Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yi Zhao and Version 2 by Dean Liu.
Eucommia ulmoides Oliver (E. ulmoides) is a popular medicinal herb and health supplement in China, Japan, and Korea, and has a variety of pharmaceutical properties. The neuroendocrine–immune (NEI) network is crucial in maintaining homeostasis and physical or psychological functions at a holistic level, consistent with the regulatory theory of natural medicine.
- Eucommia ulmoides Oliver (E. ulmoides)
- neuroendocrine–immune
- cancer
- network pharmacology
1. Introduction
Eucommia ulmoides Oliver (E. ulmoides) is a monotypic genus Eucommia, also known as tuchong in Japanese and tu-chung in Korean, and was recorded in Shen Nong Ben Cao, a classic Chinese medical book [1]. It is characterized by being resistant to cold (−40 °C) and hot (44 °C) conditions [2]. Generally, E. ulmoides is cultivated in the southern area of Qingling in China, including the Guizhou, Sichuan, Hubei, Shaanxi, Hunan, Gansu, Yunnan, Anhui, Guangxi, Henan, Zhejiang, and Jiangxi provinces (Figure 1A) [3][4][5][3,4,5]. E. ulmoides has been utilized for at least 2000 years according to ancient Chinese medical records [6]. In 1955, the first global conference on the pharmacological effects of E. ulmoides was organized in Leningrad, and the scientists there suggested that E. ulmoides was effective in decreasing blood pressure [7]. From then on, E. ulmoides has attracted intensive attention worldwide and a great deal of scientific research and clinical trials have been undertaken to study its biological functions and pharmacological effects (Figure 1B).
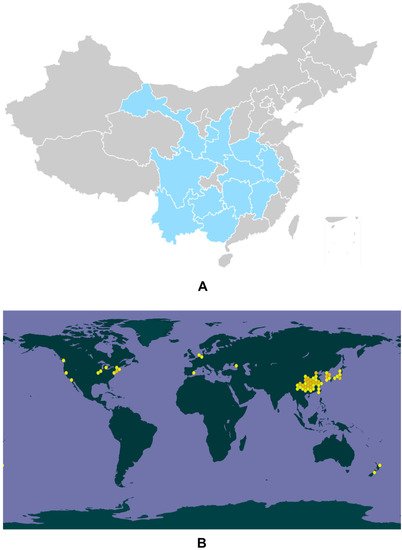
Figure 1. Genuine areas for E. ulmoides in China (A). The global biodiversity information of E. ulmoides (B).
Recently, the barks and leaves of E. ulmoides have been listed separately in the Chinese Pharmacopoeia (2020 version), with different quality control criteria, but identical functions, including nourishing the liver and kidney, and strengthening bones and muscles [8]. Additionally, Eucommia ulmoides Oliver barks (EUE) were recorded in the European Pharmacopoeia (9th Edition), Japanese Pharmacopoeia (17th Edition, English Version), Hong Kong Chinese Materia Medica Standards (Volume 3), and Taiwan Herbal Pharmacopeia (3rd Edition). In particular, among the 200 standard formulations in the Taiwan Herbal Pharmacopeia, 5 prescriptions involve EUE [9]. Traditionally, E. ulmoides has been considered to have the properties of tonifying the kidney and liver, strengthening bones and muscles, and fixing meridians from ancient records [6]. While in modern pharmacology, 204 chemical constituents have been identified from the leaves, barks, seeds, and flowers of E. ulmoides, and these compounds are divided into phenols, iridoids, lignans, flavonoids, terpenoids, sterols, gutta-percha, polysaccharides, unsaturated fatty acids, amino acids, and mineral elements [10]. Several components display vital biological functions both in vivo and in vitro, such as hypolipidemic, antihypertensive, antidiabetic, anti-inflammatory, antioxidative, neuroprotective, hepatoprotective, bone-metabolic, renoprotective, anti-aging, anti-fatigue, antidepressant, hypnotic-sedative, immune regulation, cognitive improvement, uterine smooth relaxation muscles, and erectile function enhancement [11][12][13][14][15][11,12,13,14,15]. Apart from being a medicine, E. ulmoides has also been employed as a health supplement popular in China, Japan, and Korea [16]. In short, E. ulmoides has great economic value and the potential for being used in novel drugs.
