Biodegradable metals have been extensively studied due to their potential use as temporary biomedical devices, on non-load bearing applications. These types of implants are requested to function for the healing period, and should degrade after the tissue heals. A balance between mechanical properties requested at the initial stage of implantation and the degradation rate is required. The use of temporary biodegradable implants avoids a second surgery for the removal of the device, which brings high benefits to the patients and avoids high societal costs. Among the biodegradable metals, iron as a biodegradable metal has increased attention over the last few years, especially with the incorporation of additive manufacturing processes to obtain tailored geometries of porous structures, which give rise to higher corrosion rates. Withal by mimic natural bone hierarchical porosity, the mechanical properties of obtained structures tend to equalize that of human bone.
- biodegradable metals
- iron
- porous iron
1. Introduction
2. Fabrication Techniques for Iron and Porous Iron
Although cellular structures have been widely studied driven by the pioneer work of Gibson and Ashby [4], some challenges still remain as the manufacturing of complex geometrical structures. The fabrication method is of utmost importance as it affects the microstructure and consequently the mechanical properties of a certain structure. The purpose of the methods described in athe present work is to obtain a porous structure with high surface area. A large variety of manufacturing methods has been reported in the literature to obtain iron porous structures. Some methods will be denoted by “conventional” and other will be designated as “advanced” methodologies as they rely on additive manufacturing procedures.2.1. Conventional Manufacture Techniques
Porous structures of biodegradable metals have been manufactured by traditional methods, such as direct foaming, spray foaming, chemical vapour deposition and electrophoretic deposition, powder metallurgy and melt injection moulding [5][6]. Even though these techniques control some specifications of the pores, they are not accurate in pore dimensions and, as a result, randomly organized porous structures are achievable [7]. The foaming procedures are difficult to apply on iron, due to the high density, high melting point, high surface tension and low viscosity of iron melt [7]. Powder metallurgy is another technique adequate for making porous metallic biomaterials, having the advantage of the fabrication of final-shaped products with an interconnected porous structure, which is useful for bone regeneration applications [7]. Also powder metallurgical techniques are less expensive than 3D-printing or laser sintering [7]. One of the powder metallurgy methods is hot isostatic pressure, which consists of the compaction of metallic powders through an application of a pressure, at a given temperature that compresses and sinters the parts at the same time. The compacting pressure and initial powder size are important parameters that will influence the final structure properties, being the use of finer powders useful to obtain good mechanical properties [7]. The fabrication of biodegradable iron alloy stents with powder methods was first performed by Hermawan et al. [3][8], which developed alloys that degrade faster than pure iron. For example, a Fe-Mn alloy produced by powder methods exhibits a faster in vitro degradation than the same alloy obtained by casting, due to the unavoidable presence of porosity [9]. The addition of silver to Fe-Mn alloys was evaluated with the development of Fe-(30 wt%)Mn-(1–3 wt%)Ag alloys, which were obtained by powder mixtures, mechanically alloying and sintering [10]. The same procedure of powder metallurgy with ball milling of the powders, mechanical allowing and sintering was used by Mandal et al. to develop a novel Fe-Mn-Cu alloy with enhanced antimicrobial properties [11]. The fabrication of iron composites (Fe/Mg2Si) was also performed by powder metallurgy [9], as well as the production of Fe–Ag and Fe–Au composites [12]. Gorejová et al. [13] were also able to produce porous structures from the carbonyl iron powder via the powder metallurgy process, in which polyurethane foams were impregnated by the slurry with the iron powder and thermally treated, sintered, to obtain the final structure [13]. Although traditional processing technologies possess advantages, they show poor ability in fabricating parts with complex geometrical shapes, which may be needed for bone implant in order to respond to the patient requirements.2.2. Advanced Manufacture Techniques/Additive Manufacturing
