Botulinum neurotoxin A (BoNT-A) which is generally known as anti-contraction of muscles has been reported as a successful treatment in various types of chronic ulcers.
- BoNT-A
- ischemia
- neuromodulators
1. Introduction
2. Mechanism of BoNT-A on Wound Healing
Normal physiologic wound healing is a dynamic process consisting of different continuous, overlapping, and precisely programmed phases [1][3][11]. Any disruption in the process leads to abnormal wound healing or chronic unhealed ulcers [12]. The literature review found BoNT-A can enhance wound healing in various types of ulcers. Several studies that investigated mechanisms of BoNT-A on wound healing found that BoNT-A decreased inflammatory cell infiltration during the inflammatory phase [20][21][22]. Inhibition of mast cell degranulation and reduction in number were reported [19][23]. Reduction in vascular permeability and exudation of neutrophils and macrophages were also observed. Consequently, pus deposition on ulcers was reduced [24]. Furthermore, during the proliferative phase, it could enhance blood flow and promote vascular sprouting by increasing expression in CD31+, ∝SAM (+) pericytes, as well as fibroblasts [25][26][27][28]. It could be due to a reduction of reactive oxygen species (ROS) release and endothelial nitric oxide synthase (eNOS) in the hypoxic area [25][29]. Altogether, granulation tissue was created and filled the wound. It also can inhibit releasing norepinephrine resulting in vasodilatation and increasing oxygenation to the wound [30]. In the period of the remodeling phase, lower expression of TGF-β1 by BoNT-A was found which reduces the risk of fibrosis and scar formation [24]. Figure 1 shows a mechanism of BoNT-A in wound healing for comprehensive understanding.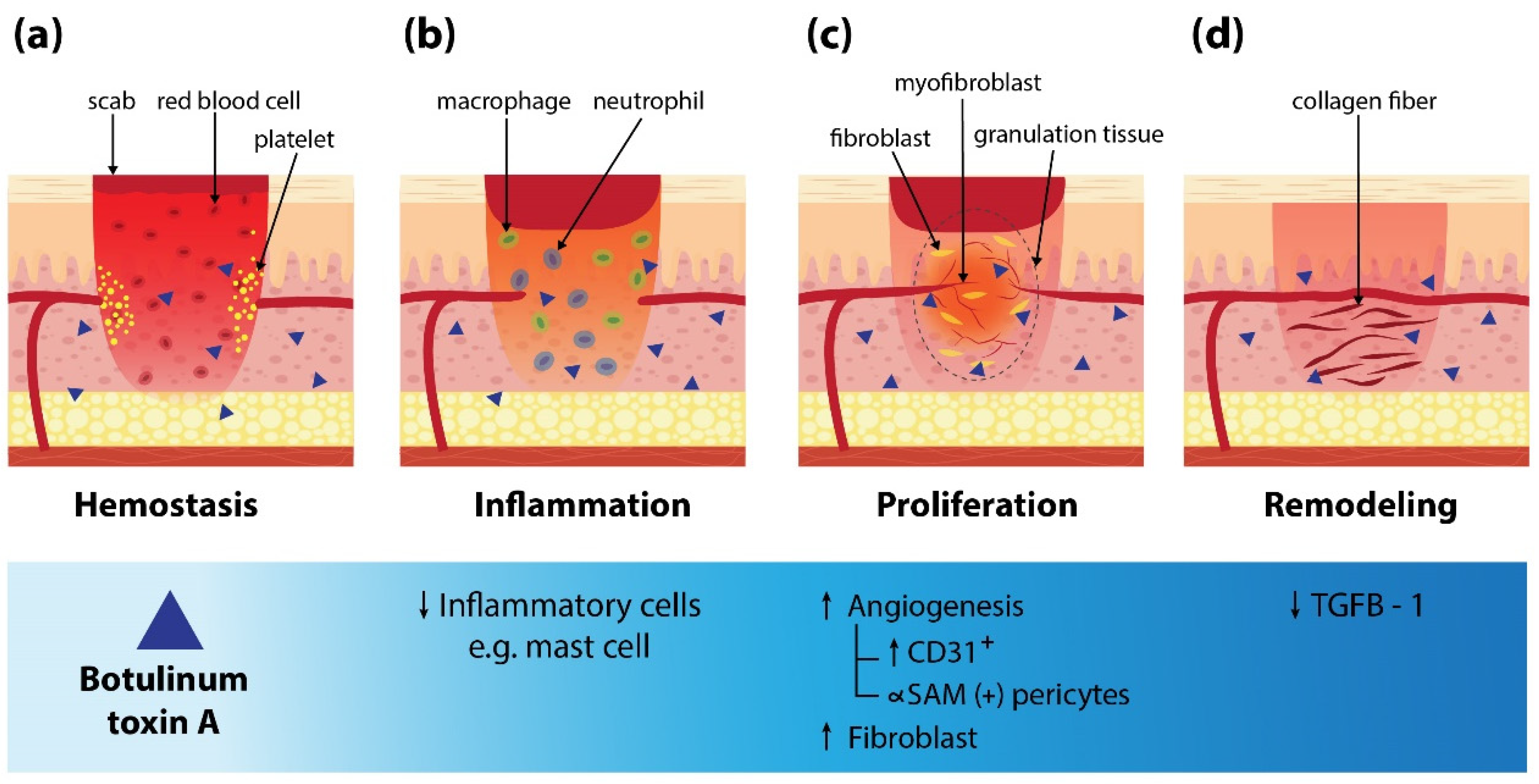
3. BoNT-A for Various Types of Skin Ulcers
Ischemic Ulcers Secondary to Raynaud’s Phenomenon (RP)
Although numerous publications demonstrated a positive effect of BoNT-A on RP [32][33][34][35][36], this entry will mainly focus on patients who had RP-associated ulcers. Based on the literature search, 12 articles (case reports, retrospective case series, and prospective case series) with a total of 104 patients who had RP-associated ulcers were identified (Table 1) [37][38][39][40][41][42][43][44][45][46][47][48]. Patients have been suffering from RP symptoms (pain, loss of function, disfigurement, and so forth) and chronic ischemic nonhealing ulcers. Moreover, some underwent sympathectomy but the clinical of ulcers did not improve [37].| Authors, Year | Study Type | N (Gender) | Age, Years (Mean or Range) | Type of BoNT-A | BoNT-A Dilution with 0.9% (NSS) | BoNT-A Dose/Location | Follow-Up Period | Results | Reinjection (Interval) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Quinatana Castanedo et al., 2020 [40] | Case report | 1 (female) | 15 | NR | NR | 32 units/Foot | 4 weeks |
