Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Evgenios Kokkinos.
Arsenic poisoning constitutes a major threat to humans, causing various health problems. The presence of arsenic in ecosystems can originate from several natural or anthropogenic activities. Arsenic can be then gradually accumulated in different food sources, such as vegetables, rice and other crops, but also in seafood, etc., and in water sources (mainly in groundwater, but also to a lesser extent in surface water), potentially used as drinking-water supplies, provoking their contamination and therefore potential health problems to the consumers.
- arsenic
- food contamination
- drinking water
- health effects
1. Introduction
Arsenic is a ubiquitous toxic metal, belonging in the metalloid group of the periodic table, found naturally in the lithosphere, hydrosphere, and atmosphere, as well as generally in the biosphere [1]. Both organic and inorganic forms of arsenic exist in nature (mostly in the form of complexes); various transportation routes in environment have been identified and rather high concentrations (mainly in water sources) have been reported in several regions around the world [2].
The World Health Organization (WHO) recommended the regulation limit (as imposed by the respective legislation) of arsenic concentration in drinking water at 10 μg/L [3]. This is also the limit of arsenic in drinking water imposed by the European Commission, the United States Environmental Protection Agency and other inter/national organizations. Contamination of water by arsenic, especially groundwater, due to arsenic’s high toxicity, is considered a major health issue in various areas worldwide. Relevant research showed that the long-term exposure to elevated concentrations of arsenic can threaten human health, causing a variety of health disorders, including skin lesions (e.g., keratosis, pigmentation) and various internal and skin cancers [4].
Nevertheless, arsenic has been used in various industries for the production of several products, such as glass, ceramics, electrical appliances, cosmetics and fireworks. In the mid-20th century, arsenic was also widely used to produce pesticides, and for the production of wood preservatives [5]. As a result of past and current uses, arsenic contamination is currently considered a problem of great concern for the scientific community, found mainly in water sources, but also in food, threatening the health of millions of people.
2. Origin of Arsenic Contamination
High concentrations of arsenic may occur naturally (e.g., due to erosion of minerals) in several areas, which are therefore contaminated, and by anthropogenic activities (e.g., industrial production and uses). The continuous exposure of humans to arsenic via food and water consumption can lead to serious health damages, because this is a carcinogenic element with high affinity for thiols. It can also replace phosphorus in biochemical reactions owing to their similar chemical properties, as they belong in the same group of the periodic table, actually being very close, indicating the highly destructive role