You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Ernest Popardowski.
Amongst the surface treatment technologies to emerge in the last few decades, UV-C radiation surface treatment is widely used in food process industries for the purpose of shelf life elongation, bacterial inactivation, and stimulation.
- UV-C radiation
- stress response
- mechanical properties
- stimulation
- potato tuber
1. Introduction
The non-thermal technique is relatively fruitful for elongating fresh foods’ shelf life. Nevertheless, its potentiality of adversely influencing sensory attributes has been highlighted [1,2,3,4,5,6,7][1][2][3][4][5][6][7]. Several non-thermal technologies for the treatment of food crops have come to emerge over the last few decades. This technology included irradiations (pulsed ultraviolet, gamma, and X-ray) pulsed electric field, pulsed electromagnetic field, cold plasma, ultra-sonication, microwave, supercritical technology, high-pressure processing, etc. [8,9,10,11][8][9][10][11]. In the classification of surface treatment, microwave surface treatment can be considered as either a thermal or non-thermal surface treatment depending on the intensity used [12,13][12][13]. It is reported that the irradiation technology is of high energy. Therefore, alongside its desirable effects, it is expected to induce undesirable alterations by interacting with different structures as well as chemical constituents of the potato tubers [14]. Among other different crop treatment techniques, such as gamma irradiation and fumigation [15], the UV-C radiation technique is one of the non-thermal technologies that is dominantly used for the surface disinfection and decontamination of crops and fruits and is by far regarded as effective [16]. UV-C radiation, emitted at wavelengths of 200–280 nm along with UV-A (320–400 nm) and UV-B (280–320 nm), is reported to be retained by the ozone layer. The different regions of UV radiation are shown in Figure 1 [17,18,19][17][18][19]. UV-C radiation, because of its high absorption level by the ozone layer, does not penetrate the earth in any appreciable amount. The shorter the wavelength, the higher the energy of the photon as depicted from the Planck relation. This short wavelength carries a higher pocket of energy which is capable of destroying microorganisms by damaging the microbial DNA, causing cross-linking between neighboring thiamine and cytosine in the same DNA strand [20,21][20][21].
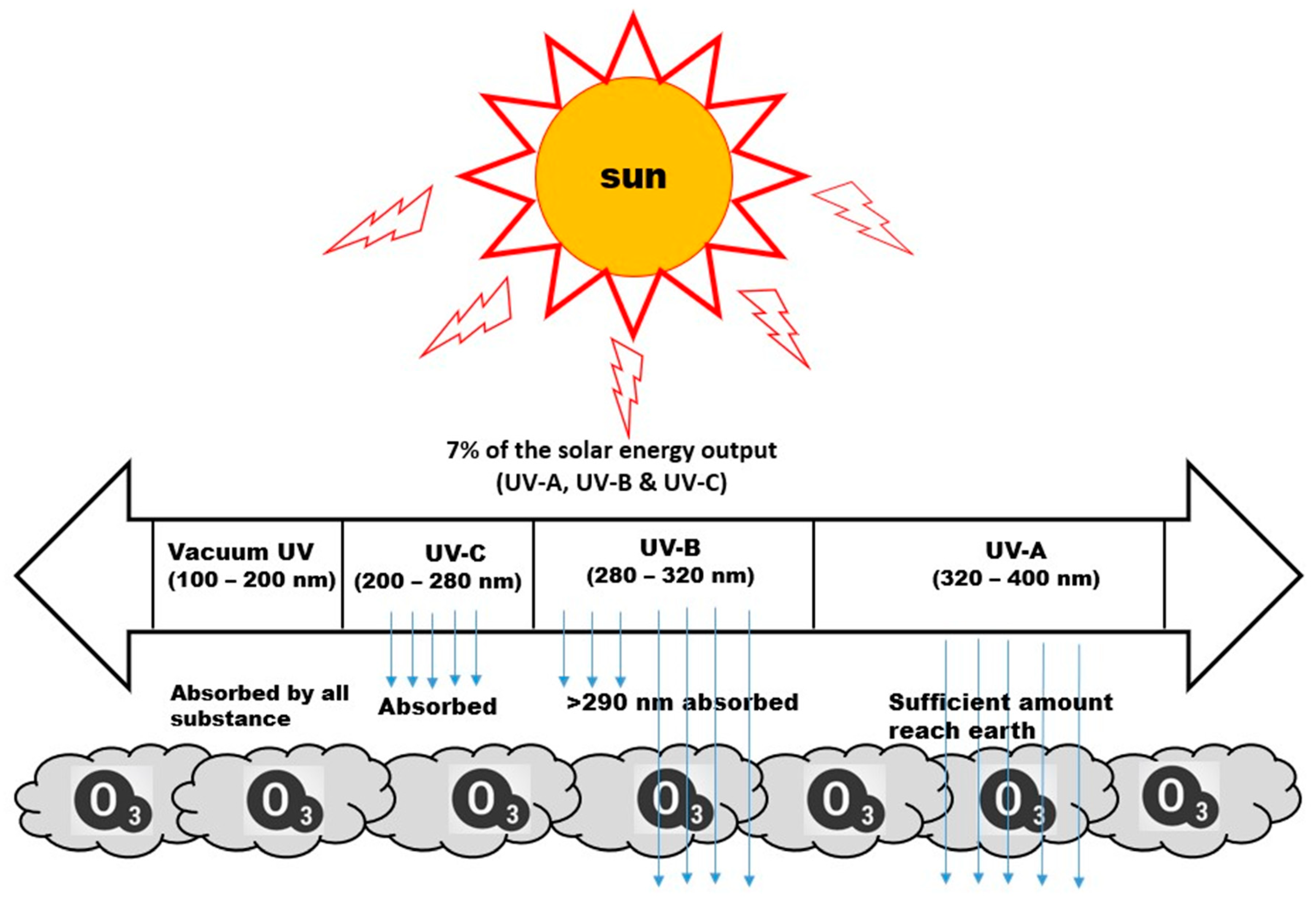
Figure 1. Different regions of UV radiation.