The emergence of additive manufacturing (AM) procedures allowed obtaining parts with a porous structure with a certain shape and geometry, that were difficult to produce through more conventional procedures. Additive manufacturing (AM) has led to a revolutionary change in manufacturing engineering for clinical applications and design for metallic implants [6][14][15][16]. AM is capable of controlling the pore size, interconnectivity, shape, and geometry of the biodegradable metallic scaffold [14]. These advanced techniques are ideal methods for medical applications in comparison with classic methods, being precisely controlled, customized to the patient needs and due to its ability to produce replicas of the CT-imaged tissue [17][18]. The application of AM to nondegradable metals, such as titanium and CoCr alloys has proven to be extremely successful [14]. Still, the use of AM to fabricate biodegradable metallic parts is beginning to be studied [14]. AM techniques produce 3D complex parts in a layer-by-layer sequence from a computer-aided design (CAD) model. For bone substitute devices, porous structures can be designed with tailoring architecture in order to minimize stress shielding, to simplify fluids transport and to promote fast healing [19]. The advantage of tailored porous scaffolds is to provide a structure that mimics the bone, allowing the permeability of physiological fluids and cells ingrowth [14][20]. Additionally, as the porous structures produced by AM have a larger surface area, they generally result in higher degradation rates [21]. Among the metal-based additive manufacturing (MAM) techniques, powder bed fusion (PBF) is the most widely used method for the production of metal implants, along with selective laser melting (SLM) and electron beam melting (EBM) [6][14]. MAM processes behaviour is dependent of transient heat transfer, powder thermal properties and melt pool temperature [6]. While in EBM the energy source is an electron beam, in SLM a laser beam with adjustable wavelength is used [6]. As a consequence, EBM can only be used in conductive metals, while SLM can be used to produce metals, ceramics and polymers [6]. Also, compared to SLM, EBM process has a larger zone affected by the heat, producing larger feature sizes [6]. Selective laser melting and electron beam melting are fast and not so expensive tools to prepare orthopaedic devices, presenting low material waste and feasibility to mix different materials with functional gradient [22]. Both electron beam melting (EBM) and selective laser melting (SLM) are able to fabricate structures with complex architecture [6]. In the literature, other studies have reported the production of iron porous structures by several advanced techniques, such as inkjet 3D printing [23][24]. 3D printing and pressure less microwave sintering [25], direct metal printing [21], laser metal deposition and selective laser melting [26][27][28]. Among the solid free-form fabrication methods, inkjet 3D printing has the advantage of manufacture metals, polymers, ceramics and composites [24]. Inkjet 3D process deposits liquid binder selectively onto layers of spread powder creating layers of the parts defined by CAD model [24]. For example, Chou et al. [24] used binder jet printing of Fe-30Mn powders to obtain porous structures that presented excellent cytocompatibility and mechanical properties close to the ones of human bone, reducing the stress-shield effects. These structures may be used in low-load-bearing applications. The 3D printed Fe-30Mn structures were found to corrode faster than pure iron [24]. The fabrication procedure consisting of 3D printing and pressure-less microwave sintering consists in the printing of a polymeric structure on which the mixture of powder is poured. Then, the set is placed in a furnace and with heating and vapourization the polymer vanishes, remaining a porous structure which afterwards, is submitted to microwave sintering process [25]. This method allowed obtaining iron structures with porosities in the range of 45.6–86.9% with ultimate compressive strength of 13.16–52.06 MPa. Li et al. [21] managed to get successful porous iron structures through direct metal printing of iron powders. The biodegradation behaviour showed that the mechanical properties of the porous structures were E = 1600–1800 MPa after 28 days of biodegradation, close to the values of trabecular bone. Electrochemical results revealed that the rate of biodegradation was 12 times higher for AM porous iron in comparison of that of cold-rolled iron [21]. Carluccio et al. [26] made a comparative study with selective laser melting, laser metal deposition and the traditional technique of casting to manufacture pure iron as biodegradable metal. Researchers found that selective laser metal produces hierarchical porosity in complex configurations, being an advantage compared to laser metal deposition [26]. The microstructural difference between these three types of manufacturing iron structures is mainly the grain size and morphology [26]. The grain size is dependent of the cooling rate, and for casting process, the cooling rate is the slowest, between 101 and 102 K/s [26]. For laser-based additive manufacturing, the cooling rates are usually higher due to small melt pools, creating a finer grain size [26]. Due to the significantly higher cooling rates of the laser based AM processes, the grain size is lower which promotes higher mechanical properties of the SLM samples [26]. SLM process for iron presented improved mechanical properties and, based on Li et al. [29] research, iron prostheses manufactured by SLM with hierarchical porosity resulted in Young’s modulus below 20 GPa [26][29].3. Biodegradation Behavior and Biocompatibility of Iron
