Mitochondria, as the main site of cellular energy metabolism and the generation of oxygen free radicals, are the key switch for mitochondria-mediated endogenous apoptosis. Ca2+ is not only an important messenger for cell proliferation, but it is also an indispensable signal for cell death. Ca2+ participates in and plays a crucial role in the energy metabolism, physiology, and pathology of mitochondria. Mitochondria control the uptake and release of Ca2+ through channels/transporters, such as the mitochondrial calcium uniporter (MCU), and influence the concentration of Ca2+ in both mitochondria and cytoplasm, thereby regulating cellular Ca2+ homeostasis. Mitochondrial Ca2+ transport-related processes are involved in important biological processes of tumor cells including proliferation, metabolism, and apoptosis.
- mitochondrial calcium
- calcium homeostasis
1. Introduction
2. Regulation of Mitochondrial Ca2+
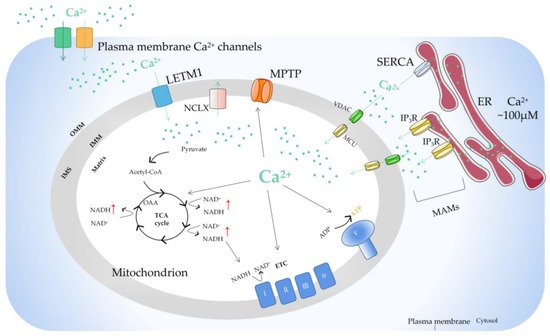
Due to the outer membrane of mitochondria possessing a high permeability for Ca2+, the concentration of Ca2+ in the membrane gap is equivalent to that in the cytoplasm [20]. In the resting state of cells, the concentration of Ca2+ in cytoplasm is about 100nM. When the cells are excited, the concentration of Ca2+ in the cytoplasm can rise to 1–3 µM. [21]. In fact, the uptake and release of Ca2+ by mitochondria can be regulated by the one-way transport mechanism or transporter [22]. The mitochondrial Ca2+ influx is mainly mediated by the mitochondrial calcium uniporter (MCU), voltage-dependent anion-selective channel (VDAC), and mitochondrial ryanodine receptor transporter. Furthermore, the mitochondrial Ca2+ efflux pathways mainly include leucine zipper/EF hand-containing transmembrane-1 (LETM1), mitochondrial Na+/Ca2+ exchanger (NCLX), and mitochondrial permeability transition pore (MPTP). MCU is a Ca2+ channel ubiquitous in mitochondrial intima [23]. It is generally considered to be a key Ca2+ transporter [24] and silencing the MCU can severely abrogate mitochondrial Ca2+ uptake [25] (Figure 1). Knockout of the MCU completely inhibited mitochondrial Ca2+ uptake triggered by several stimuli in different cell types [26]. The MCU is an ion channel with electrophysiological characteristics. Ca2+ uptake through the MCU is driven by an electrochemical gradient. The MCU and related regulating molecules, including the essential MCU regulator (EMRE), mitochondrial calcium uptake (MICU)1, MICU2, MICU3, MCU-dominant negative beta subunit (MCUb), and MCU regulator 1 (MCUR1), form a large complex to manipulate the activities of the MCU [27]. The changes in the expression of these regulators are different in different cancer cells. For example, in pancreatic cancer cells, MICU1 and MICU2 are increased, while EMRE is decreased [28]. In breast cancer cells, MCU is elevated but MCUb is reduced [29]. In ovarian cancer cells, MICU1 mRNA is enhanced [30]. In HCC cells, the MCU, MCUR1, and MICU2 are elevated, while MICU1 is in decline [31].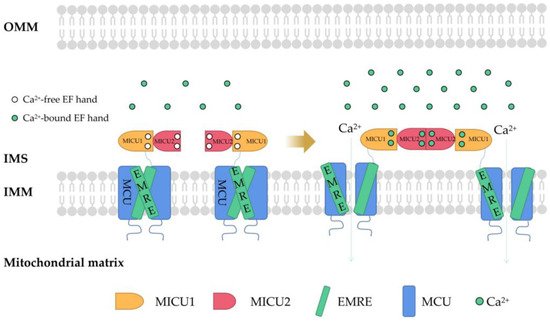
References
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Chem. Commun. 2012, 148, 1145–1159. Nunnari, J.; Suomalainen, A. Mitochondria: in sickness and in health. Chem Commun (Camb). 2012, 148, 1145-1159.
