Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Liyao Zheng.
Fragrances, short for fragrance ingredients, is a type of compounds with a sweet smell or pleasant odor that has wide applications in the fine chemical industry, especially in perfumes, cosmetics, detergents and food additives. Since the discovery of the Diels–Alder reaction, the cycloaddition of π reactants serves as one of the most powerful methods for the construction of carbocycles, which has a broad application in the fragrance industry.
- cycloaddition
- carbocycles
- fragrances
- alkenes
- alkynes
1. Introduction
Fragrances, short for fragrance ingredients, is a type of compounds with a sweet smell or pleasant odor that has wide applications in the fine chemical industry, especially in perfumes, cosmetics, detergents and food additives [1,2,3,4][1][2][3][4]. The perception of aroma and odor is a subjective phenomenon that is more difficult to describe precisely and measure quantitatively than the perception of light or sound [5,6,7,8,9,10,11,12][5][6][7][8][9][10][11][12]. Thus, fragrances with different structures are highly desirable for yielding various scents with repeated effects and long shelf life; they are key to studying the principle of olfaction, including the range and resolution of the olfactory system and the working mechanism of odorant receptors [2,5,6,7,8,9,10,11,12,13,14,15,16,17][2][5][6][7][8][9][10][11][12][13][14][15][16][17].
In ancient times, fragrances were mainly obtained from natural resources such as plant essential oils and animal secretions [3,4,18,19][3][4][18][19]. While natural ingredients still represent an important class of fragrances, they cannot meet the rapidly growing and diversified needs of consumers and perfumers. The power of organic synthesis for fragrances was unlocked in 1868 when coumarin was successfully prepared by W. H. Perkin from salicylaldehyde. With the blossoming of organic synthetic methods and the related industrial technologies over the past century, synthetic and semi-synthetic fragrances emerged to become mainstream in the flavors