Even if this surface treatment has consent from Food and Drug Administration and other institutes as recognized disinfectant technology [22[22][23],23], it is important to study its impact on the characteristics of the crop. Numerous studies have already been focused on the bactericidal and fungicidal capacity of UV-C treatment in plant products, but information on product quality attributes is scanty. Limited studies have been reporting that short wave UV-C has a pronounced effect on plants’ physiology and structure. It was reported that there induces a significant structural damaging effect on the plant cell, most specifically on the chloroplasts, mitochondria, and membranes [24,25][24][25]. The change in cellular structure is directly related to the overall alteration of the mechanical properties of the plant. This correlation is well described by a previous study conducted on the potato tuber cell structural parameters having a significant effect on the overall mechanical characteristics such as strength and modulus of elasticity [26]. Moreover, in the other study on UV-C exposed strawberries, cellular structural changes in relation to the physical properties of the fruit were studied stating that delayed softening and higher firmness are caused by changes in the activity of enzymes and proteins responsible for cell wall disassembly [27]. It was clearly stated that the change in mechanical characteristics of food derived from plants arises from three structural factors at the cellular level are turgor, cell wall rigidity, and cell-cell adhesion [28,29][28][29].
The mechanical characteristics of crops play a prominent role in deciding the acceptability in the chains of target users, such as food processing industries and people. Since plants are biological materials with complex structures that have high exposure to mechanical change, these changes can lead to irreversible changes in structure and other crop characteristics where these changes are reported to be invisible, having inner deformations [26]. Potatoes are the fourth-ranking food crop in the world in terms of calories and are extensively produced throughout the world [30,31,32][30][31][32]. The mechanical properties of the potato or derived products following surface treatment by irradiation during either growth or storage represent an imperative factor for meeting consumers’ needs, as they are required for determining other properties when characterizing food qualities. Most importantly, one of the most important features here is the texture that potentially undergoes change, since external factors are concerned [33,34,35,36,37][33][34][35][36][37].
Studies have shown the influence of different physical treatment methods inducing a change in potato mechanical characteristics. Microwave treatment methods have found extensive applications in various processing and operations [38,39,40,41][38][39][40][41]. Studies have demonstrated their influential effects on the texture, compression test, weight loss, morphological, and microstructural changes of potatoes [42,43,44][42][43][44]. In the process of extending the shelf life of potatoes, gamma radiation is also an important facility nowadays. Several studies on the effect of gamma irradiation on physicomechanical properties of potatoes have been carried out on different potato cultivars for the evaluation of sprout weight, appearance quality, texture, specific gravity, morphology, puncture test, shear test, and compressive tests at varied dosses ranging from 0.04 to 100 kGy under different storage conditions [14,45,46,47,48][14][45][46][47][48]. From the result, Gamma irradiated resulted in an intact, rigid cell wall, more cavities, and bigger lamellar structure. At lower dose, mechanical properties were maintained, and sprouting was inhibited, while increased specific gravity at higher dose was also indicated. Texture was reduced with increasing dose, and the appearance of potato tuber was firm and slightly shrivelled. One of the non-destructive evaluation techniques is an ultrasound that applies a low range of intensities (0.1–20 kHz), but higher intensities (20–100 kHz) are reported to alter the physical, mechanical, and structural characteristics of starch granules derived from potato [49,50][49][50]. The results indicated that the sonicated potato tubers showed a change in molecular order in the crystalline region. A transparent appearance of the potato starch was also found. The effect of pulsed magnetic field treatment on the firmness, energy for cutting, and smoothening of the surface of potato was investigated at lower pulses ranging from 1 to 2.5 kV. It was concluded that firmness and energy for cutting were reduced while smoothening of the surface increased [8,51][8][51].