|
Yes (every 12 months) | |
| Habib et al., 2020 [47] | Case series | 3 (female) | 23–50 | NR | NR | 32 units/Hand | 1 week |
|
No | |
| Min et al., 2020 [44] | Case report | 1 (male) | 48 | Medytoxin® (Medytox, Seoul, Korea) | 10 units/0.1 mL | 10 units/Hand | 12 weeks |
|
Yes (weekly for 3 weeks) | |
| Souk and Kim, 2019 [48] | Case report | 2 (female) | 50 and 62 | Medytoxin® (Medytox, Seoul, Korea) | 10 units/0.1 mL | 10 units/Hand | 8 weeks |
|
Yes 2/2 (4 and 5 weeks, 7 and 8 weeks) | |
| Garrido-Rios et al., 2018 [41] | Case report | 1 (female) | 30 | NR | 8–10 units/0.4 mL | 80–100 units/Hand | 2 months |
|
No | |
| Medina et al., 2018 [45] | Retrospective case series | 15 (female 14/male 1) | 35–71 | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 100 units/5 mL | Average 45 units/Hand | 3 years |
|
Yes 6/15 (annually) | 4/15 temporary decrease intrinsic muscle strength |
| Blaise et al., 2017 [43] | Case report | 1 (female) | 55 | NR | NR | 100 units/Hand | 4 months |
|
No | |
| Motegi et al., 2016 [42] | Prospective, case series | 10 (NR) | 62.5 (±3.5) | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 20 units/0.1 mL | 10 units/Hand | 16 weeks |
|
No | |
| Zhang et al., 2015 [38] | Retrospective case series | 10 (female 5/male 5) | 48–91 | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 100 units/5 mL | 60 units/Hand | 6 months (average) |
|
No | |
| Smith et al., 2012 [37] | Case report | 1 (female) | 52 | NR | 5 units/0.1 mL | 100 units/Hand | 3 months |
|
No | Mild, nonlimiting thenar muscle weakness |
| Neumeister. 2010 [46] | Retrospective case series | 33 (female 19/male 14) | 18–72 | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 100 units/20 mL | 50 units/Hand | 6 years |
|
Yes 7/33 (not reported) | - 3 patients had temporary intrinsic muscle weakness that lasted 2 months |
| Fregene et al., 2009 [39] | Retrospective case series | 26 (female 14/male 12) | 60.7 (±1.9) | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 100 units/2 mL | Average 77 units/Hand | 18 months (average) |
|
No | - Some reported intrinsic muscle weakness and 1 dysesthesia digit which resolved completely by 5 months |
BoNT-A for Pressure Ulcers
Administration of BoNT-A on pressure ulcers that were associated with muscle spasticity has been reported [51][52][53][54][55]. The rationale for the use of BoNT-A on pressure ulcers is to relieve muscle spasticity [56][57]. As a result, pressure ulcers are adequately accessed and omitted from repetitive trauma.