and fragrances industries [2,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36][2][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36]. Nowadays, fragrance molecules represent an attractive testing ground and offer a multi-billion-dollar outlet for organic synthesis, stimulating researchers in academia and fragrance manufacture to seek out new fragrances and explore more efficient, economical, scalable and environmentally friendly synthetic methods.
Similar to medicinal chemistry, some structures have been established as privileged structures in fragrance chemistry, many of which have one or more carbo- or heterocycles (Figure 1). The ring sizes range from that of cyclopropane in olibanic acid to the macrocycles in muscone and civetone. Diverse types of rings, including carbocyclic and heterocyclic, saturated and unsaturated, aromatic and non-aromatic, monocyclic and polycyclic rings exist in both natural and synthetic fragrances. With highly structural diversity and the resulting tunable odor characters, cyclic fragrances have emerged as molecular probes for exploring olfactory chemical space. New synthetic methods enable much more structural diversity of fragrances, including the scaffold diversity of different rings and their linking modes, as well as the variations of substituents and their replacement by heteroatoms. These compounds include not only derivatives and analogs of natural fragrances but also those compounds with novel scaffolds due to rational design or unexpected findings.
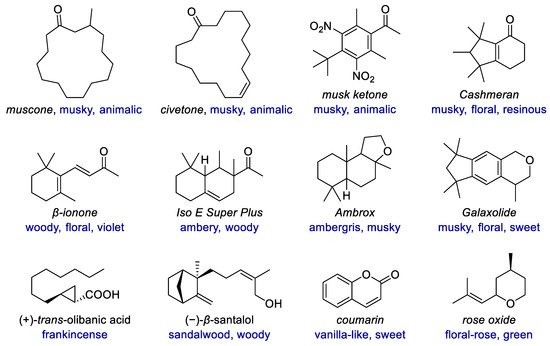
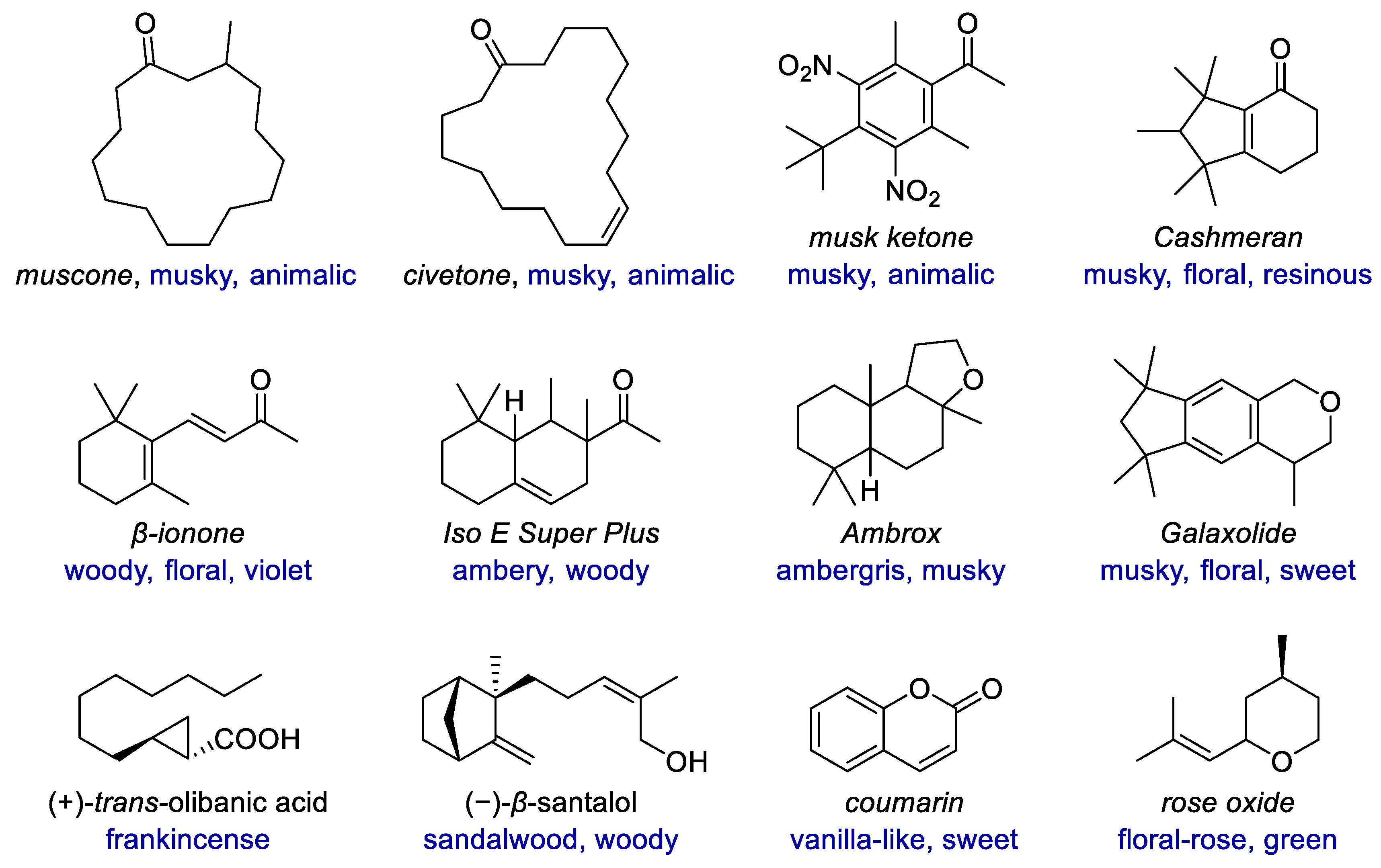
Figure 1. Examples of fragrances with diverse carbocyclic or heterocyclic scaffolds.
Examples of fragrances with diverse carbocyclic or heterocyclic scaffolds.
Unsaturated hydrocarbons, especially alkenes and alkynes, are extensively used for the construction of carbo- and heterocycles due to their adjustable reactivity and cyclization modes. Many of these building blocks can be easily obtained from natural resources or petrochemicals via reliable processes. In addition, the reactions