2. Influence of UV-C on the Physical and Mechanical Properties of Different Crops
UV-C surface treatment technology is a set of techniques by which a specific wavelength of 254 nm is delivered from a source for the purpose of microbial inactivation, disinfection, and stimulation. The short wave carries a pocket of energy necessary to inhibit microorganisms from the surface of the product by developing defense mechanisms. However, the application is highly dose dependent and results a positive and negative impact on the mechanical properties of the given product. In the quest of the current state of the art, a number of findings were reported in different types of products and varieties as well. It is apparently clear that the UV-C effect on the plant products is highly dependent on UV-C dose.2.1. Physical Properties
With a specific focus in food characterization, textural profile analysis (TPA) offers comprehensive properties important to the acceptability of a product by the end user. A wide set of parameters traced from TPA analysis includes hardness, cohesiveness, viscosity, elasticity (springiness), adhesiveness, resilience, brittleness (fracturability), chewiness, and gumminess. This is one of the most important parameters as far as the UV-C stimulation is concerned with the cell physiology. The properties of firmness, hardness, and change in mass are critically assessed in this review as they are greatly influenced by the anatomy of the plant tissue, the strength of the cell wall, and ultimately other mechanical properties. It is one of the most important parameters when it comes to UV-C stimulation and cell physiology [57][52]. The firmness of bell pepper fruit treated at 0.25 kJ∙m−2 and its control sample significantly dropped at the initial storage period, but after seven days of storage the UV-C treated sample was found to be firmer than the control. A weight loss experiment in the same fruit resulted in lower weight loss in the treated sample [58][53]. An experiment on the UV-C treated in green asparagus confirmed an appreciable increment in tissue toughness and a significant increase in cutting energy (sheer force) even at a lower UV-C dose of 1 kJ∙m−2 and 8 min exposure time [59][54]. Conversely, a low UV-C dose up to 3.8 kJ∙m−2 is indicated to have an insignificant change in textural properties [60][55]. Observations on the fresh-cut melon after UV-C exposure confirms no significant difference in the firmness as a result of UV-C application at 1.2 kJ∙m−2 followed by storage at 6 °C [61][56]. No significant difference was observed among treated and control samples in delaying softening at a lower UV-C dose. The research work on the firmness and weight loss characteristics of UV-C exposed fresh-cut apples were analyzed by different authors and resulted in no significant change in the firmness [62,63][57][58], while a decrease in weight loss by 6% was noted at an exposure time of 1 min and intensity of 1.2 kJ∙m−2 UV-C [62][57]. For fruit crops, weight loss is an important parameter as it is associated with dehydration, which significantly determines the commercial value of the fruit. The weight loss of amaranth vegetables treated at a moderate UV-C dose of 1.7 kJ∙m−2 reduced the weight loss significantly better than the control [64][59]. The weight loss experiment on different UV-C exposed tomato cultivars, namely “cherry tomato” and Elpida, showed no effect and reduced weight loss when treated at 3.7 kJ∙m−2 and 4 kJ∙m−2, respectively [65,66][60][61]. It was depicted that a lower UV-C dose of up to 4 kJ∙m−2 has a minor effect on the firmness of tomato [67][62] and blueberry fruits [68][63]. The firmness value of different tomato cultivars was enhanced when exposed to UV-C treated in the range of 3 kJ∙m−2 to 4.2 kJ∙m−2 [65,66,69,70,71,72][60][61][64][65][66][67]. An experiment conducted on the quality parameters of UV-C exposed pineapple at the dose of 4.5 kJ∙m−2 over the range of 0–90 s and followed by a 10 °C storage condition was able to retain the firmness properties better than the control samples [73][68]. Exposure to 7.11 kJ∙m2 induced higher tissue softening in lettuce and caused the development of abnormal color in cauliflower [74,75][69][70]. Research work on a comparative study of the effect of UV-A and UV-C over the temperature range of 25–100 °C on oyster mushrooms reported UV-C exposed mushroom samples had a higher increase in loss modulus and loss factor than that of UV-A exposed ones. From the outcome, it was concluded UV-C light had a greater impact on the mechanical properties of oyster mushrooms compared to UV-A light [76][71]. Storage modulus was lower for the samples exposed to both UV-A and UV-C, indicating the samples had a viscoelastic characteristic. In the quest for the previous state of the art, the most decisive factor was found to be firmness, which was studied dominantly following the UV-C irradiation and storage. It was also noted from recent research that firmness is an important sensory characteristic for consumers and is the factor that is highly affected by mechanical processes that potentially induce the ejection of intracellular fluid due to tissue rupture [77][72]. The textural aspect of mechanical property is dominantly used in the processing, as well as with raw vegetables and fruits, which is connected with the rheological characteristics of biological materials called firmness or hardness. It expresses the maximum force required to attain a specific strain in compression, puncture, and cut tests [57,78][52][73].2.2. Mechanical Properties