Regarding the point of injection, BoNT-A can be injected directly into abnormally contracted muscle or under electromyographic guidance [52][53][54]. The number of injection points was considered based on the size of muscles. According to a report by Insito et al., abobotulinum toxin with doses of 200 and 120 speywood unit (SU) was injected into orbicularis oris and masseter in a vegetative state patient with oromandibular dyskinesia [54]. Regarding the larger size of muscles such as Gluteal muscle, a high dosage of 660 SU was used [53]. Gupta and Wilson reported a dosage of onabotulinum toxin ranging from 100 to 150 units per muscle [52]. Abnormal contraction or spasticity were improved as early as 1 week after treatment [5]. All ulcers were reported as complete healing with the most delayed time period of 6-month follow-up [53]. The number of treatment sessions varied from one to two sessions. A repeated treatment session might be considered in patients with partially healed ulcers to maintain the weakness of muscles. Data regarding the use of BoNT-A in pressure ulcers is summarized in Table 2.
Table 2. A summary of articles on the treatment outcome of other types of chronic ulcer.
|
Authors, Year |
Study Type |
N (Gender) |
Age, Years (Mean or Range) |
Type of BoNT-A |
BoNT-A Dilution with 0.9% NSS |
BoNT-A Dose/Location |
Follow-up Period |
Results |
Reinjection (Interval) |
Comments |
|
Gupta and Wilson, 2020 [52] |
Case report |
1 (female) |
59 |
NR |
NR |
150 units for pectoralis major, 150 for elbow flexors, 100 for flexor digitorum superficialis |
5 months |
Completely healed ulcer |
Yes (5 months) |
Pressure ulcer |
|
Insito and Basciani, 2009 [53] |
Case report |
1 (male) |
27 |
Dysport®, Ipsen Limited, Slough, UK |
NR |
660 Speywood units (left Gluteus maximus) |
6 months |
Weaken muscle contraction Healed ulcer |
Yes (3 months) |
Pressure ulcer |
|
Insito et al., 2008 [54] |
Case report |
1 (male) |
73 |
Dysport®, Ipsen Limited, Slough, UK |
NR |
200 Speywood units for Orbicularis oris, 120 for Masseter |
3 months |
Improved dyskinetic disorder Completely healed ulcer |
Yes (2 months) |
Pressure ulcer |
|
Sillitoe et al., 2007 [55] |
Letter to editors |
1 (male) |
58 |
NR |
NR |
NR (adductor muscle bellies lower limbs) |
16 weeks |
Marked reduction in spasticity Ulcers showed signs of healing Ulcers show significant improvement Ulcers fully healed |
No |
Pressure ulcer |
|
Laarakker and Borah, 2020 [58] |
Retrospective cohort, case series |
5 (NR) |
31-71 |
NR |
NR |
80–100 units (palm and wrist) |
NR |
All Digits were preserved |
No |
Traumatic ulcer |
|
Upton et al., 2009 [59] |
Letter to editors |
1 (NR) |
4 |
NR |
NR |
10 units (palm) |
NR |
The digits were rescued |
No |
Traumatic ulcer |
|
Zhong et al., 2019 [60] |
Case series |
4 (female 1/male 3) |
16-78 |
NR |
NR |
32–48 units (face, leg, foot) |
50 days |
Ulcers healed |
No |
Chronic skin ulcer
|
|
Alsharqi et al., 2011 [61] |
Correspondence |
1 (male) |
51 |
Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) |
NR |
70 units (right foot) |
3 months |
Completely healed ulcer |
Yes (3 months) |
Neuropathic ulcer |
BoNT-A for Traumatic Ulcers
