ThEpide therapeutic landscape in patientsrmal growth factor receptor (EGFR) is a transmembrane protein with advanced non-small-cell lung cancer harboring oncogenic biomarkers has radically changed with the development of targeted therapies. Although lung cancers are known to frequently metastasize to the brain, oncogene-drivencytoplasmic kinase activity that transduces important growth factor signals from the extracellular milieu to cells. Given that more than 60% of non-small- cell lung cancer patients show a higher incidence of both brain metastases at baseline and a further risk of central nervous system progression/relapses (NSCLC) express EGFR, EGFR has emerged as an important therapeutic target for the treatment of these tumors.
- NSCLC
- oncogenic biomarkers
- brain metastases
1. Introduction
-
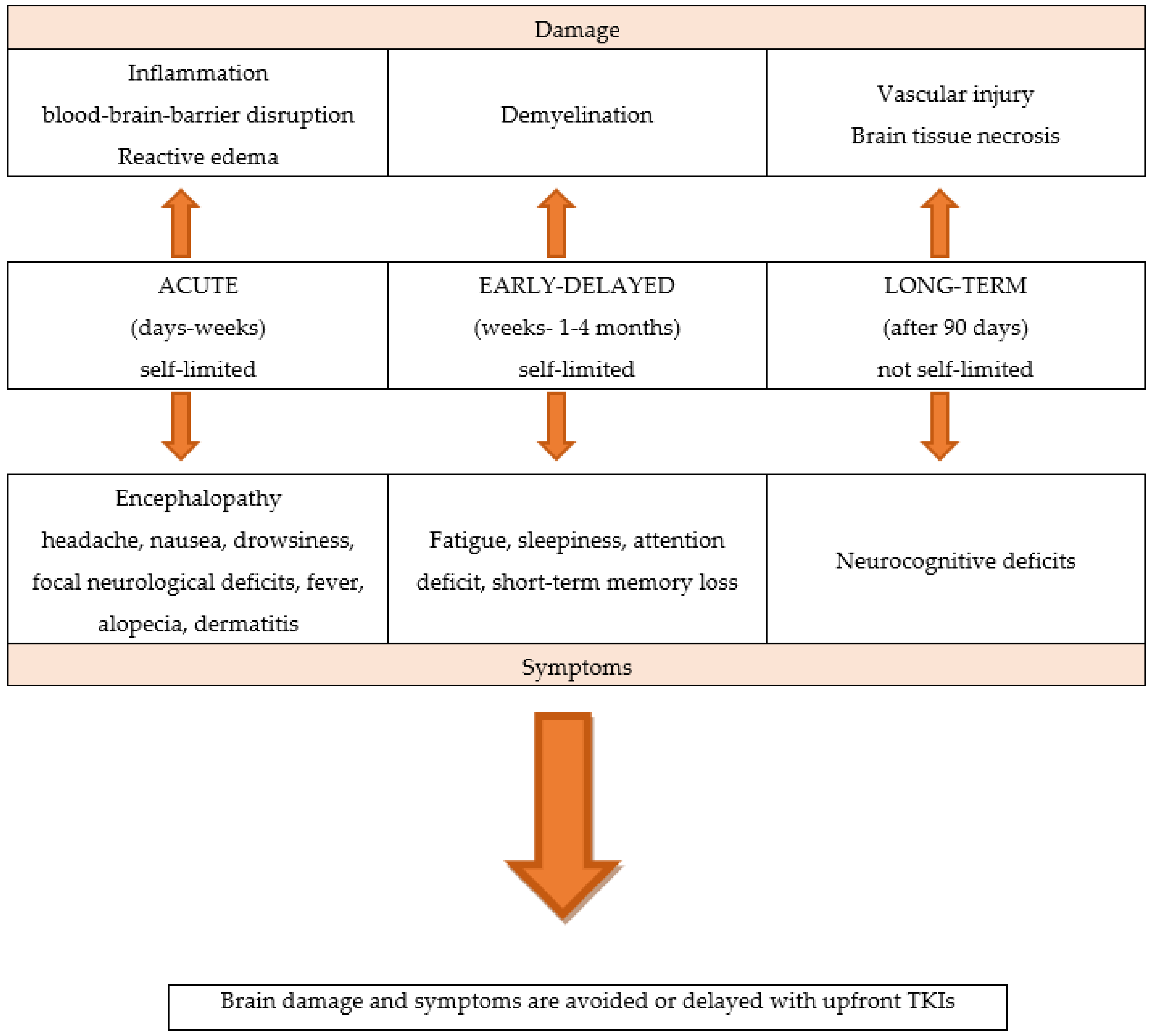
The newer generation targeted systemic therapies have demonstrated far greater CNS penetration than CT or older targeted agents; the newer molecular targeted agents are liposoluble compounds with low molecular weight and can cross the BBB; furthermore, they have the ability to penetrate cerebrospinal fluid (CSF).