involving unsaturated bonds usually have high atom economy. With classic or new odorants as targets, innovative synthetic strategies and methods have been developed recently that enrich the frontiers of alkene and alkyne chemistry. Though there are a few reviews published on fragrance synthesis [2,18[2][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36],19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], the importance of unsaturated hydrocarbons in this area is still underestimated, especially that of alkynes and enynes.
2. Synthesis of Fragrances via Cycloaddition or Formal Cycloaddition
Since the discovery of the Diels–Alder reaction, the cycloaddition of π reactants serves as one of the most powerful methods for the construction of carbocycles, which has a broad application in the fragrance industry [53][37]. One classic example is the production of Ambrelux from myrcene via the AlCl3-promoted Diels–Alder reaction [33] (Scheme 1a). Some of the attractive features of cycloaddition include perfect atom economy up to 100%, good functional group compatibility due to typically redox-neutral conditions, and the modular ability to access products via multiple substituents from readily available building blocks.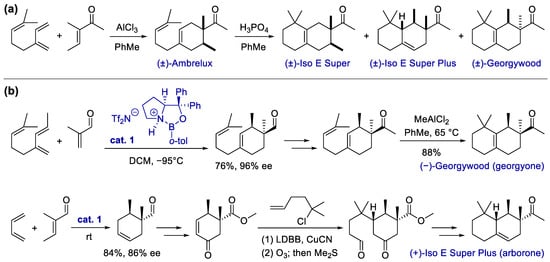
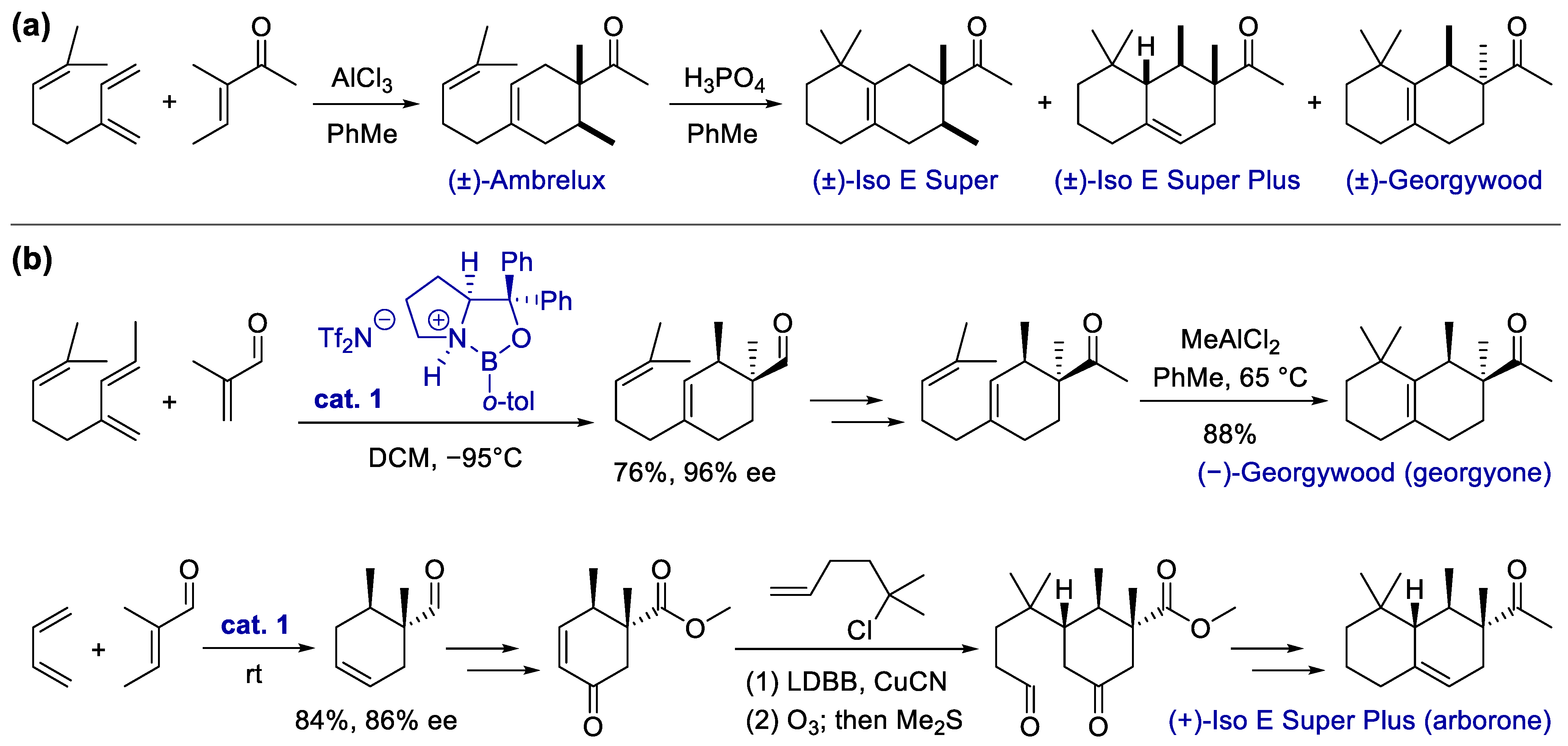 Scheme 1. Synthesis of carbocyclic fragrances via the Diels–Alder reaction. (a) Synthesis of Ambrelux and its conversion via acid-promoted annulative rearrangement; (b) total synthesis of georgyone and arborone, with the enantioselective Diels–Alder reaction as a key step.
Scheme 1. Synthesis of carbocyclic fragrances via the Diels–Alder reaction. (a) Synthesis of Ambrelux and its conversion via acid-promoted annulative rearrangement; (b) total synthesis of georgyone and arborone, with the enantioselective Diels–Alder reaction as a key step.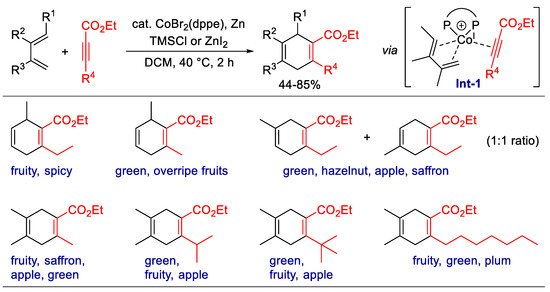
Scheme 2. Synthesis of fragrant 1,4-cyclohexadiene carboxylates via Co-catalyzed [4 + 2] cyclization.
Synthesis of fragrant 1,4-cyclohexadiene carboxylates via Co-catalyzed [4 + 2] cyclization.