Posttraumatic ulcers associated with vascular compromise (e.g., crush, direct drug injection, proximal arterial injury from catheterization, etc.) were reported successfully treated by BoNT-A [58][59]. According to a retrospective cohort study by Laarakker et al., patients with traumatic ischemic ulcers were categorized into two groups (BoNT-A treated group vs. non-BoNT-A group). In the BoNT-A treated group, 80 to 100 units of onabotulinum toxin were injected into the palm of each patient. The location of injection was at the level of the distal palmar crease and close to the radial and ulnar arteries. All digits (100%) were rescued for the BoNT-A injection group, while 83% had amputation of necrotic digits in patients without BoNT-A injection [58]. In addition, pain scores were lower in BoNT-A-treated fingers when compared to no BoNT-A injection. The postulated mechanism was the improvement of blood flow by BoNT-A. Another traumatic ulcer on the hand of a 4-year-old child was rescued without amputation by administration of 10 units BoNT-A into the proximal palm, the radial and ulnar artery locations of the distal forearm [59]. Data regarding the use of BoNT-A for traumatic ulcers is summarized in Table 2.
BoNT-A for Other Types of Chronic Ulcers
Neuropathic foot ulcer has been reported to be successfully treated by two sessions of BoNT-A injection [61]. The amount of 70 units of onabotulinum toxin was infiltrated around the ulcer with a repeated injection at 3 months with a similar dosage. The proposed mechanism was a reduction of sweat-induced maceration and optimization of the wound-healing environment.
Zhong et al. reported various types of chronic ulcers that have been successfully treated by BoNT-A administration in four patients [60]. One interesting case was a chronic infective skin ulcer on the left temporal region due to acne squeezing. After systemic antibiotics and debridement, there was a slight improvement in the ulcer. Multiple points of BoNT-A injection around the ulcer were done with 32 units in total. The wound was completely healed 20 days after injection. Data regarding the use of BoNT-A for other types of chronic ulcers is summarized in Table 2.
4. Practical Guidelines for Treatment
To date, a standard guideline for BoNT-A injection for chronic ulcers has not been established yet due to a lack of strong evidence that supports the efficacy of BoNT-A for skin ulcers. Based on the available data, BoNT-A might be offered to patients with chronic skin ulcers due to vascular compromised (i.e., RP-associated ulcer, pressure ulcers with vascular compromised), traumatic ulcers, etc. (Figure 3). BoNT-A for RP-associated ulcers seems to be the most promising efficacy and established treatment method. Nevertheless, its harmlessness and ubiquity make it worth trying for those chronic ulcers that failed standard therapy. Adverse effects were mild and temporally, an intrinsic hand muscle weakness has been reported which resolved completely within 5 months [41][43][49][50]. In terms of point of injection, the recommendation is to consider the type of ulcer including 1) ischemic ulcers: inject toward neurovascular bundles for vasodilation [41][42][43][46][49][50][51]; 2) pressure ulcers: inject into contracted and spastic muscles for muscle relaxation; and 3) other types: inject around the ulcer to optimize wound environment [60][61]. Dosage and reconstitution should be considered individually depending on wound size or volume of muscle.