Mechanical properties of harvested vegetables and fruits are important characterization throughout processing, storage, and consumption. Mechanical properties may be defined by cell structure and are dependent on the physical state, flow properties, and porosity. In the light of measurements, force-deformation methods are commonly used in textural or mechanical properties of solid foods, fresh vegetable/fruit, and their derivatives in their solid state. The basic mechanical properties that are determined from the force-deformation includes rupture force, toughness, cutting force, shear force, and strength [79][74], which are effective for different purposes, such as product standardization, transportation, handling, and design purposes as well. If wresearchers take account of the dimension of a product in the mechanical testing, stress–strain characterization, for example, can be used and some important properties can be obtained from the result of this test, such as yield strength, Young’s modulus, ultimate strength, and modulus of elasticity. Recent research conducted intensive work on mechanical changes of UV-C irradiated strawberries at a varied irradiation dose of 0.8, 2, and 4 kJ∙m−2 by changing the storage duration from 0 to eight days at 0 °C. Resistance to compression, crush resistance, and distance to tissue failure attributes dropped significantly during storage. According to the author, the resistance to compression was higher at a lower UV-C dose. At 2 kJ∙m−2 and 4 kJ∙m−2, tissue deformation reported a higher value [80][75]. In the latest work on the effect of UV-C irradiation on some of the mechanical characteristics of blueberries ‘O’Neal’ a puncture test was performed at UV-C intensities of 5.3, 8.3, and 11.4 kJ∙m−2, exposure time of 7, 11, and 15 min, and storage time from 0 to 15 days. According to the report, mechanical parameters were not affected by UV-C treatments until 15 days of storage time when irradiated samples showed higher values of rupture force, deformation, and weight loss [81][76]. In a related study, a compression test (stress –strain) was performed on UV-C exposed fresh-cut apples at an exposure time of 10, 15, and 25 min and UV-C intensity at 5.6, 8.4, and 14.1 kJ∙m−2. True stress and deformability modulus were noticeably reduced at lower exposure time and UV-C dose [63][58]. The effect of UV-C on the mechanical characterization as well as the dimensions (length, width, and height) of Faba bean during storage was studied by considering various UV-C exposure times (0, 30, 60, and 90 min) and during storage periods of 0, three, six, and nine months. The result indicated that main dimensions, mass and bulk volume, and true volume were decreased by increasing the storage period and decreasing ultraviolet irradiation time. UV-C irradiation time increased with reducing the storage time, although, length and thickness decreased slightly. From this research, very important mechanical tests, such as shear force and shear penetration, were also investigated on the UV exposed Faba bean. The authors described the decrement of both the sheer force and penetration force of seed as UV-C exposure time increased during the given storage time [82][77]. The mechanical properties of different UV-C irradiated plant commodities are briefly presented in Table 1.Table 1. Mechanical and related characteristics of UV-C exposed plant products.
| Commodity | Operational Condition | Key Finding | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sweet corn kernels | UV-C dose at 0. 1.94, and 4.01 kJ∙m | −2 | , controlled atmosphere of with %oxygen: %carbondioxide: %nitrogen ratios of 21:0.03:78, 3:10:87, and 3:15:82 at 6 °C for 20 h. | Hardness remains unchanged | [83] | [78] | ||||
| Fresh-cut green onion | UV-C exposure time at 3, 5, 10, and 15 min and storage days of 5, 10, 15 days and storage temperature of 5 °C. | Higher UV-C exposure results in higher weight loss (%). | [84] | [79] | ||||||
| Tomato ( | Lycopersicon esculentum | L.) | UV-C dose 3.7 kJ∙m | −2 | from 0 to 25 days of storage duration time at 16 °C and relative humidity of 95%. | Firmness (Newton) decreased with storage duration. Higher resistance penetration compared to the control sample. | [85] | [80] | ||
| Cucumber ( | Cucumis sativus | L.) | UV-C dose of 4.5 kJ∙m | −2 | stored for 15 days at 4 °C, a combination of UV-C with Nano-coating Nanocapsules. | The UV-C control sample brought better firmness, as the loss was delayed to day 9 of storage. | [86] | [81] | ||
| Peeled garlic ( | Allium sativum | L.) | UV-C dose of 2 kJ∙m | −2 | stored for 15 days at room temperature. | High firmness value with the UV-C treated sample. | [87] | [82] | ||
| Cherry tomato | UV-C dose of 3.7 kJ∙m | −2 | UV-C treated and control sample both mass loss and firmness were unaffected. | [65] | [60] | |||||
| Tomato | (Lycopersicon esculentum | L.) | UV-C dose of 4.2 kJ∙m | −2 | for 8 min | The firmness decreased gradually during storage in both the control and UV-C treated tomatoes. | [72] | [67] | ||
| Common dandelion and purple coneflower |
UV-C dose of 3.8 J∙m | −2 | , 1 m of distance from light source, 10 to 120 exposure time, and 21 days of storage period. | Fresh and dry weight loss recorded for both dandelion and purple coneflower is higher than the control sample. | [88] | [83] | ||||
| Strawberry | UV-C dose of 1.70 kJ∙m | −2 | for 4.8 min and Storage duration of 0, 2, and 4 days at 21 °C. | No difference in firmness between fruit from control and UV-C-treated samples. | [89] | [84] | ||||
| Mango ( | Kensington Pride | ) | UV-C dose at 4.0, 8.3, and 11.7 kJ∙m | −2 | ), room temperature (20 ± 1 °C), relative humidity at 80%, and ethylene storage duration from 3 to 12 days. | At a higher UV-C dose, the firmness is significantly higher than untreated fruits after 6 days of storage. No significant difference in weight loss with the control sample. | [90] | [85] | ||
| Tahitian lime ( | Citrus latifolia | ) | The doses were 3.4, 7.2, and 10.5 kJ∙m | −2 | . Fruits were located 20 cm from the UV-C light source. | Higher dose (10.5 kJ∙m | −2 | ) reduced weight loss. | [91] | [86] |
| Mango ( | Tommy Atkins | ) | UV-C dose of 8220 mW∙m | −2 | and exposure time of 10 and 20 min. and storage temperature 5 and 20 °C | Lower weight loss and high firmness for Samples exposed to 10 min and 5 °C. | [92] | [87] |
References
- Soares, I.G.; Silva, E.B.; Amaral, A.J.; Machado, E.C.; Silva, J.M. Physico-chemical and sensory evaluation of potato (Solanum tuberosum L.) after irradiation. An. Acad. Bras. Ciências 2016, 88, 941–950.
