Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 3 by Amina Yu.
Metal oxide nanoparticles have a higher surface area due to their smaller size, which makes them useful in various applications such as biosensors, bio-nanotechnology, and nanomedicine . These nanoparticles have many atoms on their surfaces, making them highly reactive. There is a huge demand for packaging materials that can keep food fresher for extended periods of time. The incorporation of nanoscale fillers in the polymer matrix would assists in the alleviation of packaging material challenges while also improving functional qualities.
- nanocomposite
- food packaging
- metal oxides
1. Metal Oxide Nanoparticles for Food Packaging Application
The mechanism of action of nanoparticles on bacterial cells is shown in Figure 1 [1].
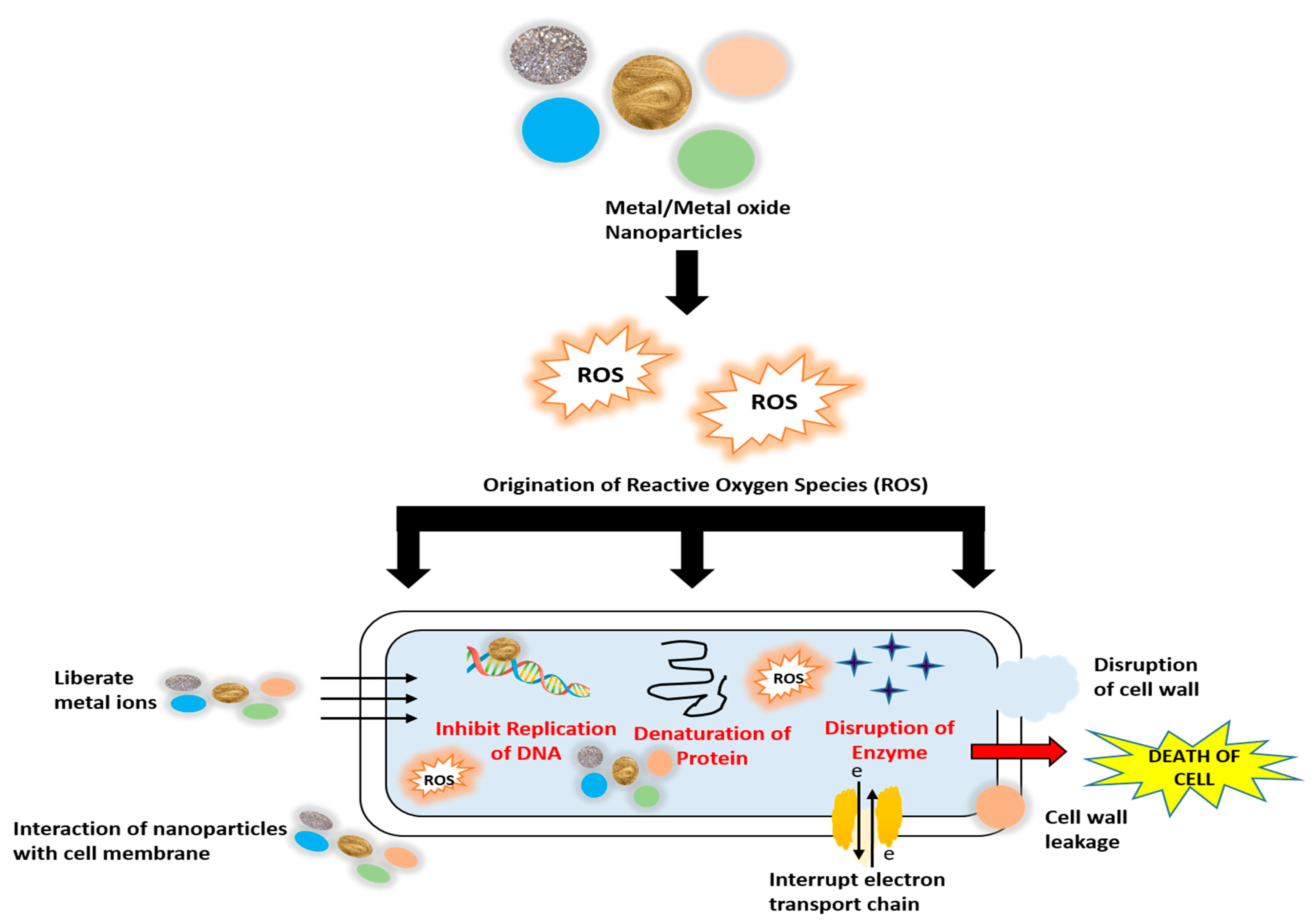
Figure 1. Mechanism of action of metal and metal oxide nanoparticles on bacterial cells.