The neuroendocrine–immune (NEI) regulatory network (Figure 2) is a complex system, incorporating the nervous system, endocrine system, and immune system, to maintain homeostasis and plays a pivotal role in the treatment of complicated diseases, with the involvement of bioregulatory signals such as neurotransmitters, hormones, and cytokines/chemokines [17][18][19][17,18,19]. RThis researchers view aims to systematically summarize the chemical components, biological activities, and pharmacological effects of E. ulmoides on NEI diseases, which will provide a reference for research, development, and application of the active components of E. ulmoides.
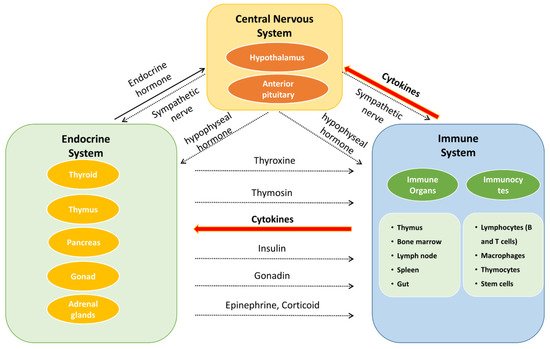
Figure 2. The interaction of neuroendocrine–immune system.
2. Neuroendocrine–Immune Regulatory Network
Besedobsky first proposed the NEI network in 1997. The interactions among the NEI system represent a complete communication circuit by sharing common signaling ligands and their receptors. In general, the nervous system regulates the immune system in two ways. One is through the release of neurotransmitters or neuropeptides such as acetylcholine, 5-HT, and opioid peptides from the endings of autonomic nerves, which act on immune cells and organs (bone marrow, thymus, lymph nodes, and gut) [20]. The second regulatory mechanism is through the hypothalamic–pituitary–adrenocortical (HPA), hypothalamic–pituitary–thyroid (HPT), hypothalamic–pituitary–gonadal (HPG), and hypothalamic–pituitary–somatotropic (HPS) axes to regulate the immune system. The release of neurohormones such as corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus can stimulate the anterior pituitary gland to secrete adrenocorticotropic hormone (ACTH). ACTH acts on the adrenal cortex and promotes the secretion of glandular hormones (e.g., glucocorticoids) to mediate the immune response [21]. On the other hand, immune cells can generate various immune mediators to trigger the activation of the nervous system in response to inflammatory and invasive stimuli. For example, IL-1 can upregulate the secretion of CRH [22], IL-1β, and TNF-α, which are regarded as potential neurotoxic substances [23], while the proinflammatory cytokine induction of interferon-γ (IFN-γ) provides neuroprotection during acute neuroinflammation by inducing the secretion of IL-6 [24].
The secretion of hormones also has a role in the nervous system. For example, thyroxine is important for the development of the brain [25], oxytocin can improve the learning and memory of mice by regulating the hippocampus [26], and vasopressin can also enhance memory [27]. As for the immune system, hormones such as glucocorticoids secreted by the adrenal cortex have both anti-inflammatory and proinflammatory effects [28]. As gonadal hormones, both androgens and estrogens can improve immunity [29][30][29,30]. In particular, estrogen can alter the immune response by binding to specific receptors on immune cells, resulting in their proliferation, and they play a feedback regulatory role on the hypothalamic–pituitary axis [29][31][29,31].
The NEI network is involved in multidirectional functions and multiple systems. Once the imbalance or alteration of the NEI network occurs, various diseases may follow, such as multiple sclerosis, fatigue, inflammation, psoriatic arthritis, systemic lupus erythematosus, depression, anxiety, cancer, and obesity [32][33][34][35][36][37][38][39][40][41][32,33,34,35,36,37,38,39,40,41]. From a holistic perspective, E. ulmoides has a “multi-components, multi-targets” profile for the treatment of various diseases. Hence, it is valuable to understand the pharmacological effects of E. ulmoides in the NEI network and fill this research gap.