- Nawara, P.; Gliniak, M.; Popardowski, E.; Szczuka, M.; Trzyniec, K. Control system of a prototype measurement system for the identification of ultra-low photonic emission of organic materials. In Proceedings of the 2018 Progress in Applied Electrical Engineering, Koscielisko, Poland, 18–22 June 2018.
- Jakubowski, T. Transfer of microwave irradiation effects of seed potatoes (Solanum tuberosum L.) to the plants of next generations. Bulg. J. Agric. Sci. 2015, 21, 1185–1193.
- Kielbasa, P.; Drozdz, T.; Nawara, P.; Drozdz, M.; Trzyniec, K. Assessment of the potential of using photon emission to identify selected qualitative features of organic matter. In Proceedings of the 2018 Applications of Electromagnetics in Modern Techniques and Medicine, Racławice, Poland, 9–12 September 2018; pp. 117–120.
- Nawara, P.; Trzyniec, K.; Dróżdż, T.; Popardowski, E.; Juliszewski, T.; Zagórda, M.; Miernik, A. Analysis of the possibility of identifying the quality parameters of the oil using ultra-weak secondary luminescence. Prz. Elektrotech. 2020, 96, 117–120. (In Polish)
- Jakubowski, T. The influence of selected physical methods on the content of starch and simple sugars in stored potato tubers. In Proceedings of the 2019 Applications of Electromagnetics in Modern Engineering and Medicine, Janow Podlaski, Poland, 9–12 June 2019; pp. 63–66.
- Jakubowski, T. The reaction of garden cress (Lepidium sativum L. to microwave radiation. In Proceedings of the 2018 Applications of Electromagnetics in Modern Techniques and Medicine, Racławice, Poland, 9–12 September 2018; pp. 81–84.
- Fauster, T.; Schlossnikl, D.; Rath, F.; Ostermeier, R.; Teufel, F.; Toepfl, S.; Jaeger, H. Impact of pulsed electric field (PEF) pretreatment on process performance of industrial French fries production. J. Food Eng. 2018, 235, 16–22.
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal technologies for food processing. Front. Nutr. 2021, 8, 657090.
- Huang, H.W.; Wu, S.J.; Lu, J.K.; Shyu, Y.T.; Wang, C.Y. Current status and future trends of high-pressure processing in food industry. Food Control 2017, 72, 1–8.
- Jakubowski, T. The effect of stimulation of seed potatoes (Solanum tuberosum L.) in the magnetic field on selected vegetation parameters of potato plants. Prz. Elektrotech. 2020, 96, 166–169.
- Jakubowski, T. The influence of microwave radiation at the frequency 2.45 GHz on the germination. Prz. Elektrotech. 2018, 94, 254–325.
- Jakubowski, T. Effect of microwave radiation on the germination of Solanum tuberosum L. tubers. Bangladesh J. Bot. 2016, 45, 1255–1257.
- Mahto, R.; Das, M. Effect of gamma irradiation on the physico-mechanical and chemical properties of potato (Solanum tuberosum L.), cv. ‘Kufri Sindhuri’, in non-refrigerated storage conditions. Postharvest Biol. Technol. 2014, 92, 37–45.
- Jamieson, L.E.; Meier, X.; Page, B.; Zulhendri, F.; Page-Weir, N.; Brash, D.; McDonald, R.M.; Stanley, J.; Woolf, A.B. A review of postharvest disinfestation technologies for selected fruits and vegetables. Plant Food Res. Client Rep. 2009, 19, 36072.
- Lu, C.; Ding, J.; Park, H.K.; Feng, H. High intensity ultrasound as a physical elicitor affects secondary metabolites and antioxidant capacity of tomato fruits. Food Control 2020, 113, 107176.
- Hockberger, P.E. A History of Ultraviolet Photobiology for Humans, Animals and Microorganisms. Photochem. Photobiol. 2002, 76, 561–579.
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry–a critical review. J. Sci. Food Agric. 2000, 80, 637–645.
- Darras, A.I.; Tsikaloudakis, G.; Lycoskoufis, I.; Dimitriadis, C.; Karamousantas, D. Low doses of UV-C irradiation affects growth, fruit yield and photosynthetic activity of tomato plants. Sci. Hortic. 2020, 267, 109357.
- Vanhaelewyn, L.; Van Der Straeten, D.; De Coninck, B.; Vandenbussche, F. Ultraviolet radiation from a plant perspective: The plant-microorganism context. Front. Plant Sci. 2020, 11, 597642.
- Rameš, J.; Chaloupecký, V.; Sojková, N.; Bencko, V. An attempt to demonstrate the increased resistance of selected bacterial strains during repeated exposure to UV radiation at 254 nm. Cent. Eur. J. Public Health 1997, 5, 30–31.
- United States Food and Drug Administration—FDA. Ultraviolet radiation for the processing and treatment of food. In Code of Federal Regulations; 21 Part, Section 179.39; FDA: Silver Spring, MD, USA, 2002.
- National Archives and Records Administration, Code of Federal Regulations. 21 CFR 179.39—Ultraviolet Radiation for the Processing and Treatment of Food. Available online: https://www.govinfo.gov/app/details/CFR-2011-title21-vol3/CFR-2011-title21-vol3-sec179-39/context (accessed on 4 May 2022).
