulcers; SLU+, patients with active sickle leg ulcers or previous history; miR, microRNA; R2: coefficient of determination; β: coefficient of regression; Bold p-values indicate significance at p < 0.05.
7. Target Gene Prediction with Biological Processes of miR-199a-5p to SCD Patients
6. Target Gene Prediction with Biological Processes of miR-199a-5p to SCD Patients
The results demonstrate interaction networks of miR-199a-5p and target genes using miRWalk analysis (Figure 4a). Correlation between level significance by the score for these genes is presented (Figure 4b), as well as biological processes (Figure 4c; Table S1).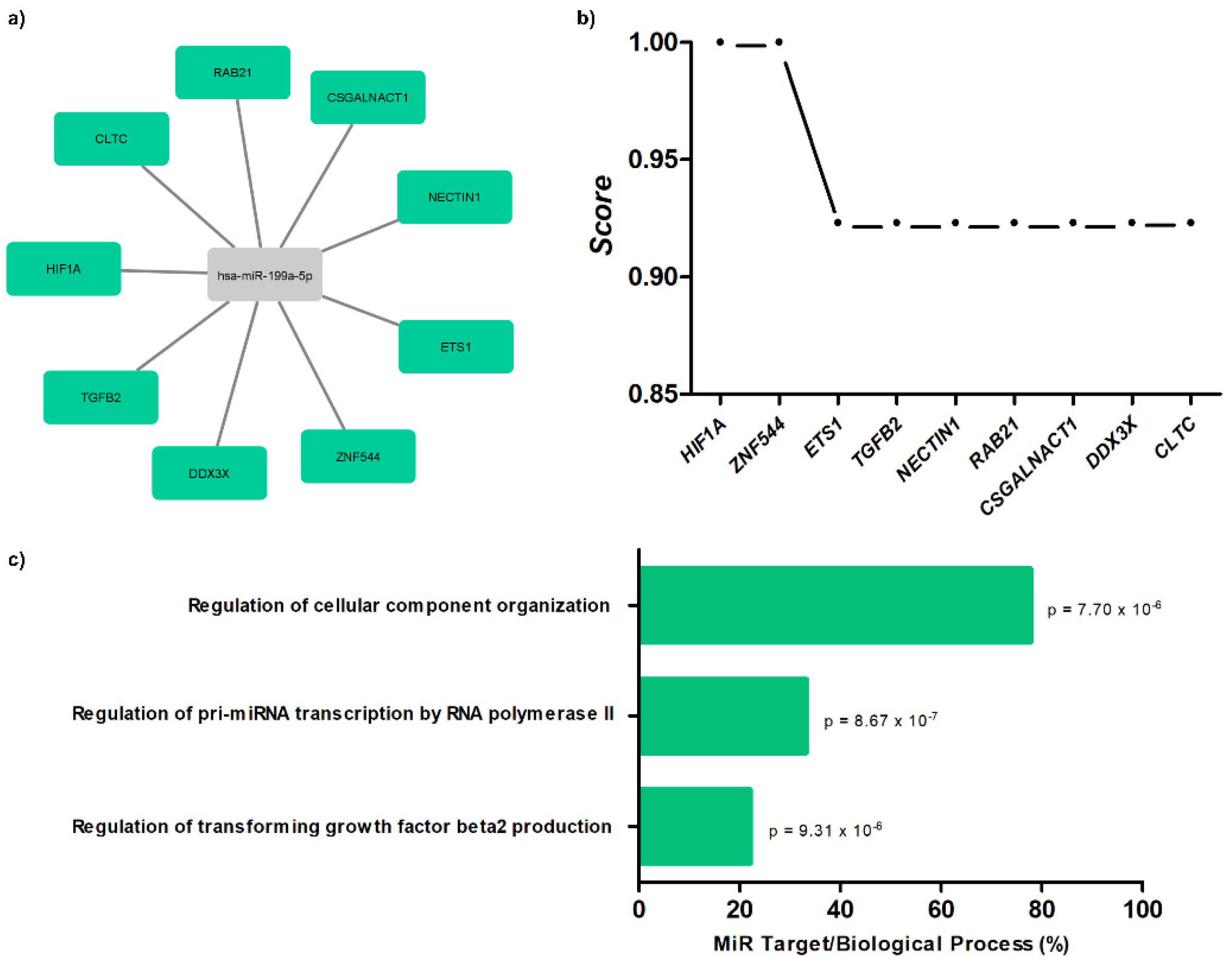
References
- Njoku, F.; Zhang, X.; Shah, B.N.; Machado, R.F.; Han, J.; Saraf, S.L.; Gordeuk, V.R. Biomarkers of clinical severity in treated and untreated sickle cell disease: A comparison by genotypes of a single center cohort and African Americans in the NHANES study. Br. J. Haematol. 2021, 194, 767–778.
- Antwi-Boasiako, C.; Andemariam, B.; Colombatti, R.; Asare, E.V.; Strunk, C.; Piccone, C.M.; Manwani, D.; Boruchov, D.; Farooq, F.; Urbonya, R.; et al. A study of the geographic distribution and associated risk factors of leg ulcers within an international cohort of sickle cell disease patients: The CASiRe group analysis. Ann. Hematol. 2020, 99, 2073–2079.
- Sickle Cell Disease: Ulcers: Prevention and Treatment, 1st ed.; Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Especializada: Brasília-DF, Brasil, 2012; ISBN 978-85-334-1957-5.
- Senet, P.; Blas-Chatelain, C.; Levy, P.; Manea, E.M.; Peschanski, M.; Mirault, T.; Stankovic-Stojanovic, K.; Debure, C.; Debbache, K.; Girot, R.; et al. Factors predictive of leg-ulcer healing in sickle cell disease: A multicentre, prospective cohort study. Br. J. Dermatol. 2017, 177, 206–211.
- Umeh, N.I.; Ajegba, B.; Buscetta, A.J.; Abdallah, K.E.; Minniti, C.P.; Bonham, V.L. The psychosocial impact of leg ulcers in patients with sickle cell disease: I don’t want them to know my little secret. PLoS ONE 2017, 12, e0186270.
- Li, B.; Zhu, X.; Ward, C.M.; Starlard-Davenport, A.; Takezaki, M.; Berry, A.; Ward, A.; Wilder, C.; Neunert, C.; Kutlar, A.; et al. MIR-144-mediated NRF2 gene silencing inhibits fetal hemoglobin expression in sickle cell disease. Exp. Hematol. 2019, 70, 85–96.
- Yang, X.; Zheng, Y.; Tan, J.; Tian, R.; Shen, P.; Cai, W.; Liao, H. miR-199a-5p–HIF-1α-STAT3 positive feedback loop contributes to the progression of non-small cell lung cancer. Front. Cell Dev. Biol. 2021, 8, 1–13.
- Le, N.-T.; Abe, J. MicroRNA 199a and the eNOS (Endothelial NO Synthase)/NO Pathway. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2278–2280.
- Pan, Q.; Zheng, J.; Du, D.; Liao, X.; Ma, C.; Yang, Y.; Chen, Y.; Zhong, W.; Ma, X. microRNA-126 priming enhances functions of endothelial progenitor cells under physiological and hypoxic conditions and their therapeutic efficacy in cerebral ischemic damage. Stem Cells Int. 2018, 2018, 1–13.