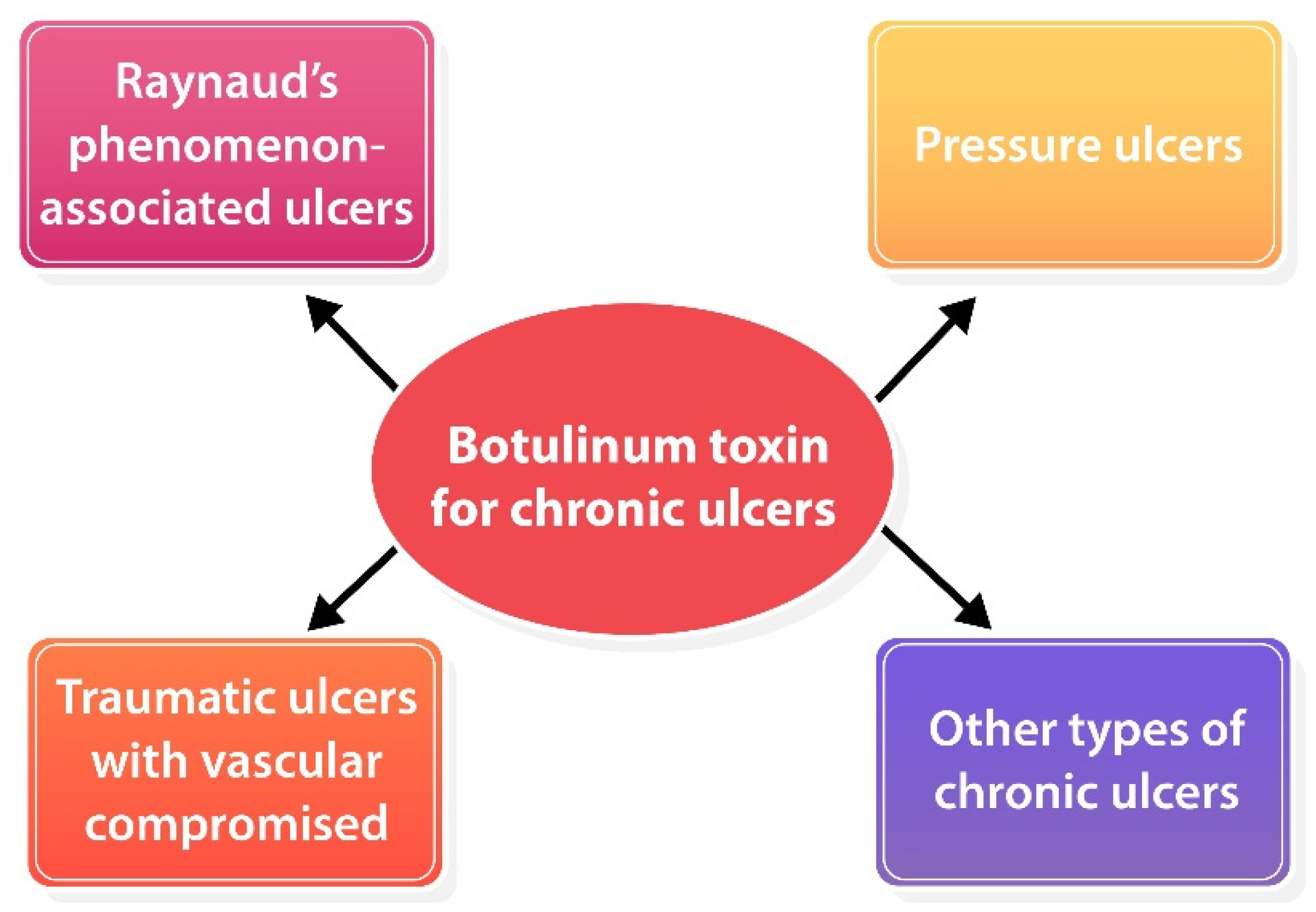
Figure 3. Potential role of BoNT-A for various types of chronic ulcers.
5. Conclusions
In summary, BoNT-A injection, a minimally invasive procedure that has a low rate of side effects can be adjunctive therapy for enhancing wound healing in various types of chronic ulcers that have been treated for underlying causes and had wound care properly as well as in ischemic ulcers associated RP in which failed conventional therapy. However, there is no randomized controlled trial study (RCT) with a large number of patients to affirm those efficacies. The amount of BoNT-A injection and the exact point of injection is still uncertain. Future randomized controlled studies should be conducted to evaluate the efficacy and safety of BoNT-A for various types of ulcers with different anatomical regions.
References
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206.
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746.
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686.
- Goldman, R. Growth factors and chronic wound healing: Past, present, and future. Adv. Skin Wound Care 2004, 17, 24–35.
- Singh, A.; Halder, S.; Menon, G.R.; Chumber, S.; Misra, M.C.; Sharma, L.K.; Srivastava, A. Meta-analysis of randomized controlled trials on hydrocolloid occlusive dressing versus conventional gauze dressing in the healing of chronic wounds. Asian J. Surg. 2004, 27, 326–332.