The degradation of metallic implants inside the body increases the ion content levels, causing cytotoxicity. To prevent cytotoxicity, the degradation rate needs to be controlled, and the absorption should occur at the same rate as the tissue is repaired [3][30], [31]. The main challenge for iron is raise degradation rate by accelerating the process. High energy grain boundaries in iron alloys and finer microstructures attend to enhance the corrosion rate [32][33]. Further methods have been used to control the degradation rate, one of them making porous scaffolds and other alloying iron with specific elements, as Pd, Mn, Ca, Ag, which promote iron corrosion and improve mechanical properties [32][34]. The main process for degradation of porous iron scaffold in SBF is diffusion process [32]. Grain refinement methods are currently for altering the way metals degrade. An advantage of these techniques is that the chemistry of the metal remains unchanged [33]. Fine grained metals have also been discovered to provoke a weaker inflammatory response in hosts, with an overall better interaction. Ralston and Birbilis have introduced a relationship between the materials average grain size and its corrosion rate, similar to Hall-Petch equation, given by [33]:3.1. Corrosion Mechanism of Iron in Physiological Conditions
Body fluids are an aqueous aggressive environment that provokes electrochemical corrosion of metals. Controlling the corrosion rate helps controlling the cytotoxicity by metals, achieving a balance between the release rate of corrosion products and the ability of the body to absorb and excrete them [6]. Ideally, degradation begins at a very slow rate to maintain optimal mechanical integrity of the implant and increases at the same rate the body is healing itself. A period of 6–12 months is expected for the remodelling process to be completed [7]. It is important to emphasize that iron degradation should not be so fast that could cause an intolerable accumulation of degradation product around the implantation site. A total period of 12–24 months after implantation is considered reasonable for the stent to be totally degraded [7][31][35]. The corrosion rate is determined by kinetic factors and corrosion tendencies are determined by thermodynamic factors [36][37]. Metal corrosion in vivo is predominantly driven by chloride ions present in body fluids, namely the contact with blood and interstitial fluid. The chloride ion concentration in plasma is 113 mEq L−1 and in interstitial fluid is 117 mEq L−1, despite the low value is capable of corroding metallic implants [38]. In addition, chemicals such as amino acids and proteins found in body fluids tend to accelerate corrosion. The pH of the body fluid changes little acting as a buffer solution. Normal blood and interstitial fluids have a pH of around 7.35–7.45, although it can decrease near surface implantation areas and isoelectric points of biomolecules, such as proteins [38]. Recent research made by Sharma et al. [39] measured the pH increase in 28 days of additive manufactured porous iron in SBF solution. The results showed an increase in pH of 0.5 ± 0.05. Li et al. [21] observed an increase of pH 7.4 to 7.8 after 28 days immersion in r-SBF solution for an iron scaffold with 80% porosity made by direct metal printing. A general representation of metallic interfaces reacting with body fluid is present in Figure 1, where the metal reacts with the environment, release positive ions (Mn+) to the environment, keeping electrons (e−) to the metal substrate. The contact of surface metal with body fluid results in oxidization of the metal to a more stable ion [40]. The reactions lead to formation of a protective metal oxide layer on the surface (yellow spots). The interactions with the body fluids may lead to deposition of calcium phosphate on the metal oxide layer, which permit that cells adhere on the surface to form tissues [40].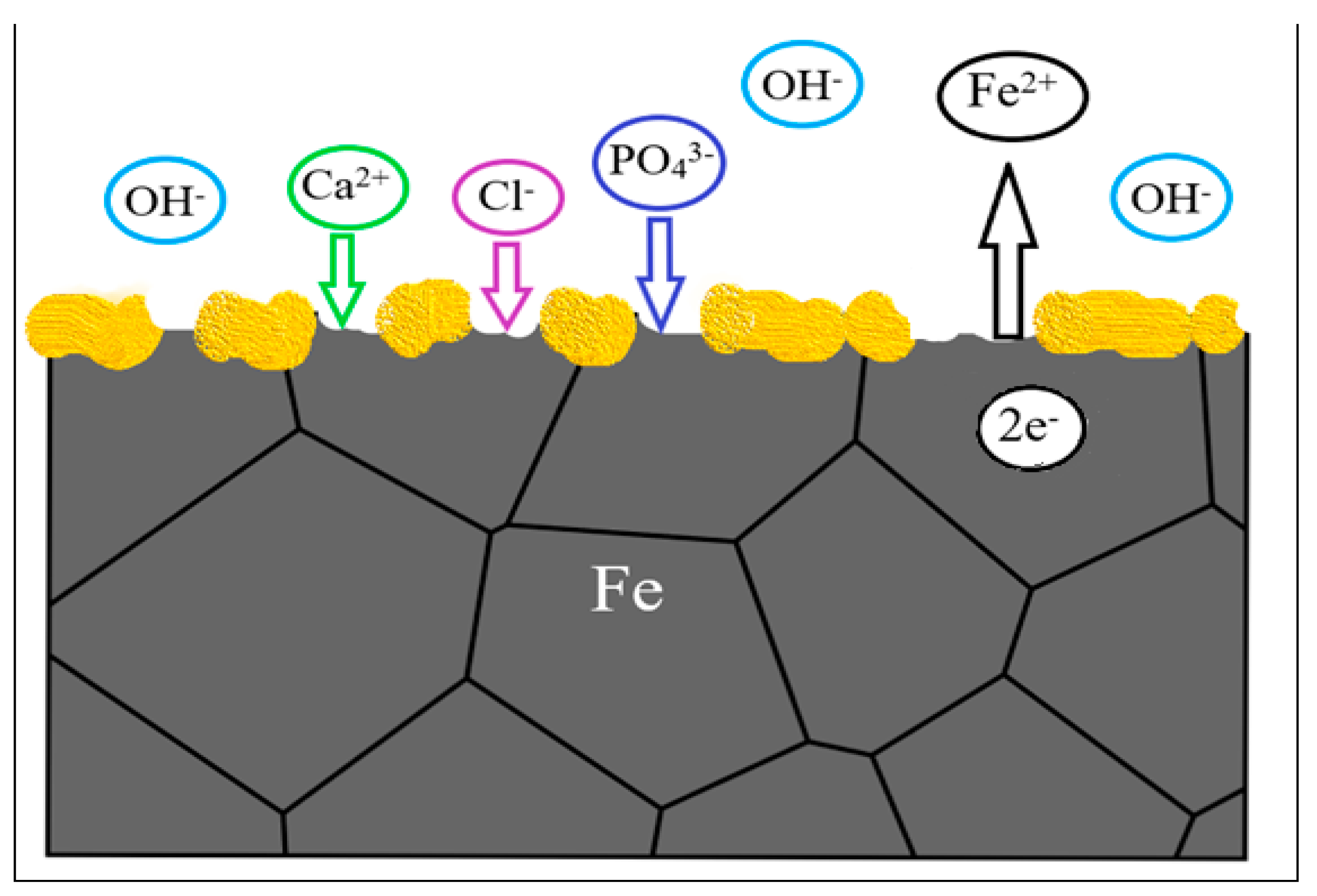
3.2. In Vitro and In Vivo Biocompatibility