of this element during DNA replication and metabolic activity [8][6]. Soil: The predominant forms of arsenic in soils are arsenate (As5+), arsenite (As3+) and organic arsenic. Usually, in soil matrixes it can be found complexed with amorphous iron and aluminum oxides [9][7]. The arsenic form in the soil varies according to the different textures. For instance, the presence of clay can increase the fixation of arsenic in the respective soil, as at circum-neutral pH values arsenic is adsorbed onto the clay particles [10][8]. Its concentration and mobility are also dependent on pH and redox potential at the specific environmental sites [11][9]. Because of the toxic effects of arsenic, it has been used (mainly in the past) for the production of specific herbicides, insecticides, various toxins and decongestants. The use of intensive phosphate fertilizers in agriculture is also considered a potential source for arsenic contamination, because arsenic is a common contaminant of most phosphate minerals, which are used for the production of these fertilizers. However, the amount of arsenic in other fertilizers(e.g., nitrogen or potash) is rather low and can be considered insignificant [12][10]. Figure 1 shows the various routes of arsenic and arsenic-related compounds that can accumulate in an ecosystem due to anthropogenic activities, resulting in environmental deterioration.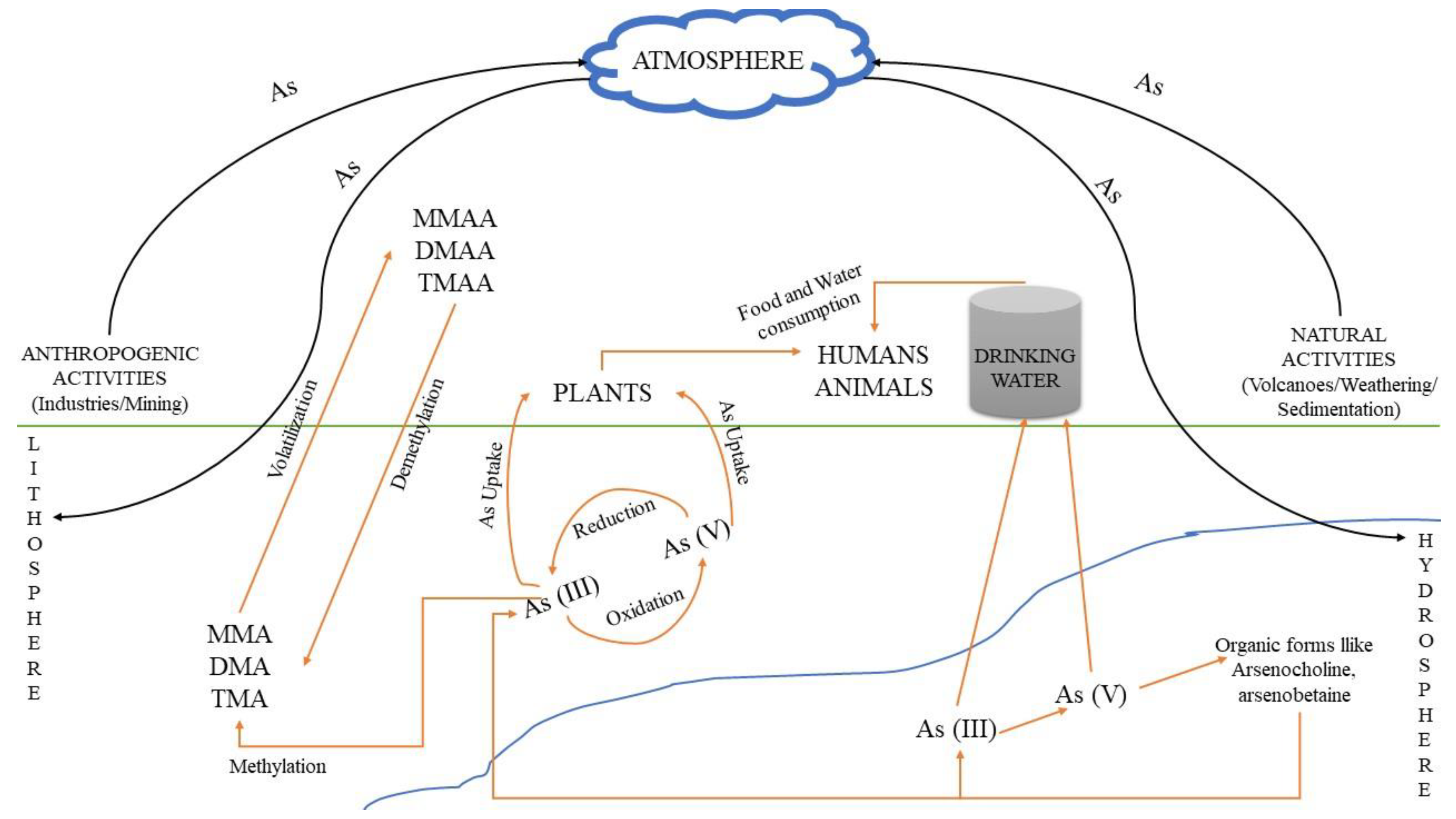
Figure 1. Pathways through which arsenic and its relevant compounds may enter the environment and contaminate soil, atmosphere and water. Human and natural activities result in As accumulation, mainly in soil and water, where As(V) and As(III) are interconverted via oxidation and reduction bio/reactions. The respective methylated products can be produced from As(III) species, i.e., MMA, DMA and TMA, resulting in the formation of MMAA, DMAA and TMAA chemical compounds, mainly through volatilization, while the reverse process occurs through demethylation.
Atmosphere: Arsenic can be released into the atmosphere by natural or anthropogenic activities. According to a rough estimation, the global annual release of arsenic in the atmosphere is 7.8 × 107 kg/year. The natural sources are expected to release 1.2 × 107 kg arsenic per year, wherein volcanoes and microbial volatilization may supplementarily contribute an additional 8.9 × 106 and 2.1 × 107 kg/year, respectively [13][11]. The amount of arsenic stored in the northern hemisphere is almost five times higher than the amount of arsenic stored in the southern hemisphere, mainly due to the more intensive industrialized conditions existing in the northern hemisphere [14][12]. Other environmental problems, potentially causing increased arsenic emissions in the atmosphere, may include deforestation, grass burning, and the use of wood as fuel. High and significant concentrations of arsenic are also connected with the emissions of industrial wastes, especially heavy/toxic metals [15][13]; the concentration of arsenic in sewage sludges is considered to be an indicator of the industrialization degree for the surrounding area.