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15.
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32.
- Nelzen, O.; Bergqvist, D.; Lindhagen, A. Venous and non-venous leg ulcers: Clinical history and appearance in a population study. Br. J. Surg. 1994, 81, 182–187.
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228.
- Brem, H.; Sheehan, P.; Rosenberg, H.J.; Schneider, J.S.; Boulton, A.J. Evidence-based protocol for diabetic foot ulcers. Plast. Reconstr. Surg. 2006, 117, 193S–209S.
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625.
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605.
- Bickers, D.R.; Lim, H.W.; Margolis, D.; Weinstock, M.A.; Goodman, C.; Faulkner, E.; Gould, C.; Gemmen, E.; Dall, T. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J. Am. Acad. Dermatol. 2006, 55, 490–500.
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125.
- Erbguth, F.J. From poison to remedy: The chequered history of botulinum toxin. J. Neural Transm. (Vienna) 2008, 115, 559–565.
- Alster, T.S.; Harrison, I.S. Alternative Clinical Indications of Botulinum Toxin. Am. J. Clin. Dermatol. 2020, 21, 855–880.
- Campanati, A.; Martina, E.; Giuliodori, K.; Consales, V.; Bobyr, I.; Offidani, A. Botulinum Toxin Off-Label Use in Dermatology: A Review. Skin Appendage Disord. 2017, 3, 39–56.
- Lewandowski, M.; Świerczewska, Z.; Barańska-Rybak, W. Off-Label Use of Botulinum Toxin in Dermatology-Current State of the Art. Molecules 2022, 27, 3143.
- Choi, J.E.; Werbel, T.; Wang, Z.; Wu, C.C.; Yaksh, T.L.; Di Nardo, A. Botulinum toxin blocks mast cells and prevents rosacea like inflammation. J. Dermatol. Sci. 2019, 93, 58–64.
- Al-Qattan, M.M.; Al-Shanawani, B.N.; Alshomer, F. Botulinum toxin type A: Implications in wound healing, facial cutaneous scarring, and cleft lip repair. Ann. Saudi Med. 2013, 33, 482–488.
- Chang, C.S.; Wallace, C.G.; Hsiao, Y.C.; Chang, C.J.; Chen, P.K. Botulinum toxin to improve results in cleft lip repair: A double-blinded, randomized, vehicle-controlled clinical trial. PLoS ONE 2014, 9, e115690.
- Gassner, H.G.; Sherris, D.A.; Otley, C.C. Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast. Reconstr. Surg. 2000, 105, 1948–1953.
- Park, T.H. The effects of botulinum toxin A on mast cell activity: Preliminary results. Burns 2013, 39, 816–817.
- Lee, B.J.; Jeong, J.H.; Wang, S.G.; Lee, J.C.; Goh, E.K.; Kim, H.W. Effect of botulinum toxin type a on a rat surgical wound model. Clin. Exp. Otorhinolaryngol. 2009, 2, 20–27.
- Uchiyama, A.; Yamada, K.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Ishikawa, O.; Motegi, S. Protective effect of botulinum toxin A after cutaneous ischemia-reperfusion injury. Sci. Rep. 2015, 5, 9072.
- Kim, Y.S.; Roh, T.S.; Lee, W.J.; Yoo, W.M.; Tark, K.C. The effect of botulinum toxin A on skin flap survival in rats. Wound Repair Regen. 2009, 17, 411–417.
- Liu, H.; Yu, Z.; Wang, J.; Zhang, X.; Lei, L.; Zhang, Y.; Su, Y.; Ma, X. Effects of Botulinum Toxin A on the Blood Flow in Expanded Rat Skin. J. Investig. Surg. 2022, 35, 1036–1043.
- Park, T.H.; Rah, D.K.; Chong, Y.; Kim, J.K. The effects of botulinum toxin A on survival of rat TRAM flap with vertical midline scar. Ann. Plast. Surg. 2015, 74, 100–106.