3. Pharmacological Effects of E. ulmoides on NEI Network-Associated Diseases
The NEI network mediators and their end products have widespread effects at the systemic and cellular levels. They are responsible for disease behavior, such as cancer, neurodegenerative diseases (Alzheimer’s disease (AD) and Parkinson’s disease (PD) which are explained in Figure 34A), metabolic disorders including obesity, insulin resistance, diabetes, cardiovascular disease, and dyslipidemia, as well as osteoporosis, fatigue, depression, and anxiety [32][33][34][35][36][37][38][39][40][41][42][43][44][32,33,34,35,36,37,38,39,40,41,48,49,50] (Figure 34B). Thus, maintaining the balance of the NEI network may bring benefits for the treatment of these diseases. Through reviewing the literature on E. ulmoides, rwesearchers found that the herb does affect these diseases, as discussed in the following sections and summarized in Table 1.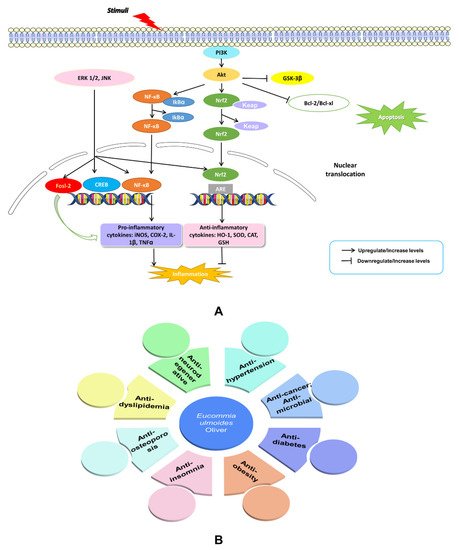
Figure 34.
An illustration of NF-kB and PI3K-Akt signaling and their effects on AD and PD (
A
). Summary of published therapeutic properties of
E. ulmoides
(
B
).
Table 1.
Summary of pharmacological effects for
Eucommia ulmoides
Oliver.
| Disease | Compound | Model | Dosage | Effect | Mechanism | Ref. | |
|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | ||||||
| Cancer | Chlorogenic acid | AGS cells | 0–2 mg/mL | Cytotoxicity | [45][51] | ||
| Total flavonoids | GBMs cells lines U251, U87, HS683 and A172 and human normal cell HA | H22 tumor-bearing mice | 50–200 mg/kg | Inhibit tumor growth Radiosensitization Induce apoptosis |
Increase Bax expression and decrease in Bcl-2 expression; Decrease the ratio of Bcl-2/Bax and downregulate the expression of HIF-1α, MMP-2 as well as Wee1. |
[46][47][52,53] | |
| Eucommicin A | iCSCL-10A-1, iCSCL-10A-2, MCF7, MDA-MB231 cells | 0–100 μM | Cytotoxicity, suppressed tumor sphere formation | [48][54] | |||
| Pentacyclic triterpenoids (betulinic acid, lupeol, and 3-O-laurylbetulinic acid) | Hela, MDA-MB-231, and T47D cells | 3–80 μM | Inhibit tumor cell growth and induce apoptosis | Induce mitochondrial fragmentation and suppress lysosome production in Hela cells. | [49][55] | ||
| Chlorogenic acid | HCT-116, LOVO | 600–1600 µg/mL | Inhibit proliferation and promote apoptosis | [50][56] | |||
| Eucommia ulmoides Oliver leaf (EUL) extract | A549, SNU-C4, HeLa, | 25–200 µg/mL | Inhibit proliferation | [51][57] | |||
| Total Polysaccharides | LLC, KMB-17 | 0.5–8.0 µg/mL | Induce apoptosis and inhibit proliferation | Activate Caspase-3 pathway. | [52][58] | ||
| E. ulmoides extract | HCT116 | 500–800 mg/L | Cytotoxicity | [53][59] | |||
| EUL extract and chlorogenic acid | HCT116, LOVO | 1600 µg/mL | Inhibit invasion and migration | [54][60] | |||
| Alzheimer’s disease (AD) and Parkinson’s disease (PD) | Eucommia ulmoides Oliver bark (EUE) extract | Lipopolysaccharide (LPS)-stimulated BV-2 microglia 6-hydroxydopamine (6-OHDA)-induced SH-SY5Y cells |
2.5–100 μg/mL | Anti-inflammatory Anti-oxidative stress |
Inhibit phosphorylation of MAPKs, PI3K/Akt, and GSK-3β, suppress NF-κB activation and induce Nrf2-dependent HO-1 activation; Inhibit reactive oxygen species (ROS) production, mitochondrial dysfunction, and phosphorylation of JNK, PI3K/Akt and GSK-3β, thereby blocking NF-κB nuclear translocation. |
[55][56][61,62] | |
| EUE extract | H2O2 -induced SH-SY5Y cells | Scopolamine-induced ICR mice | 5–20 μg/mL, 5–20 mg/kg |