-
Oncogene-addicted NSCLC patients are living several years rather than only a few months, allowing for more time for BMs to develop, as well as for adverse effects from prior RT to manifest.
-
These factors lead to a treatment strategy shift, privileging brain penetrant TKIs systemic therapies over local treatments, maintaining patient QoL by minimizing the RT-related consequences.
- The newer generation targeted systemic therapies have demonstrated far greater CNS penetration than CT or older targeted agents; the newer molecular targeted agents are liposoluble compounds with low molecular weight and can cross the BBB; furthermore, they have the ability to penetrate cerebrospinal fluid (CSF).
- Oncogene-addicted NSCLC patients are living several years rather than only a few months, allowing for more time for BMs to develop, as well as for adverse effects from prior RT to manifest.
- These factors lead to a treatment strategy shift, privileging brain penetrant TKIs systemic therapies over local treatments, maintaining patient QoL by minimizing the RT-related consequences.
2. Egfr Mutations
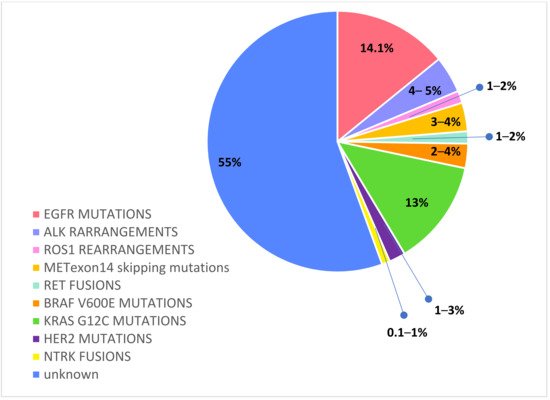
Activated EGFR mutations, predominantly exon 19 deletions and exon 21 L858R mutations, occur in approximately 14.1% of Caucasian NSCLC [31]. Among EGFR mutated NSCLC patients, BMs have an increased frequency, considering baseline incidence ranging from 23% to 32% [32,33,34,35][32][33][34][35] and a further risk of intracranial progression of about 15–20% during first-generation TKIs treatment [36,37][36][37]. These data reflect a pharmacokinetic failure of the first- and second- generation EGFR TKIs to penetrate the brain. Though erlotinib, gefitinib, and afatinib have intracranial activity, these agents have a limited BBB penetration, and they are detected in CFS only at a low concentration, in the 1 to 5 percent range of what is observed in the serum [38,39,40,41][38][39][40][41]. By contrast, third-generation irreversible TKI osimertinib achieves a greater intracerebral concentration and has shown high intracranial response rates, even against leptomeningeal carcinomatosis [42,43,44][42][43][44]. Osimertinib was first approved in the second-line setting in patients that developed a T790M mutation after failure of a first-generation TKI. Pooled data from two phase II trials—AURA extension and AURA2—in 50 T790M-positive advanced NSCLC patients with BMs progressed to prior EGFR TKI have demonstrated the significant intracranial activity of osimertinib; CNS objective response rate (ORR) and disease control rate (DCR) were 54% and 92%, respectively, and CNS response was observed regardless of prior brain irradiation [45]. In the randomized phase III AURA 3 trial osimertinib demonstrated significantly greater progression-free survival (PFS) than platinum-based doublet-CT in patients with EGFR T790M advanced NSCLC and progression on prior EGFR-TKI treatment. Among 116 patients with BMs (measurable or not), PFS was longer with osimertinib compared to CT (11.7 vs. 5.6 months, HR 0.32; and 95% CI: 0.15–0.69) and cumulative incidence of CNS progression at 6 months was lower with osimertinib compared to CT (11.5% vs. 28.2%) [46]. Subsequently, osimertinib was approved in the first-line setting on the basis of the randomized phase III FLAURA trial, which evaluated the efficacy of upfront osimertinib versus a SoC EGFR TKI (erlotinib or gefitinib) in treatment-naïve EGFR mutant (exon 19 del or L858R) advanced NSCLC patients [47]. The CNS activity of osimertinib was confirmed in a subset analysis of the randomized phase III FLAURA trial, which evaluated the efficacy of upfront osimertinib versus a SoC EGFR TKI (erlotinib or gefitinib) in treatment-naïve EGFR mutant (exon 19 del or L858R) advanced NSCLC patients. In the preplanned, exploratory analysis (CNS analysis set, N = 128), osimertinib reported that improved CNS RR (66% vs. 43%) and median CNS PFS among patients with measurable and/or non-measurable CNS lesions was longer (not reached vs. 13.9 months, HR 0.48, 95% CI: 