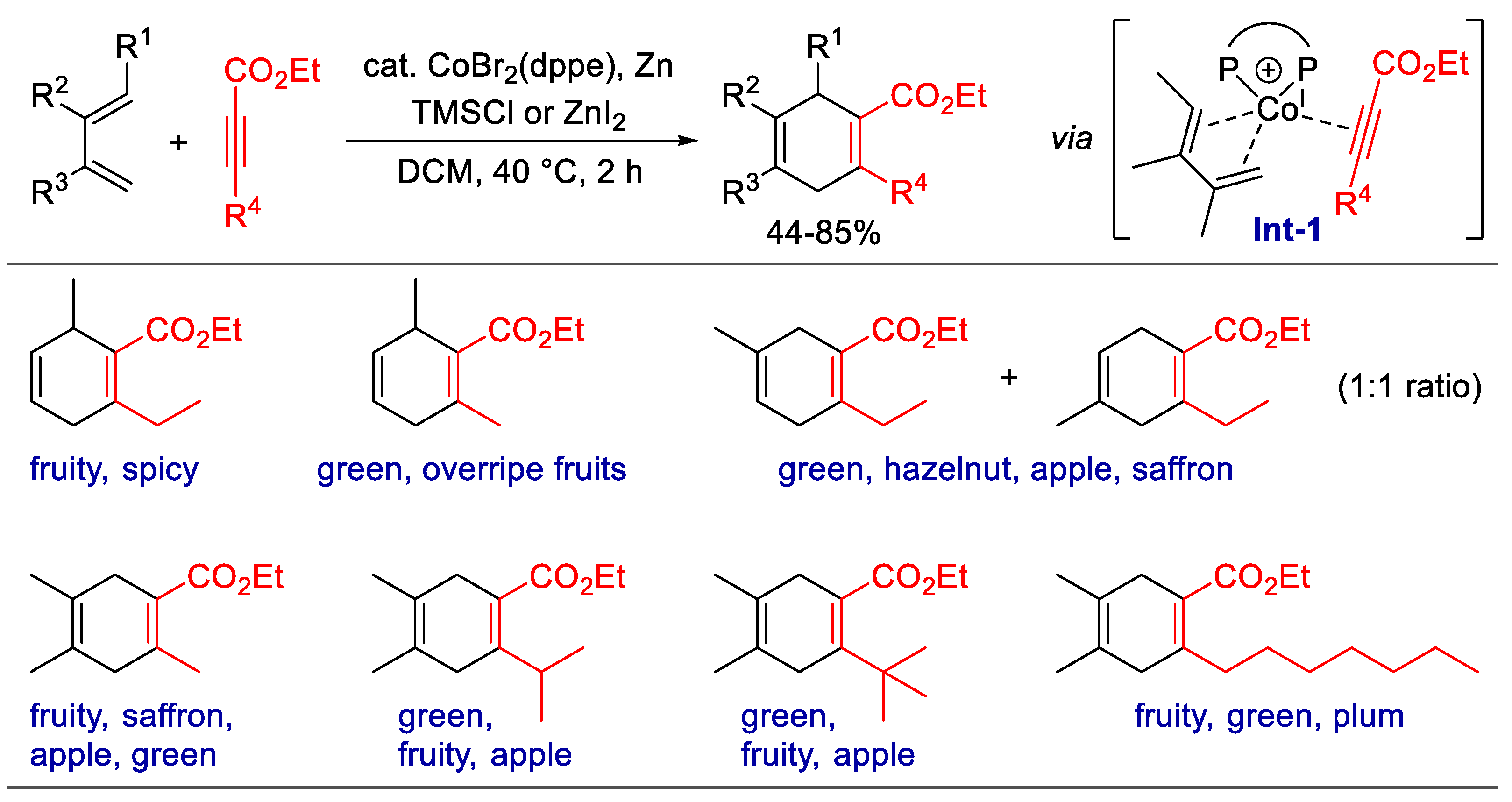
 Scheme 3. Fragrance synthesis by the List group. (a) Synthesis of hydropyran fragrances via the asymmetric hetero-Diels–Alder reaction, catalyzed by imidodiphosphorimidate (IDPi). (b) Total synthesis of the principal ingredient of vetiver oil with IDPi-catalyzed asymmetric Mukaiyama–Michael addition and Co2(CO)8-mediated Pauson–Khand [2 + 2 + 1] cyclization as the key steps.
Scheme 3. Fragrance synthesis by the List group. (a) Synthesis of hydropyran fragrances via the asymmetric hetero-Diels–Alder reaction, catalyzed by imidodiphosphorimidate (IDPi). (b) Total synthesis of the principal ingredient of vetiver oil with IDPi-catalyzed asymmetric Mukaiyama–Michael addition and Co2(CO)8-mediated Pauson–Khand [2 + 2 + 1] cyclization as the key steps.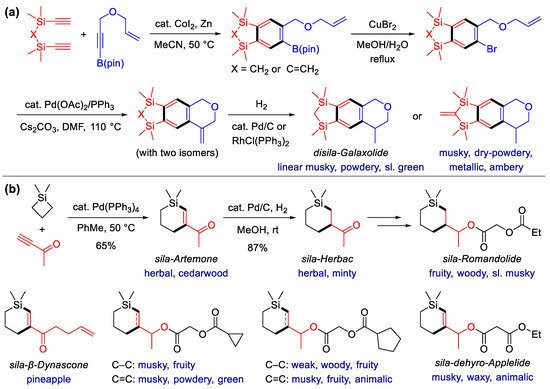
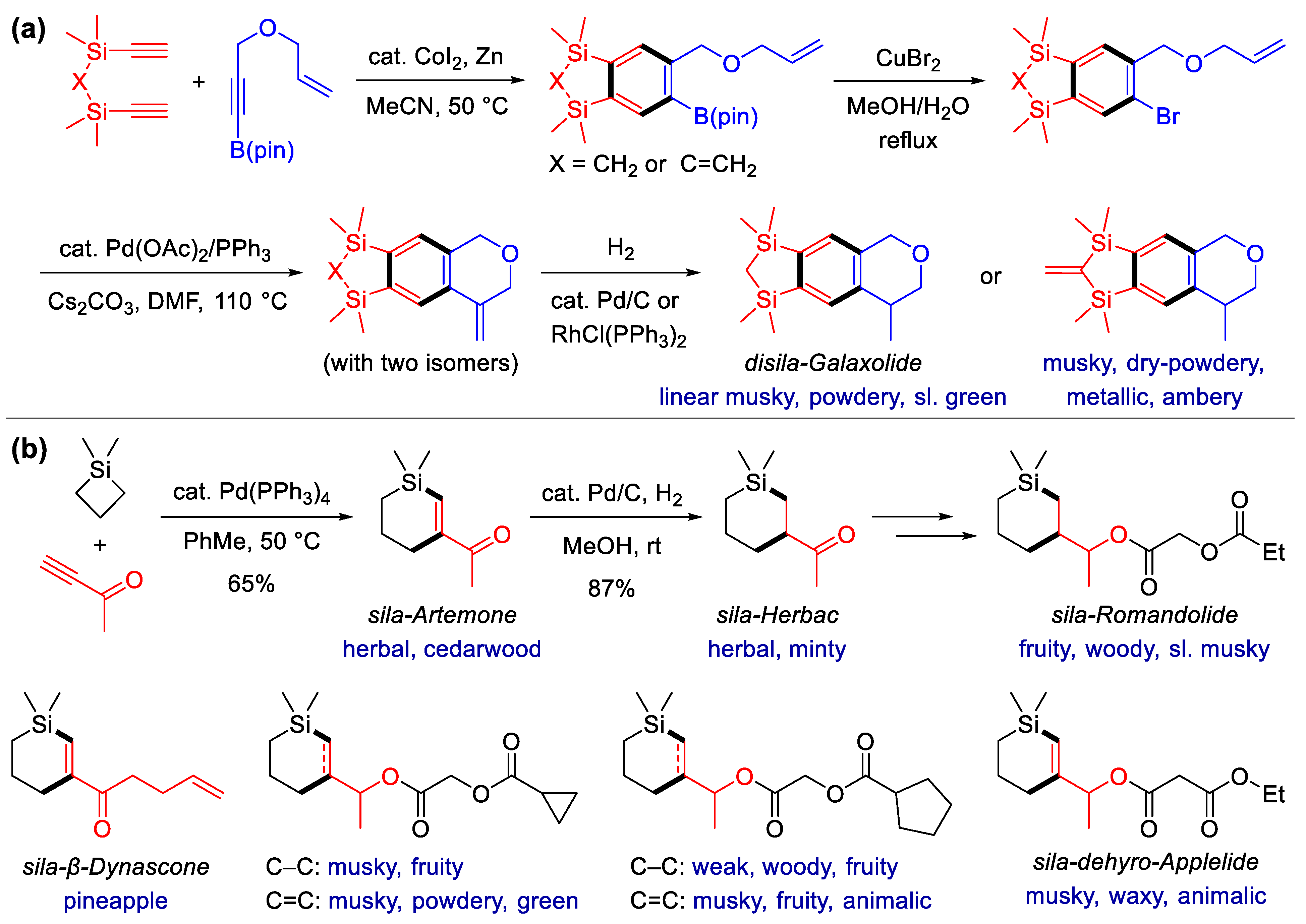 Scheme 4. Synthesis of Si-heterocyclic fragrances via alkyne annulation. (a) Synthesis of sila-analogs of galaxolide with Co-catalyzed [2 + 2 + 2] alkyne cycloaddition as the key step; (b) synthesis of fragrant six-membered silacycles via the Pd-catalyzed [4 + 2] reaction of silacyclobutanes and alkynes.
Scheme 4. Synthesis of Si-heterocyclic fragrances via alkyne annulation. (a) Synthesis of sila-analogs of galaxolide with Co-catalyzed [2 + 2 + 2] alkyne cycloaddition as the key step; (b) synthesis of fragrant six-membered silacycles via the Pd-catalyzed [4 + 2] reaction of silacyclobutanes and alkynes.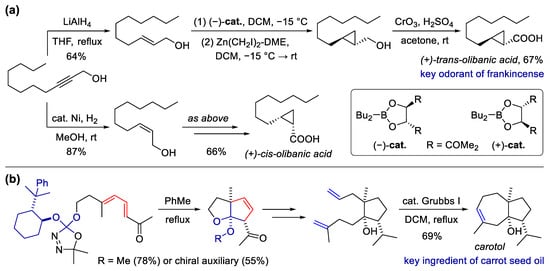