Anti-cytotoxicity Enhance cholinergic signaling |
Inhibit cytotoxicity, reduce ROS accumulation, DNA condensation, MMP stabilization, regulate Bcl-2 family proteins, inhibit MAPKs and PI3K/Akt phosphorylation; Decrease the activity of AChE and TBARS, protect BDNF and activate CREB expression. |
[57][58][63,64] | |
| EUE extract | MPTP-induced male C57BL/6J mice | 2.5–10 g/kg, 150–600 mg/kg |
Anti-neuroinflammationAnti-PD | Downregulate expression of p38, JNK, and Fosl2, reduce pro-inflammatory factors; Antagonize loss of striatal neurotransmitters and alleviate associated ambulatory motor abnormalities. |
[59][60][65,66] | ||
| Betulin, wogonin, oroxylin A, geniposidic, aucubin |
MPP+-induced SH-SY5Y cells | 10 μM | Anti-PD | Ameliorate the ubiquitin-proteasome system. | [60][66] | ||
| Geniposidic acid (GPA) | APP/PS1 mice and C57BL/6J mice | 25, 75 mg/kg | Anti-neuroinflammatory | Inhibit the activation of astrocytes and microglia, down-regulate the expression of pro-inflammatory cytokines and iNOS, upregulate the expression of anti-inflammatory cytokines and Arg-1, and block the TLR4/2-MyD88 signaling pathway by reducing the expression of HMGB-1. | [61][67] | ||
| Macranthoin G | Hydrogen peroxide (H2O2)-induced PC12 cells | 6.25–50 μM | Anti-oxidative stress-mediated cellular injury Anti-PD and anti-AD |
Decrease MDA production and ROS levels, increase MMP, restore CAT, GSH-Px and SOD activity, and inhibit NF-κB pathway and activation of IκBα, p38 and ERK. | [62][68] | ||
| Dsylipidemia | EUL extract | High-fat diet (HFD)-induced male Sprague-Dawley | 200 mg/kg | Hepatoprotective | Inhibit ER stress, enhance lysosomal function, and increase autophagic flux associated with inhibition of the mTOR-ER stress pathway. | [63][69] | |
| EUE extract, aucubin and geniposide | Palmitate-induced HepG2 cells HFD-induced female Sprague-Dawley rats |
100 μg/mL extracts, 10 μg/mL aucubin or geniposide | Anti-hepatic dyslipidemia | Inhibit ER stress by increasing V-ATPase activity, reduce hepatic lipid accumulation through secretion of apolipoprotein B and associated triglycerides and cholesterol; Enhance lysosomal activity and to regulate ER stress. |
[64][70] | ||
| EUE extract | CCl4-induced Sprague-Dawley rats | 0.25–1 g/kg | Anti-hepatic dyslipidemia | Increase lysosomal enzyme activity, reduce ER stress by improving Apo B secretion, then inhibit ROS accumulation. | [65][71] | ||
| EUE extract, aucubin, geniposide | BAX-induced HepG2 cells; | HFD-induced female Sprague-Dawley | 100 μg/mL extracts, 10 μg/mL aucubin or geniposide; 0.25–1 g/kg; |
Anti-hepatic dyslipidemia | Inhibit cell death through enhancement of lysosome activity; Enhance lysosomal activity to the regulate lysosomal BAX activation and cell death. |
[66][72] | |
| CGA enriched-EUL extract | HepG2 cells | 10–80 mg/L; 0.3–600 μM; | Lipid-lowering | Activate AMPK and inhibit SREBP2 and HMGCR to reduce TC synthesis and TG levels, increase ABCA1 and CYP7A1, and enhance TC excretion and bile acid transport, synthesis and excretion. | [67][73] | ||
| Total flavonoid | HFD-induced male Wistar rats | 10–90 mg/kg/day | Anti-hyperlipidemia | Lower serum cholesterol, triglyceride, lipoprotein, apolipoprotein, and density lipoprotein cholesterol levels, increase HDL cholesterol and apolipoprotein A. | [68][74] | ||
| Osteoporosis | Total lignans | Primary cultures of rat osteoblasts | Ovariectomy rat model | 20, 40, or 80 mg/kg/day; 300 μg/mL |