0.26–0.86, and p = 0.04). Furthermore, osimertinib reduced the risk of CNS progression in the overall study population (6% vs. 15%), regardless of the presence or absence of known or treated CNS metastases at baseline. Among patients with BMs evaluable for response (N = 41), osimertinib improved the CNS RR (91% vs. 68%) [48]. Data from this analysis show that osimertinib reveal encouraging activity against CNS involvement, with a greater intracranial response and clinical benefit both in preventing or delaying BMs. Randomized trials comparing upfront osimertinib with brain irradiation are lacking. Although retrospective data indicate that the deferral of RT may be associated with worse outcomes compared with early RT [49,50,51,52,53][49][50][51][52][53], those studies were all conducted with earlier-generation EGFR TKIs, which have less intracranial activity than osimertinib. In sum, the available data suggest that osimertinib demonstrates the greatest CNS activity and prevention of CNS progression, making it the preferred initial treatment option for EGFR-mutated NSCLC with BMs, deferring brain RT and its neurocognitive defects in case of intracranial progression.References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30.
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594.
- Waqar, S.N.; Samson, P.P.; Robinson, C.G.; Bradley, J.; Devarakonda, S.; Du, L.; Govindan, R.; Gao, F.; Puri, V.; Morgensztern, D. Non-small-cell Lung Cancer with Brain Metastasis at Presentation. Clin. Lung Cancer 2018, 19, e373–e379.
- Nishino, M.; Soejima, K.; Mitsudomi, T. Brain metastases in oncogene-driven non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8 (Suppl. 3), S298–S307.
- Moro-Sibilot, D.; Smit, E.; de Castro Carpeño, J.; Lesniewski-Kmak, K.; Aerts, J.; Villatoro, R.; Kraaij, K.; Nacerddine, K.; Dyachkova, Y.; Smith, K.T.; et al. Non-small cell lung cancer patients with brain metastases treated with first-line platinum-doublet chemotherapy: Analysis from the European FRAME study. Lung Cancer 2015, 90, 427–432.
- Sperduto, P.W.; Mesko, S.; Jing, L.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Survival in Patients with Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J. Clin. Oncol. 2020, 38, 3773–3784.
- Kadry, H.; Noorani, B.; Cucullo, L.A. Blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. BMC 2020, 17, 69.
- Morganti, J.M.; Jopson, T.D.; Liu, S.; Gupta, N.; Rosi, S. Cranial irradiation alters the brain’s microenvironment and permits CCR2+ macrophage infiltration. PLoS ONE 2014, 9, e93650.
- Yoshida, Y.; Sejimo, Y.; Kurachi, M.; Ishizaki, Y.; Nakano, T.; Takahashi, A. X-ray irradiation induces disruption of the blood-brain barrier with localized changes in claudin-5 and activation of microglia in the mouse brain. Neurochem. Int. 2018, 119, 199–206.
- Nordal, R.A.; Wong, C.S. Molecular targets in radiation-induced blood-brain barrier disruption. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 279–287.
- Perlow, H.K.; Dibs, K.; Liu, K.; Jiang, W.; Rajappa, P.; Blakaj, D.M.; Palmer, J.; Raval, R.R. Whole-Brain Radiation Therapy Versus Stereotactic Radiosurgery for Cerebral Metastases. Neurosurg. Clin. N. Am. 2020, 31, 565–573.
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990, 322, 494–500.
- Vecht, C.J.; Haaxma-Reiche, H.; Noordijk, E.M.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R.; et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann. Neurol. 1993, 33, 583–590.
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060.
- Qin, H.; Wang, C.; Jiang, Y.; Zhang, X.; Zhang, Y.; Ruan, Z. Patients with single brain metastasis from non-small cell lung cancer equally benefit from stereotactic radiosurgery and surgery: A systematic review. Med. Sci. Monit. 2015, 21, 144–152.
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491.
- Rades, D.; Schild, S.E.; Lohynska, R.; Veninga, T.; Stalpers, L.J.; Dunst, J. Two radiation regimens and prognostic factors for brain metastases in nonsmall cell lung cancer patients. Cancer 2007, 110, 1077–1082.