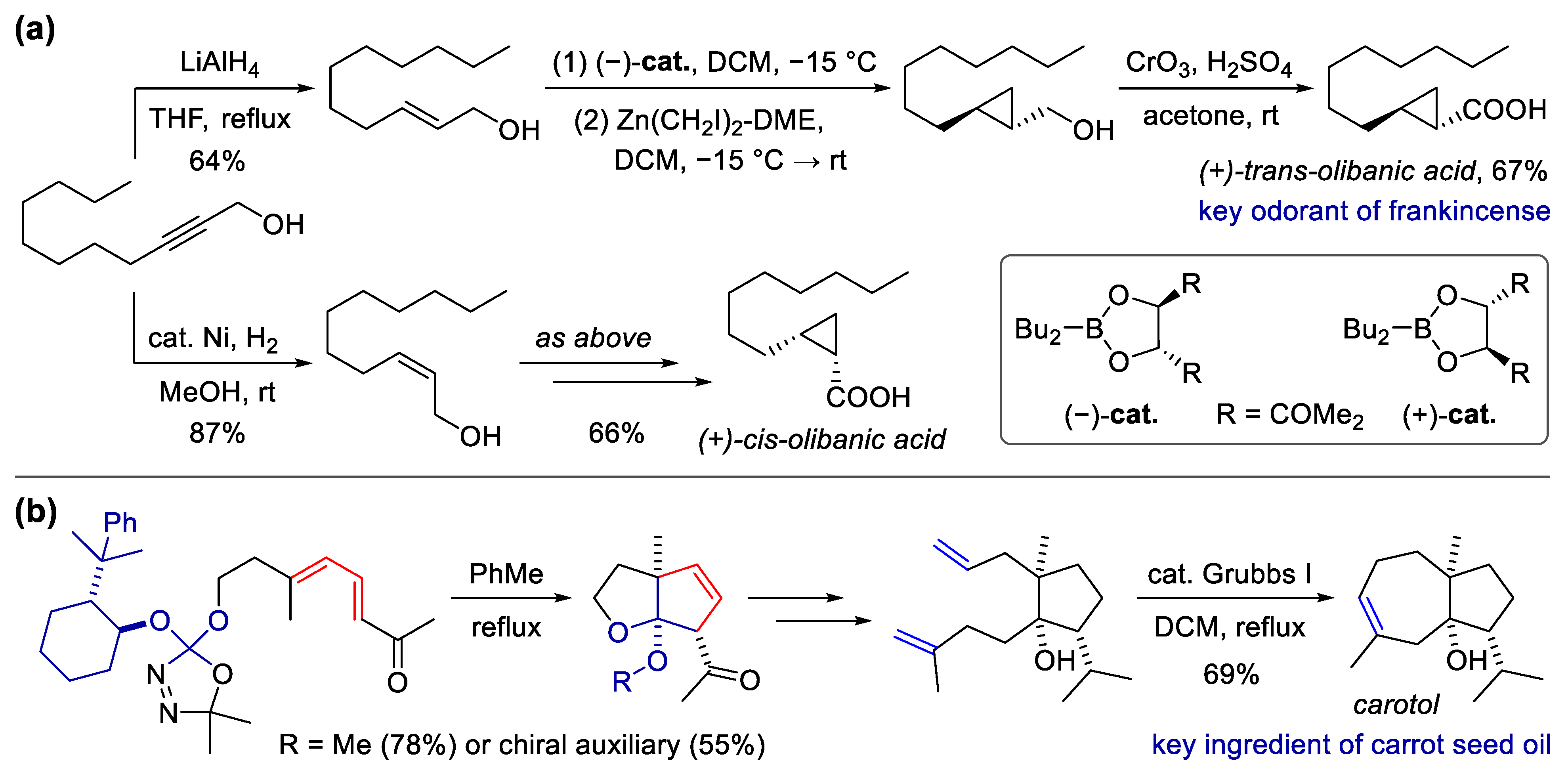 Scheme 5. Synthesis of fragrant natural products using carbene-involved cyclization. (a) Total synthesis of olibanic acids via semi-hydrogenation and cyclopropanation; (b) total synthesis of carotol via [4 + 1] cyclization and ring-closing metathesis.
Scheme 5. Synthesis of fragrant natural products using carbene-involved cyclization. (a) Total synthesis of olibanic acids via semi-hydrogenation and cyclopropanation; (b) total synthesis of carotol via [4 + 1] cyclization and ring-closing metathesis.References
- Schilling, B.; Kaiser, R.; Natsch, A.; Gautschi, M. Investigation of Odors in the Fragrance Industry. Chemoecology 2010, 20, 135–147.
- Armanino, N.; Charpentier, J.; Flachsmann, F.; Goeke, A.; Liniger, M.; Kraft, P. What’s Hot, What’s Not: The Trends of the Past 20 Years in the Chemistry of Odorants. Angew. Chem. Int. Ed. 2020, 59, 16310–16344.
- Kliszcz, A.; Danel, A.; Puła, J.; Barabasz-Krasny, B.; Możdżeń, K. Fleeting Beauty—The World of Plant Fragrances and Their Application. Molecules 2021, 26, 2473.
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666.
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218.