One of the first important studies about iron biocompatibility and degradation in vivo in coronary application was reported by Peuster et al. [46]. It was a one-year study in animal model, with pure iron and 316L stainless steel stents implanted in the aorta of pigs. Iron resulted to be a suitable metal for stent applications. Zhang et al. [42] at a pioneer study on iron compatibility with blood and cell compares 99.9 wt% purity iron with magnesium-manganese-zinc alloy and 316L stainless steel in Hank’s solution. ISO 10993-4 standard was followed and the haemolysis assay resulted in a low haemolysis ratio for iron. For the haemolysis assay, rabbit whole blood with 3.8 wt% sodium citrate was utilized. The prothrombin time assay resulted in excellent anticoagulant iron, and platelet adhesion tests in iron showed impressive anti-platelets adhesion. During cells toxicity, iron ions presented toxicity to the stem marrow cell of mouse bone. The standard ISO 10993-4 recommends the value of 5% in the haemolysis ratio to not cause haemolysis to blood system. Iron presented a ratio of 2.44%, accepted by the standard and proving iron has excellent anti-haemolysis property. Iron ions may produce reactive oxygen species in cells. Highly reactive oxygen species can react with the most molecules found in cells, making them toxic. Furthermore, free iron can react with unsaturated fatty acids, resulting in the formation of lipid hydroperoxides and subsequently alkoxyl and peroxyl radicals. These products are capable of causing cell death and impair cellular integrity. Despite these oxygen specimens are damaging, they are normally generated in reactions and the body has defensive strategies against it. However, iron level needs to be limited in cells. The iron concentration should be less than 0.075 mg/mL [42]. Zhu et al. [47] assessed the biocompatibility of pure iron and cytotoxicity on endothelial cells was performed in SBF solution for one month at 37 °C. The incubation time was almost 700 h, the degradation rate was at the highest 40 µg/(cm2 h) and the mean rate was 20.4 µg/(cm2 h). The corrosion was predominately uniform corrosion. Endothelial cells from human umbilical vein were cultured with 10% fetal bovine serum, penicillin and streptomycin. The cells were incubated with iron solution for three days, with concentrations varying from 0 until 2000 µg/mL. The accessed cell proliferation show non-toxicity until 50 µg/mL iron concentration [47]. According to the literature [48], the grain size and texture considerably affect interactions between cells and osteoblast functions and roughness also potentially influences cell growth. The attachment, orientation, migration and metabolism of the human cells are determined by the properties of the metallic implant of austenitic stainless steel. The roughness of the grains is an important factor with regard to osteoblast adhesion and protein adsorption. Proteins and focal adhesion points of cells also interact at a scale that enables them to activate signalling pathways within the cell. These pathways, in turn, have an impact on the lifespan of the cell. Increased cellular activity is implied by an increase in cell attachment and pre-osteoblast proliferation, as well as a stronger presence of fibronectin. This is turn is linked to the physico-chemical properties of the surface of the metallic implant. The attachment of cells to the implant and their growth on its surface—and thus the compatibility between them—is influenced by the chemical and morphological properties of the surface. Hydrophilicity, ionic bonding, electrostatic and van der Waals interactions are the most significant factors that drive adsorption of macromolecules and proliferation of cells on the implant surfaces. Protein adsorption, cell spreading, and cell proliferation may be assisted with high surface energy and high surface hydrophilicity and wettability. Those characteristics are controlled by the grain size of the metallic implant [48]. Another study [49] compared pure iron and cobalt chromium coronary stents in vivo in domestic pigs for 28 days. The morphometric comparison between these two types of coronaries resulted in less inflammation of iron compared to cobalt chromium, adding the vantage of iron being radio-opaque, while cobalt chromium is radiolucent. The stents did not cause harm to the vessel, no peripheral embolization or thrombosis were found with the angiography. Although the degradation of the iron coronary stents has not been evaluated, it is noticed the brown coloration of the tissue around the stent. A possible reason is the assimilation of iron salts by the tissue. The inflammation caused by iron stents and its degradation products was not worse than the inflammation caused by the cobalt chromium stent [49]. A 36 month study was presented about the degradation, absorption and biocompatibility of a nitrided iron (Fe alloyed with 0.074 wt% N) coronary stent with 70 µm height [50]. The device was compared with other stents made by PLLA-based, magnesium based, Co-Cr, pure iron scaffold and stainless steel. For in vitro corrosion