- Bravo-Sagua, R.; Parra, V.; López-Crisosto, C.; Díaz, P.; Quest, A.F.; Lavandero, S. Calcium Transport and Signaling in Mitochondria. Compr. Physiol. 2017, 7, 623–634. Bravo-Sagua, R.; Parra, V.; López-Crisosto, C.; Díaz, P.; Quest, AF.; Lavandero, S. Calcium Transport and Signaling in Mitochondria. Compr Physiol. 2017, 7, 623-634.
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int J Mol Sci. 2020, 21, 8323.
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018, 19, 713-730.
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Intracellular calcium homeostasis and signaling. Met. Ions Life Sci. 2013, 12, 119–168. Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Intracellular calcium homeostasis and signaling. Met Ions Life Sci. 2013, 12, 119-168.
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. JBC 2016, 40, 20849–20857. Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. JBC. 2016, 40, 20849-20857.
- Zavodnik, I.B. Mitochondria, calcium homeostasis and calcium signaling. Biomed. Khim. 2016, 62, 311–317. Zavodnik, IB. Mitochondria, calcium homeostasis and calcium signaling. Biomed Khim. 2016, 62, 311-317.
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003, 4, 552-565.
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018, 69, 62–72. Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, MR.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018, 69, 62-72.
- Magalhães, P.J.; Rizzuto, R. Mitochondria and calcium homeostasis: A tale of three luminescent proteins. Luminescence 2001, 16, 67–71. Magalhães, PJ.; Rizzuto, R. Mitochondria and calcium homeostasis: a tale of three luminescent proteins. Luminescence. 2001, 16, 67-71.
- Godoy, J.A.; Rios, J.A.; Picón-Pagès, P.; Herrera-Fernández, V.; Swaby, B.; Crepin, G.; Vicente, R.; Fernández-Fernández, J.M.; Muñoz, F.J. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules 2021, 11, 1012. Godoy, JA.; Rios, JA.; Picón-Pagès, P.; Herrera-Fernández, V.; Swaby, B.; Crepin, G.; Vicente, R.; Fernández-Fernández, JM.; Muñoz, FJ. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules. 2021, 11, 1012.
- Robb-Gaspers, L.; Burnett, P.; Rutter, G.; Denton, R.; Rizzuto, R.; Thomas, A. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998, 17, 4987–5000. Robb-Gaspers, L.; Burnett, P.; Rutter, G.; Denton, R.; Rizzuto, R.; Thomas, A. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998, 17, 4987-5000.
- Uhlén, P.; Fritz, N. Biochemistry of calcium oscillations. Biochem. Biophys. Res. Commun. 2010, 396, 28–32. Uhlén, P.; Fritz, N. Biochemistry of calcium oscillations. Biochem Biophys Res Commun. 2010, 396, 28-32.
- Rizzuto, R.; Brini, M.; Murgia, M.; Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 1993, 262, 744–747. Rizzuto, R.; Brini, M.; Murgia, M.; Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993, 262, 744-747.
- Hajnóczky, G.; Robb-Gaspers, L.D.; Seitz, M.B.; Thomas, A.P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 1995, 82, 415–424. Hajnóczky, G.; Robb-Gaspers, LD.; Seitz, MB.; Thomas, AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995, 82, 415-424.
- Sheu, S.S.; Jou, M.J. Mitochondrial free Ca2+ concentration in living cells. J. Bioenerg. Biomembr. 1994, 26, 487–493. Sheu, SS.; Jou, MJ. Mitochondrial free Ca2+ concentration in living cells. J Bioenerg Biomembr. 1994, 26, 487-493.