- Sell, C.S. On the unpredictability of odor. Angew. Chem. Int. Ed. 2006, 45, 6254–6261.
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans Can Discriminate More than 1 Trillion Olfactory Stimuli. Science 2014, 343, 1370–1372.
- Teixeira, M.A.; Barrault, L.; Rodriguez, O.; Carvalho, C.C.; Rodrigues, A.E. Perfumery Radar 2.0: A Step toward Fragrance Design and Classification. Ind. Eng. Chem. Res. 2014, 53, 8890–8912.
- Keller, A.; Gerkin, R.C.; Guan, Y.; Dhurandhar, A.; Turu, G.; Szalai, B.; Mainland, J.D.; Ihara, Y.; Yu, C.W.; Wolfinger, R.; et al. Predicting human olfactory perception from chemical features of odor molecules. Science 2017, 355, 820–826.
- Gutierrez, E.D.; Dhurandhar, A.; Keller, A.; Meyer, P.; Cecchi, G.A. Predicting natural language descriptions of mono- molecular odorants. Nat. Commun. 2018, 9, 4979.
- Genva, M.; Kenne Kemene, T.; Deleu, M.; Lins, L.; Fauconnier, M.L. Is It Possible to Predict the Odor of a Molecule on the Basis of its Structure? Int. J. Mol. Sci. 2019, 20, 3018.
- Ravia, A.; Snitz, K.; Honigstein, D.; Finkel, M.; Zirler, R.; Perl, O.; Secundo, L.; Laudamiel, C.; Harel, D.; Sobel, N. A measure of smell enables the creation of olfactory metamers. Nature 2020, 588, 118–123.
- Kraft, P.; Di Cristofaro, V.; Jordi, S. From Cassyrane to Cashmeran—The Molecular Parameters of Odorants. Chem. Biodivers. 2014, 11, 1567–1596.