tests, phosphate buffered saline (PBS) with pH at 7.4, flux speed at 25 ± 5 cm/s, oxygen at 4 ± 0.5 mg/L and temperature of 37 °C was used to simulate the inner environment of a coronary artery. For in vivo experiments, stents made by nitrited iron, pure iron and 316L stainless steel were implanted at the abdominal aortas of rabbits. Also, nitrited iron stents were allocated at the coronary artery of minipigs (porcines). Pure iron has lower mechanical strength in comparison with 316L stainless steel and Co-Cr alloy, alloys traditionally used for manufacturing permanent stents. Iron can stay implanted for 18 months, therefore the balance between strength, ductility and biodegradation is the key to accomplish a suitable iron stent. Some alloys can increase the corrosion rate and mechanical performance, but they compromise the cytocompatibility, so the element needs to be in lower concentrations. As Fe-Mn alloy the in vivo corrosion of nitrided iron stent, the results presented a higher corrosion rate for nitrited iron in comparison with pure iron. After 12 months the mass loss of nitrited iron was 44.5 ± 6.4 wt% while the mass loss for pure iron stent was 24.0 ± 5.6 wt%. The initial radial strength of nitrited iron stent was 171 ± 5 kPa, after six months of in vivo performance was proximately 150 kPa and after nine months it was above 120 kPa [50]. The device performance of a coronary stent in terms of foreshortening, recoiling and crossing profile should be minimum. Side-branch accessibility and expansion diameter should be maximum as possible. Mechanical properties as radial strength should be between 110 and 170 kPa, high enough to support vessels lesion but still flexible, as clinically tested for stents Φ 3.0 × 18 mm. The studied nitrite iron stents presented better performance in comparison with the stents already in the market, such as, the Co-Cr alloy stent, the Mg-based stent and polymer-based stents [50]. Corrosion products were identified by XPS and the results showed strong signals of C, O, Ca, P and Fe presented a binding energy of 709.8 eV and Fe3+ binding energy was 712.31 eV. The degradation products identified by Raman analysis were Fe3O4, α-Fe2O3 and γ-FeOOH. The endothelialisation assess of nitrited iron stent was compared to a peer of 316L stainless steel after seven days inside a rabbit abdominal aorta, analysing the neointima coverage extend. Coronary stents are prejudicial to the endothelium, which is formed by a single layer at the vascular wall of endothelial cells [50]. The damaged at the endothelium lead to neointimal hyperplasia and may lead to stent thrombosis [51]. A homogeneous endothelium layer was formed with the nitride iron coronary, lowering the risk of stent thrombosis. The 316L stainless steel coronary seemed to interrupt the endothelium natural recover [50]. The follow-up local tissue response for the minipigs and the rabbits, 53 months and 36 months, respectively, showed no pathologic changes or abnormalities of the organs. After 53 months of nitrited iron stent implementation, the images by Micro-CT 2D presented a non-uniform degradation and absorption inside the porcine coronary. The corrosion products presented a moving tendency from in situ and peri-stent areas to tunica externa, also known as tunica adventitia, the outermost layer of the blood vessel. To essay the biosorption of the corrosion products, the in vivo analysis of the stents in minipigs was chosen because porcine coronary artery is closer to human coronary artery and the life time of a minipig is higher than a rabbit. At body fluid environment with pH 7.4, it is difficult to dissolve Fe3O4, Fe3(PO4)2, Fe2O3, Fe(OH)3 and FeOOH. Following the Pourbaix diagram of iron corrosion at a pH of 7.4 and phosphate physiological environment, Fe(OH)3, FeOOH, Fe2O3 (non-magnetic) present a steady state. Other types of corrosion products with lower stability is Fe3(PO4)2 and Fe3O4, because of their slow reaction kinetics. The natural organism low concentration of iron ions could make these corrosion products easily absorbed, since the solubility equilibrium convey towards the concentration of iron ions. The bioresorption of hydroxides and ferric oxides in body solubility, is slow and long-term. The insoluble products, resulted from iron corrosion, could take five to six years to complete bioresorption [50]. The corrosion products’ morphology and composition showed iron carbonate (FeCO3) and iron protoxide (FeO) and FTIR also presented hydroxides and phosphates. Scanning electron microscope (SEM) analysis of the external structure, reveal a white layer at the surface only after 1 day of immersion. From day 7, the structure surface presented shiny white loose degradation products, and after 28 days, these degradation products covered surface structure almost completely. The scaffold geometry interfered with the degradation at the center and