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428.
- Bassham, J.A.; Calvin, M. The path of carbon in photosynthesis. In Die CO2-Assimilation. The Assimilation of Carbon Dioxide. Handbuch der Pflanzenphysiologie; Pirson, A., Ed.; Encyclopedia of Plant Physiology; Springer: Berlin/Heidelberg, Germany, 1960; p. 5.
- Konstankiewicz, K.; Pawlak, K.; Zdunek, A. Influence_of_structural_parameters. Int. Agrophys. 2000, 15, 243–246.
- Pombo, M.A.; Dotto, M.C.; Martínez, G.A.; Civello, P.M. UV-C irradiation delays strawberry fruit softening and modifies the expression of genes involved in cell wall degradation. Postharvest Biol. Technol. 2009, 51, 141–148.
- Jackman, R.L.; Stanley, D.W. Perspectives in the textural evaluation of plant foods. Trends Food Sci. Technol. 1995, 6, 187–194.
- Waldron, K.W.; Smith, A.C.; Parr, A.J.; Ng, A.; Parker, M.L. New approaches to understanding and controlling cell separation in relation to fruit and vegetable texture. Trends Food Sci. Technol. 1997, 8, 213–221.
- Ichiki, H.; Van, N.N.; Yoshinaga, K. Stone-clod Separation and Its Application to Potato Cultivation in Hokkaido. Eng. Agric. Environ. Food 2013, 6, 77–85.
- Zhang, D.Q.; Mu, T.H.; Sun, H.N.; Chen, J.W.; Zhang, M. Comparative study of potato protein concentrates extracted using ammonium sulfate and isoelectric precipitation. Int. J. Food Prop. 2017, 20, 2113–2127.
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial phytochemicals in potato—A review. Food Res. Int. 2013, 50, 487–496.
- Flegg, P.B.; Spencer, D.M.; Wood, D.A. The Biology and Technology of the Cultivated Mushroom; John Wiley & Sons Ltd.: Chichester, UK, 1985; pp. i–xii+347.
- Miernik, A.; Jakubowski, T. Selected methods for starch content determination in plant materials. J. Phys. Conf. Ser. 2021, 1782, 012019.
- Nawara, P.; Jakubowski, T.; Sobol, Z. Application of the CIE L∗a∗b∗ method for the evaluation of the colour of fried products from potato tubers exposed to C band ultraviolet light. E3S Web Conf. 2019, 132, 02004.
- Błaszczak, W.; Sadowska, J.; Fornal, J.; Vacek, J.; Flis, B.; Zagórski-Ostoja, W. Influence of cooking and microwave heating on microstructure and mechanical properties of transgenic potatoes. Food/Nahrung 2004, 48, 169–176.
- Jakubowski, T. A system for the control and recording of physical parameters inside a chamber for UV-C irradiating of biological material. E3S Web Conf. 2019, 132, 01006.
- Gliniak, M.; Tomasik, M.; Popardowski, E.; Knaga, J.; Lis, A.; Gliniak, M. Application of natural luminescence for analysis of the radionuclide migration path during hard coal combustion. In Proceedings of the 2018 Applications of Electromagnetics in Modern Techniques and Medicine, Racławice, Poland, 9–12 September 2018; pp. 61–64.
- Kharchenko, S.; Borshch, Y.; Kovalyshyn, S.; Piven, M.; Abduev, M.; Miernik, A.; Popardowski, E.; Kiełbasa, P. Modeling of aerodynamic separation of preliminarily stratified grain mixture in vertical pneumatic separation duct. Appl. Sci. 2021, 11, 4383.
- Jakubowski, T.; Syrotyuk, S.; Yankovska, K. The use of microwave radiation with a frequency of 2.45 GHz as a factor reducing the storage losses of potato tubers. J. Phys. Conf. Ser. 2021, 1782, 012011.
- Gliniak, M.; Tomasik, M.; Popardowski, E.; Knaga, J.; Lis, A.; Gliniak, M. Application of natural luminescence for assessment of hard coal quality. In Proceedings of the 2018 Applications of Electromagnetics in Modern Techniques and Medicine, Racławice, Poland, 9–12 September 2018; pp. 73–76.
- Sobol, Z.; Jakubowski, T.; Nawara, P. The effect of UV-C stimulation of potato tubers and soaking of potato strips in water on color and analyzed color by CIE l*a*b*. Sustainability 2020, 12, 3487, Correction in Sustainability 2020, 12, 7473.
- Shen, H.; Fan, D.; Huang, L.; Gao, Y.; Lian, H.; Zhao, J.; Zhang, H. Effects of microwaves on molecular arrangements in potato starch. RSC Adv. 2017, 7, 14348–14353.
- Xie, Y.; Yan, M.; Yuan, S.; Sun, S.; Huo, Q. Effect of microwave treatment on the physicochemical properties of potato starch granules. Chem. Cent. J. 2013, 7, 113.
- Teixeira, B.S.; Inamura, P.Y.; Mastro, N.L. The influence of gamma irradiation on texture, color and viscosity properties of potato starch. In Proceedings of the 2015 International Nuclear Atlantic Conference—INAC 2015, São Paulo, Brazil, 4–9 October 2015.