Water: Since arsenic is an element of the Earth’s crust, groundwater usually presents the most severe pollution problems among other water resources [16][14]. It can be found either dissolved in water or in the form of particles. Moreover, it may be transformed to dimethyl and/or trimethyl arsenic compounds by mollusks, crustaceans and fishes [17][15]. Arsenate is the predominant form found in seawater algae, which play an important role in the biological transport of inorganic arsenic species [18][16].
3. Hazards and Limits in Food and Water
Inorganic arsenic (iAs) can be found in the environment in several forms, including As(0) (metalloid arsenic), As(III) (arsenite) and As(V) (arsenate). The latter two forms are abundant in natural and drinking waters [19][17]. Arsenate is the dominant specie in oxic waters, whereas under mildly reducing conditions, the probability of arsenite prevalence increases [20][18]. As(III) is 60 times more toxic than As(V), because of its greater tendency to react with lipids, proteins and other cellular components, causing higher cellular uptake [21][19]. In addition, As(III) is more difficult to remove from water due to its higher mobility, as compared with As(V); therefore, it can be adsorbed less efficiently on solid surfaces [22][20]. Because of its toxicity, the World Health Organization (WHO) in 1993 reduced the recommended concentration limit of total As in drinking water to 10 μg/L (from the previous 50 μg/L limit) [23][21]. Equally important and hazardous is the exposure to As from food consumption. Arsenic can accumulate in plants, mainly in cereals, reducing their growth and productivity [25][22]. Irrigation needs of crops are often related to the amount of arsenic content in the seeds, due to the already contaminated groundwater or/and surface water application [26][23]. The corresponding recommended limit by Food and Agriculture Organization (FAO), in collaboration with WHO, applies currently mainly to rice grains, due to the respective higher irrigation needs, as compared with other crops, and it has been set at 0.2 mg/Kg [27][24].4. Main Effects of Arsenic Contamination on Human Health
Arsenic is considered a top priority contaminant due to its toxicity and as a carcinogenic chemical, whereas the intake by humans has been verified worldwide, through the consumption of contaminated water and food. Both organic and inorganic forms of arsenic can exist in the environment, but the latter ones are more poisonous and toxic. Inorganic As is found more in water sources and, consequently, in the relevant edible fish products. Regarding vegetation, the exposure to arsenic contamination (e.g., through contaminated soil) can cause the inhibition of plant growth along with the loss of or reduction in photosynthetic and reproductive activities. The accumulation of arsenic in the food web may lead to acute and long-term effects on human health. Vomiting, abdominal pain, diarrhea, numbness and tingling, muscle pain and cramps, and death in extreme cases are reported as the main health symptoms of elevated arsenic intake [196][25]. The long-term effects are attributed to higher levels of inorganic arsenic in the human body. The effects can be observed mainly in skin, including pigmentation, lesions formation and patches, and acting probably as precursors to skin cancer. Bladder and lung cancers are also reported to be a result of arsenicosis [197][26]. Other health issues such as diabetes, pulmonary and heart diseases may also arise, due to the long-term exposure of arsenic; e.g., Taiwan has witnessed gangrene because of black-foot disease, leading to deaths, due to elevated arsenic concentrations [198][27].5. Control, Prevention and Treatment of As in Water Sources
Regarding the prevention and control of arsenic, various measures can be proposed and applied. First, the higher or lower arsenic sources should be identified appropriately. Second, the higher arsenic-contaminated sources of groundwater should be substituted by other safer water sources, whereas the lower arsenic concentration waters can be used for various domestic purposes (probably not for dinking). Third, the higher arsenic-contaminated waters can be blended with the lower ones to achieve an average concentration, which is permissible for their respective use, according to WHO regulations. For long-term prevention and control, industrial and other wastewaters should be treated properly. The general public should be informed of the arsenicosis problem and its health effects and