at the periphery region. The degradation products at day 7 were thinner and more condensed at the centre. At the periphery, the degradations products were loose and thicker. The periphery region presented more phosphorus and calcium [21]. The mechanical properties of AM iron remain similar to the properties of trabecular bone even after 28 days of biodegradation, which is an advantage compared to other metals. The degradation rate of the topological scaffolds was found to be 12 times larger than the one of compact iron. A suitable cytocompatibility was also observed. Another mechanical requirement for an orthopaedic material that lasts from weeks to one year is to have a strain in the interval 1.1 to 2.1, [1], which was adequate [21]. Moreover, in vitro cytocompatibility and degradation of iron porous scaffolds obtained using Fe-30Mn powder and binder jet printing point out a significantly faster degradation rate of the Fe-30Mn alloy compared to pure iron [24].3.3. Influence of Surface Treatments on Biodegradation Behaviour
Surface treatments are used primarily to enhance the biocompatibility of iron, increase the corrosion rate, and also aimed for an uniform corrosion of iron. The surface functionalization of iron to improve its biocompatibility is reported in studies including ion implantation with tantalum, with lanthanum, formation of Fe-O film, plasma nitriding, coatings with calcium phosphates and polymeric coatings [1][52][53]. Cytocompatibility and osseointegration are bioactivities improved by coating the metallic surface [34]. Huang and Zheng [52] measured pure iron degradation by coating with platinum (Pt) discs arrayed in pattern by photolithography and evaporation by electron beam. The patterned was adopted to control the degradation rate and regulate cells proliferation and adhesion. Platinum was chosen for its hemocompatibility and high corrosion potential, forming galvanic cells with pure iron. Platinum presents cytotoxicity but the chemical stability prevents for ions released into the body. The platinum discs had two designs, one with 20 µm diameter, the nearest space between discs was 5 µm and the thickness was approximately 285 nm (Φ20 µm × S5 µm). The second design was Φ4 µm × S4 µm with thickness around 80 nm [52]. Aiming the improvement of iron biocompatibility, surface modification with hydroxyapatite as a coating presents great outcomes. Hydroxyapatite is widely used as coating for metallic protheses, since hydroxyapatite is the main mineral constituent of bone and presents outstanding bone integration [23]. The in vivo osteointegration and the cytocompatibility of the protheses are affected by the bonding between coating and substrate and the morphology of the coating. The morphology is expected to be homogenous and uniform, also with high strength bonding and most of all the coating must improve biocompatibility. Nano-Plotter 3D printing iron scaffolds with tailored mechanical behaviour and were coated with nanostructured hydroxyapatite by hydrothermal method[23]. The coating successfully reduced the release of Fe ions, to below 2 mg/L for 120 µm hydroxyapatite thickness, increasing the cytocompatibility of rabbit bone marrow mesenchymal stem cells (rBMSCs). The study compared the osteogenic differentiation with the analysis of alkaline phosphatase in scaffolds with and without hydroxyapatite coating. The results showed an increased activity of alkaline phosphatase (ALP) activity of rBMSCs with the hydroxyapatite coating, indicating a osteogenic bioactivity of AM iron scaffolds [23]. Another perspective about the coated porous structures is the improvement of antibacterial coating response compared to solid implants, partly because of a higher specific surface area [54]. Iron foams were coated with polyethyleneimine (PEI), an organic polymer with biological applications which was also used to enhance the cytocompatibility of iron and its degradability [13]. Hong et al. fabricated by binder-jet 3D printing alloys with Fe-35 wt% Mn and Fe-34 wt% Mn-1 wt% Ca, evaluating the cytocompatibility and degradation behaviour [55]. The mechanical properties tests were conducted following ASTM E8-04 and the electrochemical corrosion tests were performed with Hank’s solution HBSS H1387, at 37.4 °C. The corrosion current density Icorr and the corrosion potential Ecorr were determined by Tafel analysis from potentiodynamic polarization curves[55].References
- Santos, C.; Alves, M.; Montemor, M.; Carmezim, M. Bioresorbable metallic implants: Surface functionalization with nanoparticles and nanostructures. Adv. Mater. Appl. Micro Nano Scale 2017, 219–242.
- Dong, H.; Lin, F.; Boccaccini, A.R.; Virtanen, S. Corrosion behavior of biodegradable metals in two different simulated physiological solutions: Comparison of Mg, Zn and Fe. Corros. Sci. 2021, 182, 109278.
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110.