- Jouaville, L.S.; Pinton, P.; Bastianutto, C.; Rutter, G.A.; Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 1999, 96, 13807–13812. Jouaville, LS.; Pinton, P.; Bastianutto, C.; Rutter, GA.; Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999, 96, 13807-13812.
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. Berridge, MJ.; Bootman, MD.; Roderick, HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003, 4, 517-529.
- Paudel, S.; Sindelar, R.; Saha, M. Calcium Signaling in Vertebrate Development and Its Role in Disease. Int. J. Mol. Sci. 2018, 19, 3390. Paudel, S.; Sindelar, R.; Saha, M. Calcium Signaling in Vertebrate Development and Its Role in Disease. Int J Mol Sci. 2018, 19, 3390.
- Pézier, A.; Acquistapace, A.; Renou, M.; Rospars, J.P.; Lucas, P. Ca2+ stabilizes the membrane potential of moth olfactory receptor neurons at rest and is essential for their fast repolarization Chem. Senses 2007, 32, 305–317. Pézier, A.; Acquistapace, A.; Renou, M.; Rospars, JP.; Lucas, P. Ca2+ stabilizes the membrane potential of moth olfactory receptor neurons at rest and is essential for their fast repolarization Chem Senses. 2007, 32, 305-317.
- Pendin, D.; Greotti, E.; Filadi, R.; Pozzan, T. Spying on organelle Ca2+ in living cells: The mitochondrial point of view. J. Endocrinol. Investig. 2015, 38, 39–45. Pendin, D.; Greotti, E.; Filadi, R.; Pozzan, T. Spying on organelle Ca2+ in living cells: the mitochondrial point of view. J Endocrinol Invest. 2015, 38, 39-45.
- Yamamoto, T. The Molecular Mechanisms of Mitochondrial Calcium Uptake by Calcium Uniporter. Yakugaku Zasshi J. Pharm. Jpn. 2021, 141, 491–499. Yamamoto, T. The Molecular Mechanisms of Mitochondrial Calcium Uptake by Calcium Uniporter. Yakugaku Zasshi J. Pharm. Jpn. 2021, 141, 491–499.
- Stefani, D.D.; Raffaello, A.; Teardo, E.; Szabò, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. Stefani, DD.; Raffaello, A.; Teardo, E.; Szabò, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011, 476, 336-340.
- Belosludtsev, K.N.; Dubinin, M.V.; Belosludtseva, N.V.; Mironova, G.D. Mitochondrial Ca2+ Transport: Mechanisms, Molecular Structures, and Role in Cells. Biochemistry 2019, 84, 593–607. Belosludtsev, KN.; Dubinin, MV.; Belosludtseva, NV.; Mironova, GD. Mitochondrial Ca2+ Transport: Mechanisms, Molecular Structures, and Role in Cells. Biochemistry (Mosc). 2019, 84, 593-607.
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. Baughman, JM.; Perocchi, F.; Girgis, HS.; Plovanich, M.; Belcher-Timme, CA.; Sancak, Y.; Bao, XR.; Strittmatter, L.; Goldberger, O.; Bogorad, RL.; Koteliansky, V.; Mootha, VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011, 476, 341-345.
- Pan, X.; Liu, J.; Nguyen, T.; Liu, C.; Sun, J.; Teng, Y.; Fergusson, M.M.; Rovira, I.I.; Allen, M.; Springer, D.A.; et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013, 15, 1464–1472. Pan, X.; Liu, J.; Nguyen, T.; Liu, C.; Sun, J.; Teng, Y.; Fergusson, MM.; Rovira, II.; Allen, M.; Springer, DA.; Aponte, AM.; Gucek, M.; Balaban, RS.; Murphy, E.; Finkel, T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013, 15, 1464-1472.