- Schweizer, D.F.; Schweizer, R.; Zhang, S.; Kamat, P.; Contaldo, C.; Rieben, R.; Eberli, D.; Giovanoli, P.; Erni, D.; Plock, J.A. Botulinum toxin A and B raise blood flow and increase survival of critically ischemic skin flaps. J. Surg. Res. 2013, 184, 1205–1213.
- Morris, J.L.; Jobling, P.; Gibbins, I.L. Botulinum neurotoxin A attenuates release of norepinephrine but not NPY from vasoconstrictor neurons. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2627–H2635.
- Swartling, C.; Karlqvist, M.; Hymnelius, K.; Weis, J.; Vahlquist, A. Botulinum toxin in the treatment of sweat-worsened foot problems in patients with epidermolysis bullosa simplex and pachyonychia congenita. Br. J. Dermatol. 2010, 163, 1072–1076.
- Segreto, F.; Marangi, G.F.; Cerbone, V.; Persichetti, P. The Role of Botulinum Toxin A in the Treatment of Raynaud Phenomenon. Ann. Plast. Surg. 2016, 77, 318–323.
- Shwe, S.; Sharma, A.A.; Chahal, H.S.; Doan, L.T.; Rojek, N.W. Botulinum Toxin for the Treatment of Intractable Raynaud Phenomenon. Cutis 2021, 108, E11–E14.
- Ennis, D.; Ahmad, Z.; Anderson, M.A.; Johnson, S.R. Botulinum toxin in the management of primary and secondary Raynaud’s phenomenon. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101684.
- Bello, R.J.; Cooney, C.M.; Melamed, E.; Follmar, K.; Yenokyan, G.; Leatherman, G.; Shah, A.A.; Wigley, F.M.; Hummers, L.K.; Lifchez, S.D. The Therapeutic Efficacy of Botulinum Toxin in Treating Scleroderma-Associated Raynaud’s Phenomenon: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Arthritis Rheumatol. 2017, 69, 1661–1669.
- Uppal, L.; Dhaliwal, K.; Butler, P.E. A prospective study of the use of botulinum toxin injections in the treatment of Raynaud’s syndrome associated with scleroderma. J. Hand Surg. Eur. Vol. 2014, 39, 876–880.
- Smith, L.; Polsky, D.; Franks, A.G., Jr. Botulinum toxin-A for the treatment of Raynaud syndrome. Arch. Dermatol. 2012, 148, 426–428.
- Zhang, X.; Hu, Y.; Nie, Z.; Song, Y.; Pan, Y.; Liu, Y.; Jin, L. Treatment of Raynaud’s phenomenon with botulinum toxin type A. Neurol. Sci. 2015, 36, 1225–1231.
- Fregene, A.; Ditmars, D.; Siddiqui, A. Botulinum toxin type A: A treatment option for digital ischemia in patients with Raynaud’s phenomenon. J. Hand Surg. Am. 2009, 34, 446–452.
- Castanedo, L.Q.; Rodríguez, M.F.; Pedrero, R.M.; Fernández, C.C.; Laguna, R.d.L. Ischemic ulcers of the toes secondary to Raynaud’s phenomenon in a child successfully treated with botulinum toxin. Pediatr. Dermatol. 2020, 37, 681–683.
- Garrido-Ríos, A.A.; González-Olivares, M.; Navarro-Vidal, B.; Martínez-Morán, C.; Borbujo, J. Ischaemic ulcers on the toes secondary to Raynaud phenomenon in a patient with systemic sclerosis successfully treated with botulinum toxin. Clin. Exp. Dermatol. 2018, 43, 503–505.
- Motegi, S.; Yamada, K.; Toki, S.; Uchiyama, A.; Kubota, Y.; Nakamura, T.; Ishikawa, O. Beneficial effect of botulinum toxin A on Raynaud’s phenomenon in Japanese patients with systemic sclerosis: A prospective, case series study. J. Dermatol. 2016, 43, 56–62.
- Blaise, S.; Roustit, M.; Forli, A.; Imbert, B.; Cracowski, J.L. Non-healing ischaemic digital ulcer in a systemic sclerosis patient: A challenging clinical case. Int. Wound J. 2017, 14, 978–981.
- Min, H.K.; Kim, H.R.; Lee, S.H.; Park, S.H.; Oh, J.; Choi, K. Refractory Digital Ulcers Treated by Botulinum Toxin and Endothelin Receptor-1 Antagonist in Anti-MDA5-Antibody-Positive Dermatomyositis. J. Clin. Neurol. 2020, 16, 160–162.