Anti-osteoporosis, prevent OVX-induced decrease of bone mass and deterioration of trabecular microarchitecture | Induce primary osteoblastic cell proliferation and differentiation; Increase osteoprotegrin expression and decrease NF-κB ligand expression. |
[69][75] |
| EUE extract | Adolescent female rats | 30, 100 mg/kg | Increase longitudinal bone growth rate and enhance osteoblastogenesis | Promote chondrogenesis in the growth plate and increase BMP-2 and IGF-1. | [70][76] | ||
| 5-(hydroxymethyl)-2-furaldehyde (5-HMF) | Rat bone mesenchymal stem cells (bMSCs) | 0.05, 0.10, and 0.20 mg/mL | Anti-osteoporosis; inhibit adipogenesis and enhance osteoblastogenesis | Increase ALP, COL1alpha1 (7 days only), OCN and OPN expression, decrease PPARgamma, FABP4, C/EBPalpha and LPL expression. | [71][77] | ||
| Pinoresinol 4′-O-β-d-glucopyranoside, pinoresinol di-O-β-d-glucopyranoside, aucubin, wogonin, baicalein, and α-O-β-d-glucopyranosyl-4,2′,4′-trihydroxydihydrochalcone | MCF-7 cells; MDA-MB-231 cells; Hela cells | 10−6 M, 10−5 M, and 10−4 M | Prevent estrogen deficiency-induced osteoporosis | Activate ER-dependent transcription of estrogen target genes; Exhibit significant difference in ER subtype (α vs. β) selectivity; Proliferation effect on breast cancer cells mediated by the genomic action of Erα. Stimulation of endogenous estrogen-responsive genes (pS2). |
[72][78] | ||
| EUL extracts | Rat osteoblastic MC3T3-E1 cells | 6.25, 12.5, 25, 50, and 100 µg/mL | Anti-osteoporosis, restrain cell oxidative damage and increase cell survival rate in a dose-dependent manner | Decrease the expression of caspases 3, 6, 7, and 9. | [73][79] | ||
| Insomnia | Astragalin; Eucommiol | KM mice | 5, 10 and 20 mg/kg; 50, 100, and 200 mg/kg |
Reduce spontaneous activity, increase sleep ratio, shorten sleep latency and lengthen sleep time; Reduce the convulsion rate and prolong convulsion latency. |
[74][75][80,81] | ||
| Hypertension | Total flavonoid | Human glioblastoma cells (U251, U87, HS683 and A172) | 0.5–32 μg/mL | Enhance the radiotherapy effect, decrease the cell viability, inhibit migration and invasion, | HIF-α/MMP-2 pathway and intrinsic apoptosis pathway. | [46][52] | |
| Male flower extract | Male spontaneously hypertensive rats, Sprague Dawley rats | 0.05, 0.10, 0.20 g/mL | Reduce blood pressure, promote the expression of ACE2 | Activate the ACE2-Ang-(1–7)-Mas signaling pathways. | [76][82] | ||
| EUL extract | Wistar-Kyoto rats | 5% (w/w, extract/high-fat diet) | Reduce blood pressure, prevent aortic media hypertrophy | [11] | |||
| Diabetes mellitus | EUE extract | Streptozotocin (STZ)-induced diabetic rat model | 1.4 g/kg | Reduce the level of plasma glucose | Prohibit the reduction of superoxide dismutase (SOD) activity; Suppress the elevation of malondialdehyde (MDA). |
[77][83] | |
| EUL extract and EUL powder | HFD-induced male SD rats | 3%, 9% EUL 3%, 9% EGLP |
Improve insulin resistance and decrease plasma glucose level, reduce the production of ATP and the level of triacylglyceride, and regulate fatty acid oxidation | Enhance the use of circulating blood glucose in skeletal muscles. | [78][84] | ||
| Asperuloside | HFD-induced male SD rats | 0.03, 0.1, 0.3 ASP; 5% ELE | Reduce body weight, visceral fat, food intake, and circulating levels of glucose, insulin, triacylglyceride and nonesterified fatty acid | Increase mRNA levels of Cs, Idh3α, Ogdh, Sdha, Comp I, Comp IV, and Comp V in skeletal muscles; Reduce ATP production in WAT; Increase mRNA level of FA transport protein, Cpt1α and Acadvl, suppress Fas mRNA, and activate FA β-oxidation. | [79][85] | ||