- Garbincius, J.; Elrod, J. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 2022, 102, 893–992. Garbincius, J.; Elrod, J. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022, 102, 893-992.
- Chen, L.; Sun, Q.; Zhou, D.; Song, W.; Yang, Q.; Ju, B.; Zhang, L.; Xie, H.; Zhou, L.; Hu, Z.; et al. HINT2 triggers mitochondrial Ca2+ influx by regulating the mitochondrial Ca2+ uniporter (MCU) complex and enhances gemcitabine apoptotic effect in pancreatic cancer. Cancer Lett. 2017, 411, 106–116. Chen, L.; Sun, Q.; Zhou, D.; Song, W.; Yang, Q.; Ju, B.; Zhang, L.; Xie, H.; Zhou, L.; Hu, Z.; Yao, H.; Zheng, S.; Wang, W. HINT2 triggers mitochondrial Ca2+ influx by regulating the mitochondrial Ca2+ uniporter (MCU) complex and enhances gemcitabine apoptotic effect in pancreatic cancer. Cancer Lett. 2017, 411, 106–116.
- Curry, M.C.; Peters, A.A.; Kenny, P.A.; Roberts-Thomson, S.J.; Monteith, G.R. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochem. Biophys. Res. Commun. 2013, 434, 695–700. Curry, MC.; Peters, AA.; Kenny, PA.; Roberts-Thomson, SJ.; Monteith, GR. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochemical and Biophysical Research Communications. 2013, 434, 695-700.
- Sancak, Y.; Markhard, A.; Kitami, T.; Kovacs-Bogdan, E.; Kamer, K.; Udeshi, N.; Carr, S.A.; Chaudhuri, D.; Clapham, D.E.; Li, A.A.; et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 2013, 342, 1379–1382. Sancak, Y.; Markhard, A.; Kitami, T.; Kovacs-Bogdan, E.; Kamer, K.; Udeshi, N.; et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013, 342, 1379-1382.
- Wang, W.; Xie, Q.; Zhou, X.; Yao, J.; Zhu, X.; Huang, P.; Zhang, L.; Wei, J.; Xie, H.; Zhou, L.; et al. Mitofusin-2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Lett. 2015, 358, 47–58. Wang, W.; Xie, Q.; Zhou, X.; Yao, J.; Zhu, X.; Huang, P.; Zhang, L.; Wei, J.; Xie, H.; Zhou, L.; Zheng, S. Mitofusin-2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Lett. 2015, 358, 47–58.
- Zhuo, W.; Zhou, H.; Guo, R.; Yi, J.; Zhang, L.; Yu, L.; Sui, Y.; Zeng, W.; Wang, P.; Yang, M. Structure of intact human MCU supercomplex with the auxiliary MICU subunits. Protein Cell. 2020, 12, 220–229. Zhuo, W.; Zhou, H.; Guo, R.; Yi, J.; Zhang, L.; Yu, L.; et al. Structure of intact human MCU supercomplex with the auxiliary MICU subunits. Protein & Cell. 2020, 12, 220-229.
- Fan, M.; Zhang, J.; Tsai, C.; Benjamin, J.; Rodriguez, M.; Xu, Y.; Liao, M.; Tsai, M.; Feng, L. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature 2020, 582, 129–133. Fan, M.; Zhang, J.; Tsai, C.; Benjamin, J.; Rodriguez, M.; Xu, Y.; Liao, M.; Tsai, M.; Feng, L. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature. 2020, 582, 129-133.
- Tomar, D.; Elrod, J.W. Metabolite regulation of the mitochondrial calcium uniporter channel. Cell Calcium. 2020, 92, 102288. Tomar, D.; Elrod, JW. Metabolite regulation of the mitochondrial calcium uniporter channel. Cell Calcium. 2020, 92, 102288.