- Mulvenna, P.; Nankivell, M.; Barton, R.; Faivre-Finn, C.; Wilson, P.; McColl, E.; Moore, B.; Brisbane, I.; Ardron, D.; Holt, T.; et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet 2016, 388, 2004–2014.
- Li, J.; Bentzen, S.M.; Li, J.; Renschler, M.; Mehta, M.P. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 64–70.
- van den Bent, M.J. The role of chemotherapy in brain metastases. Eur. J. Cancer 2003, 39, 2114–2120.
- Cortes, J.; Rodriguez, J.; Aramendia, J.M.; Salgado, E.; Gurpide, A.; Garcia-Foncillas, J.; Aristu, J.J.; Claver, A.; Bosch, A.; Lopez-Picazo, J.M.; et al. Front-line paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology 2003, 64, 28–35.
- Tsao, A.S.; Scagliotti, G.V.; Bunn, P.A., Jr.; Carbone, D.P.; Warren, G.W.; Bai, C.; de Koning, H.J.; Yousaf-Khan, A.U.; McWilliams, A.; Tsao, M.S.; et al. Scientific Advances in Lung Cancer 2015. J. Thorac. Oncol. 2016, 1, 613–638.
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. National comprehensive cancer network. NCCN clinical Practice guidelines in oncology 2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266.
- Jordan, E.J.; Kim, H.R.; Arcila, M.E.; Barron, D.; Chakravarty, D.; Gao, J.; Chang, M.T.; Ni, A.; Kundra, R.; Jonsson, P.; et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov. 2017, 7, 596–609.
- Chevallier, M.; Borgeaud, M.; Addeo, A.; Friedlaender, A. Oncogenic driver mutations in non-small cell lung cancer: Past, present and future. World J. Clin. Oncol. 2021, 12, 217–237.
- Pesce, G.A.; Klingbiel, D.; Ribi, K.; Zouhair, A.; von Moos, R.; Schlaeppi, M.; Caspar, C.B.; Fischer, N.; Anchisi, S.; Peters, S.; et al. Outcome, quality of life and cognitive function of patients with brain metastases from non-small cell lung cancer treated with whole brain radiotherapy combined with gefitinib or temozolomide. A randomised phase II trial of the Swiss Group for Clinical Cancer Research (SAKK 70/03). Eur. J. Cancer. 2012, 48, 377–384.
- Ma, S.; Xu, Y.; Deng, Q.; Yu, X. Treatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer. 2009, 5, 198–203.
- Doherty, M.K.; Korpanty, G.J.; Tomasini, P.; Alizadeh, M.; Jao, K.; Labbé, C.; Mascaux, C.M.; Martin, P.; Kamel-Reid, S.; Tsao, M.S.; et al. Treatment options for patients with brain metastases from EGFR/ALK-driven lung cancer. Radiother. Oncol. 2017, 123, 195–202.
- Zhang, I.; Zaorsky, N.G.; Palmer, J.D.; Mehra, R.; Lu, B. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015, 16, e510–e521.
- Zhang, Y.L.; Yuan, J.Q.; Wang, K.F.; Fu, X.H.; Han, X.R.; Threapleton, D.; Yang, Z.Y.; Mao, C.; Tang, J.L. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 78985–78993.
- Iuchi, T.; Shingyoji, M.; Itakura, M.; Yokoi, S.; Moriya, Y.; Tamura, H.; Yoshida, Y.; Ashinuma, H.; Kawasaki, K.; Hasegawa, Y.; et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int. J. Clin. Oncol. 2015, 20, 674–679.
- Rangachari, D.; Yamaguchi, N.; VanderLaan, P.A.; Folch, E.; Mahadevan, A.; Floyd, S.R.; Uhlmann, E.J.; Wong, E.T.; Dahlberg, S.E.; Huberman, M.S.; et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015, 88, 108–111.
- Hendriks, L.; Smit, E.F.; Vosse, B.A.; Mellema, W.W.; Heideman, D.A.; Bootsma, G.P.; Westenend, M.; Pitz, C.; de Vries, G.J.; Houben, R.; et al. EGFR mutated non-small cell lung cancer patients: More prone to development of bone and brain metastases? Lung Cancer 2014, 84, 86–91.
- Remon, J.; Besse, B. Brain Metastases in Oncogene-Addicted Non-Small Cell Lung Cancer Patients: Incidence and Treatment. Front. Oncol. 2018, 8, 88.