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure & Properties; Cambridge University Press: Cambridge, UK, 1999.
- Andani, M.T.; Moghaddam, N.S.; Haberland, C.; Dean, D.; Miller, M.J.; Elahinia, M. Metals for bone implants. Part 1. Powder metallurgy and implant rendering. Acta Biomater. 2014, 10, 4058–4070.
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141.
- Čapek, J.; Vojtěch, D.; Oborná, A. Microstructural and mechanical properties of biodegradable iron foam prepared by powder metallurgy. Mater. Des. 2015, 83, 468–482.
- Hermawan, H.; Dubé, D.; Mantovani, D. Developments in metallic biodegradable stents. Acta Biomater. 2010, 6, 1693–1697.
- Sikora-Jasinska, M.; Chevallier, P.; Turgeon, S.; Paternoster, C.; Mostaed, E.; Vedani, M.; Mantovani, D. Long-term in vitro degradation behaviour of Fe and Fe/Mg 2 Si composites for biodegradable implant applications. RSC Adv. 2018, 8, 9627–9639.
- Sotoudehbagha, P.; Sheibani, S.; Khakbiz, M.; Ebrahimi-Barough, S.; Hermawan, H. Novel antibacterial biodegradable Fe-Mn-Ag alloys produced by mechanical alloying. Mater. Sci. Eng. C 2018, 88, 88–94.
- Mandal, S.; Ummadi, R.; Bose, M.; Balla, V.K.; Roy, M. Fe–Mn–Cu alloy as biodegradable material with enhanced antimicrobial properties. Mater. Lett. 2019, 237, 323–327.
- Huang, T.; Cheng, J.; Bian, D.; Zheng, Y. Fe-Au and Fe-Ag composites as candidates for biodegradable stent materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 225–240.
- Gorejová, R.; Oriňaková, R.; Orságová Králová, Z.; Baláž, M.; Kupková, M.; Hrubovčáková, M.; Haverová, L.; Džupon, M.; Oriňak, A.; Kaľavský, F.; et al. In vitro corrosion behavior of biodegradable iron foams with polymeric coating. Materials 2020, 13, 184.
- Qin, Y.; Wen, P.; Guo, H.; Xia, D.; Zheng, Y.; Jauer, L.; Poprawe, R.; Voshage, M.; Schleifenbaum, J.H. Additive manufacturing of biodegradable metals: Current research status and future perspectives. Acta Biomater. 2019, 98, 3–22.
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350.
- Yusop, A.H.M.; al Sakkaf, A.; Nur, H. Modifications on porous absorbable Fe-based scaffolds for bone applications: A review from corrosion and biocompatibility viewpoints. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 18–44.
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196.
- Debroy, T.; Wei, H.; Zuback, J.; Mukherjee, T.; Elmer, J.; Milewski, J.O.; Beese, A.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224.
- Zadpoor, A.A. Additively manufactured porous metallic biomaterials. J. Mater. Chem. B 2019, 7, 4088–4117.
- Zadpoor, A. Frontiers of Additively Manufactured Metallic Materials. Materials 2018, 11, 1566.
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.I.; Leeflang, M.A.; Pouran, B.; Gonzalez-Garcia, Y.; Weinans, H.; et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393.
- Sing, L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385.
- Yang, C.; Huan, Z.; Wang, X.; Wu, C.; Chang, J. 3D Printed Fe Scaffolds with HA Nanocoating for Bone Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 608–616.
- Chou, D.-T.; Wells, D.; Hong, D.; Lee, B.; Kuhn, H.; Kumta, P.N. Novel processing of iron–manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013, 9, 8593–8603.
- Sharma, P.; Pandey, P.M. Morphological and mechanical characterization of topologically ordered open cell porous iron foam fabricated using 3D printing and pressureless microwave sintering. Mater. Des. 2018, 160, 442–454.
- Carluccio, D.; Bermingham, M.; Kent, D.; Demir, A.G.; Previtali, B.; Dargusch, M.S. Comparative study of pure iron manufactured by selective laser melting, laser metal deposition, and casting processes. Adv. Eng. Mater. 2019, 21, 1900049.
- Carluccio, D.; Xu, C.; Venezuela, J.; Cao, Y.; Kent, D.; Bermingham, M.; Demir, A.G.; Previtali, B.; Ye, Q.; Dargusch, M. Additively manufactured iron-manganese for biodegradable porous load-bearing bone scaffold applications. Acta Biomater. 2020, 103, 346–360.
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio 2019, 3, 100024.
- Li, Y.; Jahr, H.; Pavanram, P.; Bobbert, F.S.L.; Puggi, U.; Zhang, X.-Y.; Pouran, B.; Leeflang, M.A.; Weinans, H.; Zhou, J.; et al. Additively manufactured functionally graded biodegradable porous iron. Acta Biomater. 2019, 96, 646–661.
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34.
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57.