- Pluskal, T.; Weng, J.K. Natural product modulators of human sensations and mood: Molecular mechanisms and therapeutic potential. Chem. Soc. Rev. 2018, 47, 1592–1637.
- Block, E. Molecular Basis of Mammalian Odor Discrimination: A Status Report. J. Agric. Food Chem. 2018, 66, 13346–13366.
- Bierling, A.L.; Croy, I.; Hummel, T.; Cuniberti, G.; Croy, A. Olfactory Perception in Relation to the Physicochemical Odor Space. Brain Sci. 2021, 11, 563.
- Sharma, A.; Saha, B.K.; Kumar, R.; Varadwaj, P.K. OlfactionBase: A repository to explore odors, odorants, olfactory receptors and odorant–receptor interactions. Nucleic Acids Res. 2022, 50, D678–D686.
- Burger, P.; Plainfosse, H.; Brochet, X.; Chemat, F.; Fernandez, X. Extraction of Natural Fragrance Ingredients: History Overview and Future Trends. Chem. Biodivers. 2019, 16, e1900424.
- Baldovini, N.; Chaintreau, A. Identification of key odorants in complex mixtures occurring in nature. Nat. Prod. Rep. 2020, 37, 1589–1626.
- Chapuis, C.; Jacoby, D. Catalysis in the preparation of fragrances and flavours. Appl. Catal. A 2001, 221, 93–117.
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective perception of chiral odorants. Tetrahedron Asymmetry 2003, 14, 1–42.
- Sell, C.S. Scent through the Looking Glass. Chem. Biodivers. 2004, 1, 1899–1920.
- Saudan, L.A. Hydrogenation Processes in the Synthesis of Perfumery Ingredients. Acc. Chem. Res. 2007, 40, 1309–1319.
- Coulombel, L.; Grau, F.; Weiwer, M.; Favier, I.; Chaminade, X.; Heumann, A.; Bayon, J.C.; Aguirre, P.A.; Duñach, E. Lewis Super-Acid Catalyzed Cyclizations: A New Route to Fragrance Compounds. Chem. Biodivers. 2008, 5, 1070–1082.
- Ciappa, A.; Bovo, S.; Bertoldini, M.; Scrivanti, A.; Matteoli, U. Homogeneous Asymmetric Catalysis in Fragrance Chemistry. Chem. Biodivers. 2008, 5, 1058–1069.
- Brenna, E.; Fuganti, C.; Gatti, F.G.; Serra, S. Biocatalytic Methods for the Synthesis of Enantioenriched Odor Active Compounds. Chem. Rev. 2011, 111, 4036–4072.
- Brenna, M.E.; Fuganti, C. Recent advances in the synthesis of fragrances. Curr. Org. Chem. 2011, 15, 987–1005.
- Lemiere, G.; Dunach, E. Catalytic Activation of Olefins by Metal Triflates and Triflimides: Application to Fragrance Chemistry. Chem. Eur. J. 2013, 19, 3270–3280.
- Narula, A.P.S. The Search for New Amber Ingredients. Chem. Biodivers. 2014, 11, 1629–1638.
- Gusevskaya, E.V.; Jiménez-Pinto, J.; Börner, A. Hydroformylation in the Realm of Scents. ChemCatChem. 2014, 6, 382–411.
- Schröder, F. Present and Future of Cyclopropanations in Fragrance Chemistry. Chem. Biodivers. 2014, 11, 1734–1751.
- Doro, F.; Akeroyd, N.; Schiet, F.; Narula, A. The Prins Reaction in the Fragrance Industry: 100th Anniversary (1919–2019). Angew. Chem. Int. Ed. 2019, 58, 7174–7179.
- Stepanyuk, A.; Kirschning, A. Synthetic Terpenoids in the World of Fragrances: Iso E Super® is the Showcase. Beilstein J. Org. Chem. 2019, 15, 2590–2602.
- David, O.R.P. A chemical history of polycyclic musks. Chem. Eur. J. 2020, 26, 7537–7555.
- Sytniczuk, A.; Milewski, M.; Kajetanowicz, A.; Grela, K. Preparation of macrocyclic musks via olefin metathesis: Comparison with classical syntheses and recent advances. Russ. Chem. Rev. 2020, 89, 469.
- Firda, P.B.D.; Bahruji, H.; Bakar, M.B. Review on heterogeneous catalysts for the synthesis of perfumery chemicals via isomerization, acetalization and hydrogenation. Flavour Fragr. J. 2021, 36, 509–525.
- Funel, J.A.; Abele, S. Industrial Applications of the Diels-Alder Reaction. Angew. Chem. Int. Ed. 2013, 52, 3822–3863.
- Hong, S.; Corey, E.J. Enantioselective Syntheses of Georgyone, Arborone, and Structural Relatives. Relevance to the Molecular-Level Understanding of Olfaction. J. Am. Chem. Soc. 2006, 128, 1346–1352.
- Fráter, G.; Müller, U.; Schröder, F. Synthesis and olfactory properties of (−)-(1R,2S)-Georgywood. Tetrahedron Asymmetry 2004, 15, 3967–3972.