- Gottschalk, B.; Madreiter-Sokolowski, C.T.; Graier, W.F. Cristae junction as a fundamental switchboard for mitochondrial ion signaling and bioenergetics. Cell Calcium. 2022, 101, 102517. Gottschalk, B.; Madreiter-Sokolowski, CT.; Graier, WF. Cristae junction as a fundamental switchboard for mitochondrial ion signaling and bioenergetics. Cell Calcium. 2022, 101, 102517.
- Gottschalk, B.; Klec, C.; Leitinger, G.; Bernhart, E.; Rost, R.; Bischof, H.; Madreiter-Sokolowski, C.T.; Radulović, S.; Eroglu, E.; Sattler, W.; et al. MICU1 controls cristae junction and spatially anchors mitochondrial Ca2+ uniporter complex. Nat. Commun. 2019, 10, 3732. Gottschalk, B.; Klec, C.; Leitinger, G.; Bernhart, E.; Rost, R.; Bischof, H.; Madreiter-Sokolowski, CT.; Radulović, S.; Eroglu, E.; Sattler, W.; Waldeck-Weiermair, M.; Malli, R.; Graier, WF. MICU1 controls cristae junction and spatially anchors mitochondrial Ca2+ uniporter complex. Nat Commun. 2019, 10, 3732.
- Chakraborty, P.K.; Mustafi, S.B.; Xiong, X.; Dwivedi, S.K.D.; Nesin, V.; Saha, S.; Zhang, M.; Dhanasekaran, D.; Jayaraman, M.; Mannel, R.; et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat. Commun. 2017, 8, 14634. Chakraborty, PK.; Mustafi, SB.; Xiong, X.; Dwivedi, SKD.; Nesin, V.; Saha, S.; Zhang, M.; Dhanasekaran, D.; Jayaraman, M.; Mannel, R.; Moore, K.; McMeekin, S.; Yang, D.; Zuna, R.; Ding, K.; Tsiokas, L.; Bhattacharya, R.; Mukherjee, P. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat Commun. 2017, 8 14634.
- Trenker, M.; Malli, R.; Fertschai, I.; Levak-Frank, S.; Graier, W.F. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 2007, 9, 445–452. Trenker, M.; Malli, R.; Fertschai, I.; Levak-Frank, S.; Graier, WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007, 9, 445-452.
- Madreiter-Sokolowski, C.T.; Klec, C.; Parichatikanond, W.; Stryeck, S.; Gottschalk, B.; Pulido, S.; Rost, R.; Eroglu, E.; Hofmann, N.A.; Bondarenko, A.I.; et al. PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca(2+) uptake in immortalized cells. Nat. Commun. 2016, 7, 12897. Madreiter-Sokolowski, CT.; Klec, C.; Parichatikanond, W.; Stryeck, S.; Gottschalk, B.; Pulido, S.; Rost, R.; Eroglu, E.; Hofmann, NA.; Bondarenko, AI.; Madl, T.; Waldeck-Weiermair, M.; Malli, R.; Graier, WF. PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca(2+) uptake in immortalized cells. Nat Commun. 2016, 7, 12897.
- Madreiter-Sokolowski, C.T.; Győrffy, B.; Klec, C.; Sokolowski, A.A.; Rost, R.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. UCP2 and PRMT1 are key prognostic markers for lung carcinoma patients. Oncotarget. 2017, 8, 80278–80285. Madreiter-Sokolowski, CT.; Győrffy, B.; Klec, C.; Sokolowski, AA.; Rost, R.; Waldeck-Weiermair, M.; Malli, R.; Graier, WF. UCP2 and PRMT1 are key prognostic markers for lung carcinoma patients. Oncotarget. 2017, 8, 80278-80285.
- Jarrold, J.; Davies, C.C. PRMTs and Arginine Methylation: Cancer’s Best-Kept Secret? Trends Mol. Med. 2019, 25, 993–1009. Jarrold, J.; Davies, CC. PRMTs and Arginine Methylation: Cancer's Best-Kept Secret? Trends Mol Med. 2019, 25, 993-1009.
