Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Evgenios Kokkinos and Version 2 by Lindsay Dong.
Arsenic poisoning constitutes a major threat to humans, causing various health problems. The presence of arsenic in ecosystems can originate from several natural or anthropogenic activities. Arsenic can be then gradually accumulated in different food sources, such as vegetables, rice and other crops, but also in seafood, etc., and in water sources (mainly in groundwater, but also to a lesser extent in surface water), potentially used as drinking-water supplies, provoking their contamination and therefore potential health problems to the consumers.
- arsenic
- food contamination
- drinking water
- health effects
1. Introduction
Arsenic is a ubiquitous toxic metal, belonging in the metalloid group of the periodic table, found naturally in the lithosphere, hydrosphere, and atmosphere, as well as generally in the biosphere [1]. Both organic and inorganic forms of arsenic exist in nature (mostly in the form of complexes); various transportation routes in environment have been identified and rather high concentrations (mainly in water sources) have been reported in several regions around the world [2].
The World Health Organization (WHO) recommended the regulation limit (as imposed by the respective legislation) of arsenic concentration in drinking water at 10 μg/L [3]. This is also the limit of arsenic in drinking water imposed by the European Commission, the United States Environmental Protection Agency and other inter/national organizations. Contamination of water by arsenic, especially groundwater, due to arsenic’s high toxicity, is considered a major health issue in various areas worldwide. Relevant research showed that the long-term exposure to elevated concentrations of arsenic can threaten human health, causing a variety of health disorders, including skin lesions (e.g., keratosis, pigmentation) and various internal and skin cancers [4].
Nevertheless, arsenic has been used in various industries for the production of several products, such as glass, ceramics, electrical appliances, cosmetics and fireworks. In the mid-20th century, arsenic was also widely used to produce pesticides, and for the production of wood preservatives [5]. As a result of past and current uses, arsenic contamination is currently considered a problem of great concern for the scientific community, found mainly in water sources, but also in food, threatening the health of millions of people.
2. Origin of Arsenic Contamination
High concentrations of arsenic may occur naturally (e.g., due to erosion of minerals) in several areas, which are therefore contaminated, and by anthropogenic activities (e.g., industrial production and uses). The continuous exposure of humans to arsenic via food and water consumption can lead to serious health damages, because this is a carcinogenic element with high affinity for thiols. It can also replace phosphorus in biochemical reactions owing to their similar chemical properties, as they belong in the same group of the periodic table, actually being very close, indicating the highly destructive role of this element during DNA replication and metabolic activity [6][8]. Soil: The predominant forms of arsenic in soils are arsenate (As5+), arsenite (As3+) and organic arsenic. Usually, in soil matrixes it can be found complexed with amorphous iron and aluminum oxides [7][9]. The arsenic form in the soil varies according to the different textures. For instance, the presence of clay can increase the fixation of arsenic in the respective soil, as at circum-neutral pH values arsenic is adsorbed onto the clay particles [8][10]. Its concentration and mobility are also dependent on pH and redox potential at the specific environmental sites [9][11]. Because of the toxic effects of arsenic, it has been used (mainly in the past) for the production of specific herbicides, insecticides, various toxins and decongestants. The use of intensive phosphate fertilizers in agriculture is also considered a potential source for arsenic contamination, because arsenic is a common contaminant of most phosphate minerals, which are used for the production of these fertilizers. However, the amount of arsenic in other fertilizers(e.g., nitrogen or potash) is rather low and can be considered insignificant [10][12]. Figure 1 shows the various routes of arsenic and arsenic-related compounds that can accumulate in an ecosystem due to anthropogenic activities, resulting in environmental deterioration.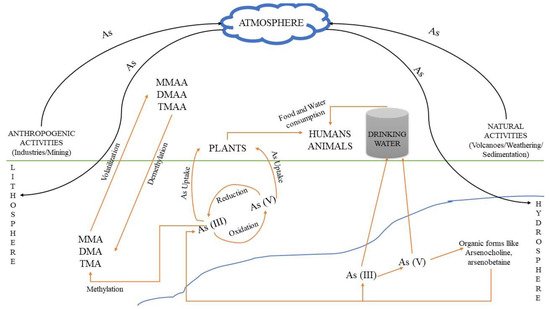
Figure 1. Pathways through which arsenic and its relevant compounds may enter the environment and contaminate soil, atmosphere and water. Human and natural activities result in As accumulation, mainly in soil and water, where As(V) and As(III) are interconverted via oxidation and reduction bio/reactions. The respective methylated products can be produced from As(III) species, i.e., MMA, DMA and TMA, resulting in the formation of MMAA, DMAA and TMAA chemical compounds, mainly through volatilization, while the reverse process occurs through demethylation.
Atmosphere: Arsenic can be released into the atmosphere by natural or anthropogenic activities. According to a rough estimation, the global annual release of arsenic in the atmosphere is 7.8 × 107 kg/year. The natural sources are expected to release 1.2 × 107 kg arsenic per year, wherein volcanoes and microbial volatilization may supplementarily contribute an additional 8.9 × 106 and 2.1 × 107 kg/year, respectively [11][13]. The amount of arsenic stored in the northern hemisphere is almost five times higher than the amount of arsenic stored in the southern hemisphere, mainly due to the more intensive industrialized conditions existing in the northern hemisphere [12][14]. Other environmental problems, potentially causing increased arsenic emissions in the atmosphere, may include deforestation, grass burning, and the use of wood as fuel. High and significant concentrations of arsenic are also connected with the emissions of industrial wastes, especially heavy/toxic metals [13][15]; the concentration of arsenic in sewage sludges is considered to be an indicator of the industrialization degree for the surrounding area.
Water: Since arsenic is an element of the Earth’s crust, groundwater usually presents the most severe pollution problems among other water resources [14][16]. It can be found either dissolved in water or in the form of particles. Moreover, it may be transformed to dimethyl and/or trimethyl arsenic compounds by mollusks, crustaceans and fishes [15][17]. Arsenate is the predominant form found in seawater algae, which play an important role in the biological transport of inorganic arsenic species [16][18].