- Charpentier, J.; Voirol, F.; Flachsmann, F.; Tanner, S.; Aeberli, N.; Brunner, G.; Goeke, A. Synthesis of 1, 4-Cyclohexadiene Carboxylates through a Formal -Cycloaddition of Propiolates under Cobalt Catalysis. Helv. Chim. Acta. 2020, 103, e2000175.
- Bao, R.L.Y.; Yin, J.; Shi, L.; Zheng, L. Rh(I)-Catalyzed enantioselective and scalable cycloaddition of 1,3-dienes with dialkyl acetylenedicarboxylates. Org. Biomol. Chem. 2020, 18, 2956–2961.
- Schreyer, L.; Properzi, R.; List, B. IDPi Catalysis. Angew. Chem. Int. Ed. 2019, 58, 12761–12777.
- Liu, L.; Kim, H.; Xie, Y.; Fares, C.; Kaib, P.S.J.; Goddard, R.; List, B. Catalytic Asymmetric -Cycloaddition of Dienes with Aldehydes. J. Am. Chem. Soc. 2017, 139, 13656–13659.
- Ouyang, J.; Bae, H.; Jordi, S.; Dao, Q.M.; Dossenbach, S.; Dehn, S.; Lingnau, J.B.; De, C.K.; Kraft, P.; List, B. The smelling principle of vetiver oil, unveiled by chemical synthesis. Angew. Chem. Int. Ed. 2021, 60, 5666–5672.
- Gatzenmeier, T.; Kaib, P.S.J.; Lingnau, J.B.; Goddard, R.; List, B. The Catalytic Asymmetric Mukaiyama–Michael Reaction of Silyl Ketene Acetals with α,β-Unsaturated Methyl Esters. Angew. Chem. Int. Ed. 2018, 57, 2464–2468.
- Dorrich, S.; Bauer, J.B.; Lorenzen, S.; Mahler, C.; Schweeberg, S.; Burschka, C.; Baus, J.A.; Tacke, R.; Kraft, P. Disila-galaxolide and derivatives: Synthesis and olfactory characterization of silicon-containing derivatives of the musk odorant galaxolide. Chem. Eur. J. 2013, 19, 11396–11408.
- Metz, S.; Nätscher, J.B.; Burschka, C.; Götz, K.; Kaupp, M.; Kraft, P.; Tacke, R. Disila-Phantolide and Derivatives: Synthesis and Olfactory Characterization of Silicon-Containing Derivatives of the Musk Odorant Phantolide. Organometallics 2009, 28, 4700–4712.
- Yang, F.; Jin, Y.; Wang, C. Nickel-Catalyzed Asymmetric Intramolecular Reductive Heck Reaction of Unactivated Alkenes. Org. Lett. 2019, 21, 6989–6994.
- Liang, R.-X.; Jia, Y.-X. Aromatic π-Components for Enantioselective Heck Reactions and Heck/Anion-Capture Domino Sequences. Acc. Chem. Res. 2022, 55, 734–745.
- Liu, J.; Zhang, Q.; Li, P.; Qu, Z.; Sun, S.; Ma, Y.; Su, D.; Zong, Y.; Zhang, J. Six-Membered Silacycle Odorants: Synthesis and Olfactory Characterization of Si Analogues of Artemone, β-Dynascone, and Herbac. Eur. J. Inorg. Chem. 2014, 2014, 3435–3440.
- Liu, J.; Zou, Y.; Fan, W.; Mao, J.; Chai, G.; Li, P.; Qu, Z.; Zong, Y.; Zhang, J.; Kraft, P. Synthesis and Olfactory Properties of Silicon-Containing Analogs of Rosamusk, Romandolide, and Applelide: Insights into the Structural Parameters of Linear Alicyclic Musks. Eur. J. Org. Chem. 2016, 2016, 976–982.
- Brunner, G.; Elmer, S.; Schröder, F. Transition-Metal-Catalyzed Cyclopropanation of Nonactivated Alkenes in Dibromomethane with Triisobutylaluminum. Eur. J. Org. Chem. 2011, 2011, 4623–4633.
- Cerutti-Delasalle, C.; Mehiri, M.; Cagliero, C.; Rubiolo, P.; Bicchi, C.; Meierhenrich, U.J.; Baldovini, N. The (+)-cis- and (+)-trans-olibanic acids: Key odorants of frankincense. Angew. Chem. Int. Ed. 2016, 55, 13719–13723.
- Ebner, C.; Carreira, E.M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679.
- Knight, A.M.; Kan, S.B.J.; Lewis, R.D.; Brandenberg, O.F.; Chen, K.; Arnold, F.H. Diverse engineered heme proteins enable stereodivergent cyclopropanation of unactivated alkenes. ACS Cent. Sci. 2018, 4, 372–377.
- Gund, M.; Déry, M.; Amzallag, V.; Spino, C. Dialkoxycarbenes in (4+1) Cycloadditions: Application to the Synthesis of Carotol. Org. Lett. 2016, 18, 4280–4283.
- Kula, J.; Izydorczyk, K.; Czajkowska, A.; Bonikowski, R. Chemical composition of carrot umbel oils from Daucus carota L. ssp. sativus cultivated in Poland. Flavour Fragr. J. 2006, 21, 667–669.
- Sieniawska, E.; Swiatek, Ł.; Rajtar, B.; Kozioł, E.; Polz-Dacewicz, M.; Skalicka-Wozniak, K. Carrot seed essential oil—Source of carotol and cytotoxicity study. Ind. Crops Prod. 2016, 92, 109–115.
More