the population at high-risk of arsenic toxicity should be monitored regularly [3]. Fourth, the necessary arsenic treatment/removal systems should be installed. These systems, such as adsorption, precipitation, coagulation, ion-exchange, membrane filtration techniques, among others, may be centralized or applied locally. Reducing the concentration of arsenic to below the permitted concentration limits requires the application of effective methods, since the arsenic removal mechanisms may pose certain difficulties. According to the speciation diagram of this element, the dominant As(III) specie in waters is the neutral form, for which it is more difficult to apply a selective removal mechanism. A common solution proposed in the literature and employed in practice is the preliminary As(III) oxidation to the As(V) form, which is negatively charged in the water pH range usually encountered [200][28]. Oxidation may be achieved chemically, or biologically by using the appropriate microorganisms [201][29]. On the other hand, and regarding the applied treatment technology, most of the aforementioned treatment methods present specific limitations, such as higher cost, lower selectivity, insufficient removal (i.e., the As residual concentration may be higher than the 10 μg/L concentration limit), higher energy consumption, or production of large amounts of toxic sludge. Among them, the most promising seems to be the adsorption process, since by applying the appropriate sorption materials, such as conventional iron- or aluminum-based adsorbents [202][30], or better yet, the novel engineered inorganic nanoparticles [203][31], it is possible to overcome most of the previous limitations. The adsorption-based technologies are also those that have found extensive applications in the highly affected As-contaminated areas of Southeast Asia [204][32]. In addition, it is possible to combine the respective removal mechanism with an oxidation mechanism, producing a unique step process, and therefore, increasing the method’s effectiveness [205][33].6. Conclusions
Arsenic concentration in food and drinking water above the maximum permissible concentration limit is a common water pollution problem in both developed and developing countries. The exposure to higher concentrations of arsenic may be life threatening. Water sources for drinking, such as surface water or groundwater, and food sources, such as fish, crops and cereals, can play a notorious role in exposing humans to arsenic. Taking into account these problems, the continuous monitoring of arsenic levels in water and food sources is mandatory in the future, allowing the application of proper treatment/removal process and the prevention of humans from arsenic intake.References
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618.
- Medunić, G.; Fiket, Ž.; Ivanić, M. Arsenic Contamination Status in Europe, Australia, and Other Parts of the World. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer: Singapore, 2020; Volume 1, pp. 183–233.
- World Health Organization (WHO). Arsenic. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 10 March 2022).
- Shahid, M.; Dumat, C.; Khan Niazi, N.; Khalid, S.; Natasha, N. Global scale arsenic pollution: Increase the scientific knowledge to reduce human exposure. VertigO 2018, 31, 21331.
- Ishiguro, S. Industries using arsenic and arsenic compounds. Appl. Organomet. Chem. 1992, 6, 323–331.
- Singh, A.; Giri, K. Effect of arsenate substitution on phosphate repository of cell: A computational study. R. Soc. Open Sci. 2018, 5, 181565.
- Sowers, T.D.; Nelson, C.M.; Blackmon, M.D.; Jerden, M.L.; Kirby, A.M.; Diamond, G.L.; Bradham, K.D. Interconnected soil iron and arsenic speciation effects on arsenic bioaccessibility and bioavailability: A scoping review. J. Toxicol. Environ. Health Part B Crit. Rev. 2022, 25, 1–22.
- Almeida, C.C.; Fontes, M.P.F.; Dias, A.C.; Pereira, T.T.C.; Ker, J.C. Adsorption and desorption of arsenic and its immobilization in soils. Sci. Agric. 2020, 78, 1–11.
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors—A review. Water Res. 2018, 140, 403–414.
- Gao, P.; Huang, J.; Wang, Y.; Li, L.; Sun, Y.; Zhang, T.; Peng, F. Effects of nearly four decades of long-term fertilization on the availability, fraction and environmental risk of cadmium and arsenic in red soils. J. Environ. Manag. 2021, 295, 113097.
- Harvey, P.J.; Handley, H.K.; Taylor, M.P. Widespread copper and lead contamination of household drinking water, New South Wales, Australia. Environ. Res. 2016, 151, 275–285.