- Sharma, P.; Pandey, P.M. Corrosion behaviour of the porous iron scaffold in simulated body fluid for biodegradable implant application. Mater. Sci. Eng. C. 2019, 99, 838–852.
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005.
- Gorejová, R.; Haverová, L.; Oriňaková, R.; Oriňak, A.; Oriňak, M. Recent advancements in Fe-based biodegradable materials for bone repair. J. Mater. Sci. 2019, 54, 1913–1947.
- Vaz, M.F.; Canhão, H.; Fonseca, J.E. Bone: A composite natural material. In Advances in Composite Materials: Analysis of Natural and Man-Made Materials; Tesinova, P., Ed.; InTechOpen: London, UK, 2011; p. 586.
- Kruger, J. Fundamental aspects of the corrosion of metallic implants. In Corrosion and Degradation of Implant Materials; ASTM International: West Conshohocken, PA, USA, 1979; pp. 107–127.
- Pourbaix, M. Electrochemical corrosion of metallic biomaterials. Biomaterials 1984, 5, 122–134.
- Hanawa, T. In vivo metallic biomaterials and surface modification. Mater. Sci. Eng. A 1999, 267, 260–266.
- Sharma, P.; Jain, K.G.; Pandey, P.M.; Mohanty, S. In vitro degradation behaviour, cytocompatibility and hemocompatibility of topologically ordered porous iron scaffold prepared using 3D printing and pressureless microwave sintering. Mater. Sci. Eng. C 2020, 106, 110247.
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.; Seok, H.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71.
- Liu, Y.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental theory of biodegradable metals—Definition, criteria, and design. Adv. Funct. Mater. 2019, 29, 1805402.
- Zhang, E.; Chen, H.; Shen, F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010, 21, 2151–2163.
- Zhen, Z.; Xi, T.; Zheng, Y. A review on in vitro corrosion performance test of biodegradable metallic materials. Trans. Nonferrous Met. Soc. China 2013, 23, 2283–2293.
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407.
- Tolouei, R.; Harrison, J.; Paternoster, C.; Turgeon, S.; Chevallier, P.; Mantovani, D. The use of multiple pseudo-physiological solutions to simulate the degradation behavior of pure iron as a metallic resorbable implant: A surface-characterization study. Phys. Chem. Chem. Phys. 2016, 18, 19637–19646.
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962.
- Zhu, S.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.; Sun, H.; Leng, Y. Biocompatibility of pure iron: In vitro assessment of degradation kinetics and cytotoxicity on endothelial cells. Mater. Sci. Eng. C 2009, 29, 1589–1592.
- Misra, R.D.K.; Nune, C.; Pesacreta, T.C.; Somani, M.C.; Karjalainen, L.P. Understanding the impact of grain structure in austenitic stainless steel from a nanograined regime to a coarse-grained regime on osteoblast functions using a novel metal deformation–annealing sequence. Acta Biomater. 2013, 9, 6245–6258.
- Waksman, R.; Pakala, R.; Baffour, R.; Seabron, R.; Hellinga, D.; Tio, F.O. Short-Term Effects of Biocorrodible Iron Stents in Porcine Coronary Arteries. J. Interv. Cardiol. 2008, 21, 15–20.
- Lin, W.; Qin, L.; Qi, H.; Zhang, D.; Zhang, G.; Gao, R.; Qiu, H.; Xia, Y.; Cao, P.; Wang, X.; et al. Long-term in vivo corrosion behavior, biocompatibility and bioresorption mechanism of a bioresorbable nitrided iron scaffold. Acta Biomater. 2017, 54, 454–468.
- Ong, A.T.L.; Aoki, J.; Kutryk, M.J.; Serruys, P.W. How to accelerate the endothelialization of stents. Arch. Mal. Coeur. Vaiss. 2005, 98, 123–126.
- Huang, T.; Zheng, Y. Uniform and accelerated degradation of pure iron patterned by Pt disc arrays. Sci. Rep. 2016, 6, 23627.
- Gorejová, R.; Oriňaková, R.; Macko, J.; Oriňak, A.; Kupková, M.; Hrubovčáková, M.; Džupon, M.; Sopčák, T.; Ševc, J.; Maskaľová, I.; et al. Electrochemical behavior, biocompatibility and mechanical performance of biodegradable iron with PEI coating. J. Biomed. Mater. Res. Part A 2022, 110, 659–671.
- van Hengel, I.; Riool, M.; Fratila-Apachitei, L.E.; Witte-Bouma, J.; Farrell, E.; Zadpoor, A.A.; Zaat, S.; Apachitei, I. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials 2017, 140, 1–15.
- Hong, D.; Chou, D.; Velikokhatnyi, O.I.; Roy, A.; Lee, B.; Swink, I.; Issaev, I.; Kuhn, H.A.; Kumta, P.N. Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater. 2016, 45, 375–386.