| 5% chlorogenic acids contained in ELE | HepG2 cells | 200, 400, 500 μg/mL | Promote glucose uptake | Inhibit glucose-6-phosphate displacement enzyme and α-glucosidase. | [80][86] | ||
| E. ulmoides | STZ induced- type 1-like DM rats | 1 g/kg/day oral administration | Decrease the level of blood urea nitrogen and creatinine, improve renal fibrosis, without influencing blood glucose level | Inhibit TGF-β/Smad signaling pathway and suppress expression of TGF-β/connective tissue growth factor. | [81][87] | ||
| EUE extract | STZ-induced mice | 200 mg/kg oral administration | Inhibit production of advanced glycation end products (AGEs) and AGEs receptors | Increase the Glo1 expression and activity; Elevate Nrf2 protein expression and reduce RAGE expression. |
[82][88] | ||
| Isoquercetin, 6″-O-acetyl-astragalin, kaempferol, quercetin, rutin, kaempferol 3-O-rutinoside, astragalin | Ribose-gelatin | 0.01, 0.1, 1, 10, 100 μg/mL | Inhibit the formation of AGEs | Block the formation of CML and CMA. | [83][89] | ||
| Lignans | RF/6A cells | STZ-induced male C57BL/6 mice | 25, 50, 75, and 100 μg/mL | Protect endothelial function from AGEs injury and oxidative stress | Regulate Nrf2/HO-1 signaling pathway. | [16] | |
| Lignans | RMCs (HBZY-1 cells) | 20, 40, and 80 mg/L | Inhibit the proliferation of mesangial cells | Reduce the mRNA expression of Col I, Col III, Col IV, and fibronectin; Reverse the elevation of aldose reductase. |
[84][90] | ||
| Obesity | Asperuloside | Male C57BL/6J mice | 0.25% (w/w) | Reduce liver, epididymal, and mesenteric white adipose tissue, decrease serum triglyceride level |
Increase Akkermansia, Parabacteroides, Bacteroides, Sutterella, Anaerostipes, Roseburia, and Coprobacillus abundance Change metabolic level of cecum, Inhibit GLP-1; Reduce the level of tumor necrosis factor alpha (TNFα), monocyte chemoattractant protein 1 (MCP1), and collagen type 1 alpha1 (Col1a1) Increase lipoprotein lipase (Lpl) and carnitine palmitoyl transferase 1 (Cpt1). |
[85][91] | |
| EUL extract Asperuloside |
HFD-induced male SD rats | 0.03, 0.1, 0.3 ASP; 5% ELE | ASP reduce the body weight, visceral fat, food take, triacylglyceride and nonestesterified fatty acid | Diminish dehydrogenase;Increase Glut4, succinyl CoA synthase; Increase mRNA levels of Cs, Idh3α, Ogdh, Sdha, Comp I, Comp IV and Comp V in skeletal muscles; Increase uncoupling protein 1 in brown adipose tissue mRNA;Reduce ATP production in WAT; Increase mRNA level of FA transport protein, Cpt1α and Acadvl, suppress Fas mRNA, and activate FA β-oxidation. |
[79][85] | ||
| Quercetin | Reduce fat accumulation in liver | Decrease the level of plasma lipid. | [86][92] | ||||
| ELE, ELE aroma | Male SD rats | 5% ELE | Promote metabolism of lipid | Elevate the level of Cpt2, Acad, complex II and V mRNA in liver; Increase expression of brain-derived neurotrophic factor, protein kinase, and phospholipase Cγ in hypothalamus. |
[87][93] | ||
| ELE extract | Male Wistar-Kyoto rats | 5% ELE | Reduce the body weight gain, visceral and perirenal fat | [11] | |||
| CGA-enriched extract from EUE | HepG2 cells | 10, 20, 25, 40, 60, and 80 mg/L | Reduce the lipid in HepG2 cells | Elevate the expression of ABCA1, CYP7A1, and AMPKα2; Reduce the level of SREBP2 and inhibit mRNA and expression of HMGCR. |
[67][73] | ||