- Lee, K.; Han, C.; Hong, S.B.; Jun, S.J.; Han, Y.; Xiao, C.; Du, Z.; Hur, S.D.; Lee, J.I.; Boutron, C.F.; et al. A 300-Year High-Resolution Greenland Ice Record of Large-Scale Atmospheric Pollution by Arsenic in the Northern Hemisphere. Environ. Sci. Technol. 2019, 53, 12999–13008.
- Vishwakarma, Y.K.; Tiwari, S.; Mohan, D.; Singh, R.S. A review on health impacts, monitoring and mitigation strategies of arsenic compounds present in air. Clean. Eng. Technol. 2021, 3, 100115.
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079.
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.R.; Francesconi, K.A. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017, 580, 266–282.
- Hussain, M.M.; Wang, J.; Bibi, I.; Shahid, M.; Niazi, N.K.; Iqbal, J.; Mian, I.A.; Shaheen, S.M.; Bashir, S.; Shah, N.S.; et al. Arsenic speciation and biotransformation pathways in the aquatic ecosystem: The significance of algae. J. Hazard. Mater. 2021, 403, 124027.
- Katsoyiannis, I.A.; Mitrakas, M.; Zouboulis, A.I. Arsenic occurrence in Europe: Emphasis in Greece and description of the applied full-scale treatment plants. Desalin. Water Treat. 2015, 54, 2100–2107.
- Thakur, J.K.; Thakur, R.K.; Ramanathan, A.L.; Kumar, M.; Singh, S.K. Arsenic contamination of groundwater in Nepal—An overview. Water 2011, 3, 1.
- Ventura-Lima, J.; Bogo, M.R.; Monserrat, J.M. Arsenic toxicity in mammals and aquatic animals: A comparative biochemical approach. Ecotoxicol. Environ. Saf. 2011, 74, 211–218.
- Katsoyiannis, I.A.; Zouboulis, A.I. Use of iron- and manganese-oxidizing bacteria for the combined removal of iron, manganese and arsenic from contaminated groundwater. Water Qual. Res. J. Can. 2006, 41, 117–129.
- World Health Organization (WHO). Arsenic in Drinking Water; Fact Sheet No. 210; WHO: Geneva, Switzerland, 1999.
- Abedi, T.; Mojiri, A. Arsenic uptake and accumulation mechanisms in rice species. Plants 2020, 9, 129.
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59.
- Food and Agriculture Organization (FAO). Codex Alimentarius Commission—Geneva 14–18 July 2014. Available online: https://www.fao.org/news/story/en/item/%20238558/icode/ (accessed on 14 March 2022).
- Zeng, Q.; Zhang, A. Assessing potential mechanisms of arsenic-induced skin lesions and cancers: Human and in vitro evidence. Environ. Pollut. 2020, 260, 113919.
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539.
- Okechukwu, C.E. Exposure to a high level of arsenic in drinking water and the risk of bladder cancer in Taiwan. Cancer Res. Stat. Treat. 2021, 4, 149–151.
- Weerasundara, L.; Ok, Y.S.; Bundschuh, J. Selective removal of arsenic in water: A critical review. Environ. Pollut. 2021, 268, 115668.
- Das, S.; Mukherjee, S. Implementation of Biotechnological Techniques in Treatment of Groundwater Contaminated with Arsenic. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 993–1000.
- Carneiro, M.A.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Current trends of arsenic adsorption in continuous mode: Literature review and future perspectives. Sustainability 2021, 13, 1186.
- Simeonidis, K.; Martinez-Boubeta, C.; Zamora-Pérez, P.; Rivera-Gil, P.; Kaprara, E.; Kokkinos, E.; Mitrakas, M. Implementing nanoparticles for competitive drinking water purification. Environ. Chem. Lett. 2019, 17, 705–719.
- Uppal, J.S.; Zheng, Q.; Le, X.C. Arsenic in drinking water—Recent examples and updates from Southeast Asia. Curr. Opin. Environ. Sci. Health 2019, 7, 126–135.
- Tresintsi, S.; Simeonidis, K.; Estradé, S.; Martinez-Boubeta, C.; Vourlias, G.; Pinakidou, F.; Katsikini, M.; Paloura, E.C.; Stavropoulos, G.; Mitrakas, M. Tetravalent manganese feroxyhyte: A novel nanoadsorbent equally selective for As(III) and As(V) removal from drinking water. Environ. Sci. Technol. 2013, 47, 9699–9705.
More