- Wang, J.; Chao, Y. Effect of gamma irradiation on quality of dried potato. Radiat. Phys. Chem. 2003, 66, 293–297.
- Chung, H.J.; Liu, Q. Molecular structure and physicochemical properties of potato and bean starches as affected by gamma-irradiation. Int. J. Biol. Macromol. 2010, 47, 214–222.
- Rezaee, M.; Almassi, M.; Minaei, S.; Paknejad, F. Impact of post-harvest radiation treatment timing on shelf life and quality characteristics of potatoes. J. Food Sci. Technol. 2013, 50, 339–345.
- Zhu, J.; Li, L.; Chen, L.; Li, X. Study on supramolecular structural changes of ultrasonic treated potato starch granules. Food Hydrocoll. 2012, 29, 116–122.
- Chung, K.M.; Moon, T.W.; Kim, H.; Chun, J.K. Physicochemical properties of sonicated mung bean, potato, and rice starches. Cereal Chem. 2002, 79, 631–633.
- Liu, C.; Grimi, N.; Lebovka, N.; Vorobiev, E. Effects of preliminary treatment by pulsed electric fields and convective air-drying on characteristics of fried potato. Innov. Food Sci. Emerg. Technol. 2018, 47, 454–460.
- Peleg, M. On fundamental issues in texture evaluation and texturization—A view. Food Hydrocoll. 2006, 20, 405–414.
- Ma, L.; Wang, Q.; Li, L.; Grierson, D.; Yuan, S.; Zheng, S.; Wang, Y.; Wang, B.; Bai, C.; Fu, A.; et al. UV-C irradiation delays the physiological changes of bell pepper fruit during storage. Postharvest Biol. Technol. 2021, 180, 111506.
- Huyskens-Keil, S.; Hassenberg, K.; Herppich, W.B. Impact of postharvest UV-C and ozone treatment on textural properties of white asparagus (Asparagus officinalis L.). J. Appl. Bot. Food Qual. 2012, 84, 229–234.
- Poubol, J.; Lichanporn, I.; Puthmee, T.; Kanlayanarat, S. Effect of ultraviolet-C irradiation on quality and natural microflora of asparagus spears. Acta Hortic. 2010, 875, 257–262.
- Manzocco, L.; da Pieve, S.; Maifreni, M. Impact of UV-C light on safety and quality of fresh-cut melon. Innov. Food Sci. Emerg. Technol. 2011, 12, 13–17.
- Manzocco, L.; Da Pieve, S.; Bertolini, A.; Bartolomeoli, I.; Maifreni, M.; Vianello, A.; Nicoli, M.C. Surface decontamination of fresh-cut apple by UV-C light exposure: Effects on structure, colour and sensory properties. Postharvest Biol. Technol. 2011, 61, 165–171.
- Gómez, P.L.; Alzamora, S.A.; Castro, M.A.; Salvatori, D.M. Effect of ultraviolet-C light dose on quality of cut-apple: Microorganism, color and compression behavior. J. Food Eng. 2010, 98, 60–70.
- Gogo, E.O.; Opiyo, A.M.; Hassenberg, K.; Ulrichs, C.; Huyskens-Keil, S. Postharvest UV-C treatment for extending shelf life and improving nutritional quality of African indigenous leafy vegetables. Postharvest Biol. Technol. 2017, 129, 107–117.
- Vunnam, R.; Hussain, A.; Nair, G.; Bandla, R.; Gariepy, Y.; Donnelly, D.J.; Kubow, S.; Raghavan, G.S. Physico-chemical changes in tomato with modified atmosphere storage and UV treatment. J. Food Sci. Technol. 2014, 51, 2106–2112.
- Cote, S.; Rodoni, L.; Miceli, E.; Concellón, A.; Civello, P.M.; Vicente, A.R. Effect of radiation intensity on the outcome of postharvest UV-C treatments. Postharvest Biol. Technol. 2013, 83, 83–89.
- Tzortzakis, N.; Borland, A.; Singleton, I.; Barnes, J. Impact of atmospheric ozone-enrichment on quality-related attributes of tomato fruit. Postharvest Biol. Technol. 2007, 45, 317–325.
- Perkins-Veazie, P.; Collins, J.K.; Howard, L. Blueberry fruit response to postharvest application of ultraviolet radiation. Postharvest Biol. Technol. 2008, 47, 280–285.
- Stevens, C.; Liu, J.; Khan, V.A.; Lu, J.Y.; Kabwe, M.K.; Wilson, C.L.; Igwegbe, E.C.; Chalutz, E.; Droby, S. The effects of low-dose ultraviolet light-C treatment on polygalacturonase activity, delay ripening and Rhizopus soft rot development of tomatoes. Crop Prot. 2004, 23, 551–554.
- Tiecher, A.; de Paula, L.A.; Chaves, F.C.; Rombaldi, C.V. UV-C effect on ethylene, polyamines and the regulation of tomato fruit ripening. Postharvest Biol. Technol. 2013, 86, 230–239.
- Obande, M.A.; Tucker, G.A.; Shama, G. Effect of preharvest UV-C treatment of tomatoes (Solanum lycopersicon Mill.) on ripening and pathogen resistance. Postharvest Biol. Technol. 2011, 62, 188–192.