- Payne, R.; Hoff, H.; Roskowski, A.; Foskett, J. MICU2 restricts spatial crosstalk between InsP3R and MCU channels by regulating threshold and gain of MICU1-mediated inhibition and activation of MCU. Cell Reports. 2017, 21, 3141–3154. Payne, R.; Hoff, H.; Roskowski, A.; Foskett, J. MICU2 restricts spatial crosstalk between InsP3R and MCU channels by regulating threshold and gain of MICU1‐mediated inhibition and activation of MCU. Cell Reports. 2017, 21, 3141–3154.
- Patron, M.; Granatiero, V.; Espino, J.; Rizzuto, R.; De Stefani, D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019, 26, 179–195. Patron, M.; Granatiero, V.; Espino, J.; Rizzuto, R.; De Stefani, D. MICU3 is a tissue‐specific enhancer of mitochondrial calcium uptake. Cell Death and Differentiation. 2019, 26, 179–195.
- Raffaello, A.; De Stefani, D.; Sabbadin, D.; Teardo, E.; Merli, G.; Picard, A.; Checchetto, V.; Moro, S.; Szabò, I.; Rizzuto, R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013, 32, 2362–2376. Raffaello, A.; De Stefani, D.; Sabbadin, D.; Teardo, E.; Merli, G.; Picard, A.; Checchetto, V.; Moro, S.; Szabò, I.; Rizzuto, R. The mitochondrial calcium uniporter is a multimer that can include a dominant‐negative pore‐forming subunit. The EMBO Journal. 2013, 32, 2362–2376.
- Tomar, D.; Dong, Z.; Shanmughapriya, S.; Koch, D.; Thomas, T.; Hoffman, N.; Timbalia, S.; Goldman, S.; Breves, S.; Corbally, D.; et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep. 2016, 15, 1673–1685. Tomar, D.; Dong, Z.; Shanmughapriya, S.; Koch, D.; Thomas, T.; Hoffman, N.; Timbalia, S.; Goldman, S.; Breves, S.; Corbally, D.; et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep. 2016, 15, 1673-1685.
- Chaudhuri, D.; Artiga, D.J.; Abiria, S.A.; Clapham, D.E. Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proc. Natl. Acad. Sci. USA 2016, 113, E1872–E1880. Chaudhuri, D.; Artiga, D. J.; Abiria, S. A.; Clapham, D. E. Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proceedings of the National Academy of Sciences of the United States of America. 2016, 113, E1872–E1880.
- Roy, S.; Dey, K.; Hershfinkel, M.; Ohana, E.; Sekler, I. Identification of residues that control Li+ versus Na+ dependent Ca2+ exchange at the transport site of the mitochondrial NCLX. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 997–1008. Roy, S.; Dey, K.; Hershfinkel, M.; Ohana, E.; Sekler, I. Identification of residues that control Li+ versus Na+ dependent Ca2+ exchange at the transport site of the mitochondrial NCLX. Biochim Biophys Acta Mol Cell Res. 2017, 1864, 997-1008.
- Starkov, A.A.; Chinopoulos, C.; Starkova, N.N.; Konrad, C.; Kiss, G.; Stepanova, A.; Popov, V.N. Divalent cation chelators citrate and EDTA unmask an intrinsic uncoupling pathway in isolated mitochondria. J. Bioenerg. Biomembr. 2017, 49, 3–11. Starkov, AA.; Chinopoulos, C.; Starkova, NN.; Konrad, C.; Kiss, G.; Stepanova, A.; Popov, VN. Divalent cation chelators citrate and EDTA unmask an intrinsic uncoupling pathway in isolated mitochondria. J Bioenerg Biomembr. 2017, 49, 3-11.
- Wilson, J.A.; Lau, Y.S.; Gleeson, J.G.; Wilson, J.S. The action of MPTP on synaptic transmission is affected by changes in Ca2+ concentrations. Brain Res. 1991, 541, 342–346. Wilson, JA.; Lau, YS.; Gleeson, JG.; Wilson, JS. The action of MPTP on synaptic transmission is affected by changes in Ca2+ concentrations. Brain Res. 1991, 541, 342-346.