- Medina, S.; Gómez-Zubiaur, A.; Valdeolivas-Casillas, N.; Polo-Rodríguez, I.; Ruíz, L.; Izquierdo, C.; Guirado, C.; Cabrera, A.; Trasobares, L. Botulinum toxin type A in the treatment of Raynaud’s phenomenon: A three-year follow-up study. Eur. J. Rheumatol. 2018, 5, 224–229.
- Neumeister, M.W. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J. Hand Surg. Am. 2010, 35, 2085–2092.
- Habib, S.M.; Brenninkmeijer, E.E.A.; Vermeer, M.H.; de Vries-Bouwstra, J.K.; Velthuis, P.J. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. Dermatol. Ther. 2020, 33, e14182.
- Souk, J.W.; Kim, H.S. Effects of botulinum toxin injection on systemic sclerosis-related digital ulcers. Korean J. Intern. Med. 2019, 34, 1169–1170.
- Zhong, J.; Lan, Y.; Fu, S.; Zhang, J.; Lu, S.; He, Y.; Zhang, J.M. Botulinum Toxin A Injection for Treatment of Chronic Skin Ulcer: A Case Series and Literature Review. Int. J. Low. Extrem. Wounds 2019, 18, 97–103.
- Merkel, P.A.; Herlyn, K.; Martin, R.W.; Anderson, J.J.; Mayes, M.D.; Bell, P.; Korn, J.H.; Simms, R.W.; Csuka, M.E.; Medsger, T.A., Jr.; et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 2002, 46, 2410–2420.
- Merkel, P.A.; Herlyn, K.; Martin, R.W.; Anderson, J.J.; Mayes, M.D.; Bell, P.; Korn, J.H.; Simms, R.W.; Csuka, M.E.; Medsger, T.A., Jr.; et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 2002, 46, 2410–2420.
- Gupta, A.D.; Wilson, D.H. Use of botulinum toxin to heal atypical pressure ulcers in the palm. Med. J. Aust. 2020, 212, 55–65.e1.
- Intiso, D.; Basciani, M. Botulinum toxin type A in the healing of a chronic buttock ulcer in a patient with spastic paraplegia after spinal cord injury. J. Rehabil. Med. 2009, 41, 1100–1102.
- Intiso, D.; Basciani, M.; Di Rienzo, F.; Tolfa, M.; Grimaldi, G.; Fiore, P. Botulinum toxin type A in the healing of ulcer following oro-mandibular dyskinesia in a patient in a vegetative state. J. Rehabil. Med. 2008, 40, 315–316.
- Sillitoe, A.T.; Bains, R.D.; Stanley, P.R.W. Botulinum toxin as a treatment for leg ulcers. Plast. Reconstr. Surg. 2007, 119, 1633.
- Turkel, C.C.; Bowen, B.; Liu, J.; Brin, M.F. Pooled analysis of the safety of botulinum toxin type A in the treatment of poststroke spasticity. Arch. Phys. Med. Rehabil. 2006, 87, 786–792.
- Bjornson, K.; Hays, R.; Graubert, C.; Price, R.; Won, F.; McLaughlin, J.F.; Cohen, M. Botulinum toxin for spasticity in children with cerebral palsy: A comprehensive evaluation. Pediatrics 2007, 120, 49–58.
- Laarakker, A.S.; Borah, G. Botulinum Toxin A Salvage of Ischemic Hand Trauma. Plast. Reconstr. Surg. 2020, 145, 161–164.
- Upton, J.; Garcia, J.; Liao, E. Botox to the rescue. Plast. Reconstr. Surg. 2009, 123, 38e.
- Zhong, J.; Lan, Y.; Fu, S.; Zhang, J.; Lu, S.; He, Y.; Zhang, J.M. Botulinum Toxin A Injection for Treatment of Chronic Skin Ulcer: A Case Series and Literature Review. Int. J. Low. Extrem. Wounds 2019, 18, 97–103.
- Alsharqi, A.; Curley, R.; Winhoven, S. Botulinum toxin type A in the management of a neuropathic foot ulcer. Clin. Exp. Dermatol. 2011, 36, 915–916.