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol. Technol. 2013, 86, 337–345.
- Pan, Y.G.; Zu, H. Effect of UV-C radiation on the quality of fresh-cut pineapples. Procedia Eng. 2012, 37, 113–119.
- Allende, A.; McEvoy, J.L.; Luo, Y.; Artes, F.; Wang, C.Y. Effectiveness of two-sided UV-C treatments in inhibiting natural microflora and extending the shelf-life of minimally processed ‘Red Oak Leaf’ lettuce. Food Microbiol. 2006, 23, 241–249.
- Artés, F.; Gómez, P.; Aguayo, E.; Escalona, V.; Artés-Hernández, F. Sustainable sanitation techniques for keeping quality and safety of fresh-cut plant commodities. Postharvest Biol. Technol. 2009, 51, 287–296.
- Edward, T.L.; Kirui, M.S.K.; Omolo, J.O.; Ngumbu, R.G.; Odhiambo, P.M. Effect of Ultraviolet-A (UV-A) and Ultraviolet-C (UV-C) Light on Mechanical Properties of Oyster Mushrooms during Growth. J. Biophys. 2014, 2014, 687028.
- Meng, X.; Zhang, M.; Zhan, Z.; Adhikari, B. Changes in Quality Characteristics of Fresh-cut Cucumbers as Affected by Pressurized Argon Treatment. Food Bioprocess Technol. 2014, 7, 693–701.
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G.V. Rheology for the food industry. J. Food Eng. 2005, 67, 147–156.
- Ohwovoriole, E.N.; Oboli, S.; Mgbeke, A.C. Studies and preliminary design for a cassava tuber peeling machine. Trans. ASAE 1988, 31, 380–0385.
- Araque, L.C.O.; Ortiz, C.M.; Darré, M.; Rodoni, L.M.; Civello, P.M.; Vicente, A.R. Role of UV-C irradiation scheme on cell wall disassembly and surface mechanical properties in strawberry fruit. Postharvest Biol. Technol. 2019, 150, 122–128.
- Jaramillo Sánchez, G.; Contigiani, E.V.; Coronel, M.B.; Alzamora, S.M.; García-Loredo, A.; Nieto, A.B. Study of UV-C treatments on postharvest life of blueberries ‘O’Neal’ and correlation between structure and quality parameters. Heliyon 2021, 7, e07190.
- Mohammed, H.E.S.H.; Suliman, A.E.R.E.; Ahmed, A.E.R.; Ebrahim, M.A. Ultraviolet effect on faba bean seed quality during storage. Asian J. Plant Sci. 2020, 19, 26–34.
- Chudhangkura, A.; Teangpook, C.; Sikkhamondhol, C.; Jariyavattanavijit, C. Effects of ultraviolet C, controlled atmosphere, and ultrasound pretreatment on free ferulic acid in canned sweet corn kernels. J. Food Sci. Technol. 2018, 55, 4167–4173.
- Kasim, M.U.; Kasim, R.; Erkal, S. UV-C treatments on fresh-cut green onions enhanced antioxidant activity, maintained green color and controlled ‘telescoping’. J. Food Agric. Environ. 2008, 6, 63–67.
- Ait Barka, E.; Kalantari, S.; Makhlouf, J.; Arul, J. Impact of UV-C irradiation on the cell wall-degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. J. Agric. Food Chem. 2000, 48, 667–671.
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Leyva-Gómez, G.; Urbán-Morlán, Z. Effects of uv-c and edible nano-coating as a combined strategy to preserve fresh-cut cucumber. Polymer 2021, 13, 3705.
- Park, M.H.; Kim, J.G. Low-dose UV-C irradiation reduces the microbial population and preserves antioxidant levels in peeled garlic (Allium sativum L.) during storage. Postharvest Biol. Technol. 2015, 100, 109–112.
- Castronuovo, D.; Sofo, A.; Lovelli, S.; Candido, V.; Scopa, A. Effects of UV-C radiation on common dandelion and purple coneflower: First results. Int. J. Plant Biol. 2017, 8, 61–64.
- Forges, M.; Bardin, M.; Urban, L.; Aarrouf, J.; Charles, F. Impact of UV-C radiation applied during plant growth on pre-and postharvest disease sensitivity and fruit quality of strawberry. Plant Dis. 2020, 104, 3239–3247.
- Pristijono, P.; Golding, J.B.; Bowyer, M.C. Postharvest UV-C treatment, followed by storage in a continuous low-level ethylene atmosphere, maintains the quality of ‘Kensington pride’ mango fruit stored at 20 °C. Horticulturae 2019, 5, 1.
- Pristijono, P.; Bowyer, M.C.; Papoutsis, K.; Scarlett, C.J.; Vuong, Q.V.; Stathopoulos, C.E.; Golding, J.B. Improving the storage quality of Tahitian limes (Citrus latifolia) by pre-storage UV-C irradiation. J. Food Sci. Technol. 2019, 56, 1438–1444.
- González-Aguilar, G.A.; Wang, C.Y.; Buta, J.G.; Krizek, D.T. Use of UV-C irradiation to prevent decay and maintain postharvest quality of ripe ‘Tommy Atkins’ mangoes. Int. J. Food Sci. Technol. 2008, 36, 767–773.
More