- Heon, S.; Yeap, B.Y.; Britt, G.J.; Costa, D.B.; Rabin, M.S.; Jackman, D.M.; Johnson, B.E. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin. Cancer Res. 2010, 16, 5873–5882.
- Patel, S.H.; Rimner, A.; Foster, A.; Zhang, Z.; Woo, K.M.; Yu, H.A.; Riely, G.J.; Wu, A.J. Patterns of initial and intracranial failure in metastatic EGFR-mutant non-small cell lung cancer treated with erlotinib. Lung Cancer 2017, 108, 109–114.
- Chiu, C.H.; Tsai, C.M.; Chen, Y.M.; Chiang, S.C.; Liou, J.L.; Perng, R.P. Gefitinib is active in patients with brain metastases from non-small cell lung cancer and response is related to skin toxicity. Lung Cancer 2005, 47, 129–138.
- Wei, Y.F.; Lim, C.K.; Tsai, M.S.; Huang, M.S.; Chen, K.Y. Intracranial Responses to Afatinib at Different Doses in Patients With EGFR-mutated Non-small- cell Lung Carcinoma and Brain Metastases. Clin. Lung Cancer 2019, 20, e274–e283.
- Zee, Y.K.; Chin, T.M.; Wong, A.S.C. Fatal cystic change of brain metastasis after response to gefitinib in non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, e145–6.
- Togashi, Y.; Masago, K.; Fukudo, M.; Terada, T.; Fujita, S.; Irisa, K.; Sakamori, Y.; Kim, Y.H.; Mio, T.; Inui, K.I.; et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 950–955.
- Colclough, N.; Chen, K.; Johnström, P.; Strittmatter, N.; Yan, Y.; Wrigley, G.L.; Schou, M.; Goodwin, R.; Varnäs, K.; Adua, S.J.; et al. Preclinical Comparison of the Blood-brain barrier Permeability of Osimertinib with Other EGFR TKIs. Clin. Cancer Res. 2021, 27, 189–201.
- Ballard, P.; Yates, J.W.T.; Yang, Z.; Kim, D.W.; Yang, J.C.H.; Cantarini, M.; Pickup, K.; Jordan, A.; Hickey, M.; Grist, M. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin. Cancer Res. 2016, 22, 5130–5140.
- Yang, J.C.H.; Kim, S.W.; Kim, D.W.; Lee, J.S.; Cho, B.C.; Ahn, J.S.; Lee, D.H.; Kim, T.M.; Goldman, J.W.; Natale, R.B.; et al. Osimertinib in Patients with Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J. Clin. Oncol. 2020, 38, 538–547.
- Goss, G.; Tsai, C.M.; Shepherd, F.A.; Ahn, M.J.; Bazhenova, L.; Crinò, L.; de Marinis, F.; Felip, E.; Morabito, A.; Hodge, R.; et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: Pooled data from two phase II trials. Ann. Oncol. 2018, 29, 687–693.
- Mok, T.; Ahn, M.J.; Han, J.Y.; Kang, J.H.; Katakami, N.; Kim, H.; Hodge, R.; Ghiorghiu, D.C.; Cantarini, M.; Wu, Y.L.; et al. CNS response to osimertinib in patients (pts) with T790M-positive advanced NSCLC: Data from a randomized phase III trial (AURA3). J. Clin. Oncol, 2017; 35.
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125.
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297.
- Iuchi, T.; Shingyoji, M.; Sakaida, S.; Hatano, K.; Nagano, O.; Itakura, M.; Kageyama, H.; Yokoi, S.; Hasegawa, Y.; Kawasaki, K.; et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013, 82, 282–287.
- Grommes, C.; Oxnard, G.N.; Kris, M.G.; Miller, V.A.; Pao, W.; Holodny, A.I.; Clarke, J.L.; Lassman, A.B. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011, 13, 1364–1369.
- Gerber, N.K.; Yamada, Y.; Rimner, A.; Shi, W.; Riely, G.J.; Beal, K.; Yu, H.A.; Chan, T.A.; Zhang, Z.; Wu, A.J. Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 322–329.
- Magnuson, W.J.; Lester-Coll, N.H.; Wu, A.J.; Yang, T.J.; Lockney, N.A.; Gerber, N.K.; Beal, K.; Amini, A.; Patil, T.; Kavanagh, B.D.; et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J. Clin. Oncol. 2017, 35, 1070–1077.
- Welsh, J.W.; Komaki, R.; Amini, A.; Munsell, M.F.; Unger, W.; Allen, P.K.; Chang, J.Y.; Wefel, J.S.; McGovern, S.L.; Garland, L.L.; et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 895–902.