- Kolomiiets’, O.; Danylovych, I.; Danylovych, H. H+-Ca2+-exchanger in the myometrium mitochondria: Modulation of exogenous and endogenous compounds. Fiziol. Zh. 2014, 60, 33–42. Kolomiiets', O.; Danylovych, I.; Danylovych, H. H+-Ca2+-exchanger in the myometrium mitochondria: modulation of exogenous and endogenous compounds. Fiziol Zh. 2014, 60, 33-42.
- Gomez-Suaga, P.; Paillusson, S.; Stoica, R.; Noble, W.; Hanger, D.P.; Miller, C.C. The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr. Biol. 2017, 27, 371–385. Gomez-Suaga, P.; Paillusson, S.; Stoica, R.; Noble, W.; Hanger, DP.; Miller, CC. The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr Biol. 2017, 27, 371-385.
- Giorgi, C.; Bonora, M.; Sorrentino, G.; Missiroli, S.; Poletti, F.; Suski, J.M.; Galindo Ramirez, F.; Rizzuto, R.; Di Virgilio, F.; Zito, E.; et al. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc. Natl. Acad. Sci. USA 2015, 112, 1779–1784. Giorgi, C.; Bonora, M.; Sorrentino, G.; Missiroli, S.; Poletti, F.; Suski, JM.; Galindo Ramirez, F.; Rizzuto, R.; Di Virgilio, F.; Zito, E.; Pandolfi, PP.; Wieckowski, MR.; Mammano, F.; Del Sal, G.; Pinton, P. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci U S A. 2015, 112, 1779-1784.
- Betz, C.; Stracka, D.; Prescianotto-Baschong, C.; Frieden, M.; Demaurex, N.; Hall, M.N. Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc. Natl. Acad. Sci. USA 2013, 110, 12526–12534. Betz, C.; Stracka, D.; Prescianotto-Baschong, C.; Frieden, M.; Demaurex, N.; Hall, MN. Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci U S A. 2013, 110, 12526-12534.
- Rimessi, A.; Marchi, S.; Patergnani, S.; Pinton, P. H-Ras-driven tumoral maintenance is sustained through caveolin-1-dependent alterations in calcium signaling. Oncogene 2014, 33, 2329–2340. Rimessi, A.; Marchi, S.; Patergnani, S.; Pinton, P. H-Ras-driven tumoral maintenance is sustained through caveolin-1-dependent alterations in calcium signaling. Oncogene. 2014, 33, 2329-2340.
- Glitsch, M. Mechano- and pH-sensing convergence on Ca2+-mobilising proteins - A recipe for cancer? Cell Calcium. 2019, 80, 38–45. Glitsch, M. Mechano- and pH-sensing convergence on Ca2+-mobilising proteins - A recipe for cancer? Cell Calcium. 2019, 80, 38-45.
- Trapani, V.; Wolf, F.I. Dysregulation of Mg2+ homeostasis contributes to acquisition of cancer hallmarks. Cell Calcium. 2019, 83, 102078. Trapani, V.; Wolf, FI. Dysregulation of Mg2+ homeostasis contributes to acquisition of cancer hallmarks. Cell Calcium. 2019, 83, 102078.
- Santoni, G.; Morelli, M.B.; Marinelli, O.; Nabissi, M.; Santoni, M.; Amantini, C. Calcium Signaling and the Regulation of Chemosensitivity in Cancer Cells: Role of the Transient Receptor Potential Channels. Adv. Exp. Med. Biol. 2020, 1131, 505–517. Santoni, G.; Morelli, MB.; Marinelli, O.; Nabissi, M.; Santoni, M.; Amantini, C. Calcium Signaling and the Regulation of Chemosensitivity in Cancer Cells: Role of the Transient Receptor Potential Channels. Adv Exp Med Biol. 2020, 1131, 505-517.