2. Zinc Oxide (ZnO) Nanoparticles for Food Packaging
ZnO has a wide range of applications because of its strong optical, electrical, piezoelectrical, semiconducting, and chemical sensing capabilities [2][3]. It was observed that increased antibiotic resistance of bacteria, its mutation, and unavailability of vaccine causes many health hazards to human beings. The high annual mortality rate has been attributed to the consumption of food with bacterial contamination. Hence, the development of antibacterial agents against food pathogens such as Salmonella typhii, Clostridium perfringes, and Pseudomonas aeruginosa and so on have become an important objective for current research. It has been carried out to examine the antibacterial activity of ZnO nanoparticles and the viability of the bacteria under ZnO applied conditions. Also, factors related to their antibacterial activity, mechanism of toxicity of ZnO nanoparticles towards these bacteria, and their applications in food have been focused on during several research projects. It has been found in many researches that ZnO acts as an antibacterial agent when it is reduced to its micrometer as well as nanometer size. ZnO nanoparticles react with the surface and core of the bacteria, thus causing a bactericidal effect [4]. These nanoparticles also show high photochemical activities and catalytical activities along with antibacterial and antifungal properties. As ZnO absorbs UVA in the range of 315–400 nm and UVB in 280–315 nm, it enhances the antibacterial responses [5].
The antibacterial property of ZnO nanoparticles is also influenced by their concentration and particle size. It has been seen that the antibacterial activity increased with an increase in surface area and concentration [6][7]. With a decrease in the size, the specific surface area gets increased. Hence, ZnO nanoparticles can easily penetrate the bacterial cell membranes [8][9][10]. It was also found that with an increase in the concentration of ZnO nanoparticles, the death of cells increased. When the concentration increased, it caused interruption of the function of mitochondria, leakage of lactate dehydrogenase, and a change in the morphology of the cell [11]. Thus, it can be concluded that increased surface area and concentration lead to increased antibacterial activity of ZnO nanoparticles. It was shown that when the concentration of ZnO particles was kept 2 mM along with small-sized nanoparticles, the growth of bacteria was reduced by 99% [12]. In another one on oral bacteria, ZnO demonstrated bacteriostatic effect against Lactobacillus salivarius and Streptococcus sobrinus, and showed inhibitions against other strains, i.e., P. aeruginosa, Streptococcus mutans, and S. aereus [11]. E. coli growth was inhibited when it was exposed to a concentration of 10 mM ZnO for 30 min [13]. When Salmonella enterica, Enter idis, other Salmonella strains, and E. coli O157:H7 were exposed to a lower concentration of ZnO nanoparticles with size 30 nm, a total of 100% bactericidal effect was observed [14].
One of the mechanisms on which the antibacterial activity of ZnO nanoparticles depends is reactive oxygen species formation. In one of these, it was observed that exposure of ZnO nanoparticles to UV produces reactive species such as hydrogen peroxide, hydroxide, and superoxide anions, which cause damage to cellular components, i.e., proteins, lipids, and DNA, and cause internalization of cell membrane of bacteria [15]. The antibacterial activity of ZnO nanoparticles is also based on the mechanisms of release of Zn ions in the medium consisting of both ZnO nanoparticles and bacteria [16][17][18], wherein the released Zn ions (from the ZnO nanoparticles) are responsible for its toxicity. This toxic interaction leads to their widespread application, mostly in the food packaging industries. It has also been observed that ZnO nanoparticles have toxic interaction with bacteria but are not toxic to human cells [19].
The morphology of ZnO nanoparticles affects the internalization mechanism. Different morphologies of ZnO nanoparticles are produced depending on the application; hence, the synthesis technique varies. These ZnO nanoparticles are manufactured using several chemical and/or physical procedures, but the chemical method is favored as it provides precise control of the shape and size of the nanoparticles [20]. Methods of fabrication require the use of various parameters that may be chemical or physical, including type of solvent, pH, temperature, etc. [21]. ZnO nanoparticles are available in different configurations compared to other metal oxides, including nano-cages, nano-combs, nano-helixes, nano-belts, etc. [22]. In addition, by modifying the growing conditions, different shapes of ZnO nanoparticles can be obtained, such as flowers, spheres, snowflakes, boxes, plates, spirals, drums, etc. [23]. The wire and rod-shaped ZnO nanostructures can penetrate the bacterial cells more easily than the spherical shaped ZnO nanoparticles [10]. It has also been shown that the biocidal activity of flower-shaped ZnO nanoparticles is higher than that of spherical and rod-shaped ZnO nanoparticles [24]. Different methods for achieving various ZnO nano-particles are summarized in Table 1.
Table 1. Methods of fabrication of ZnO nanoparticles.
| Shapes | Methods | References |
|---|---|---|
| Flower | Solution process at low temperature (90 °C) using zinc acetate dehydrate and NaOH | [25] |
| Flower, prism, snowflakes | Solution process at high temperature (180 °C for 13 h) | [26] |
| Prism like and prickly sphere like | Decomposition method at 100 °C for 13 h | [21] |
| Spherical | Non hydrolytic solution process using zinc acetate | [27] |
| Spherical | Soft chemical solution process | [28] |
| Nanorods of hexagonal prismatic and hexagonal pyramid like | Hydrothermal treatment with stabilizing agents | [13] |
| Nanowires | UV light decomposition process | [29] |
It was carried out to develop packaging films made up of agar incorporated with zinc nanoparticles to improve mechanical and functional properties. Being transparent and flexible, agar can be used to synthesize packaging films. ZnO nanoparticles were made from the extract of plant Mimusops elengi and added to the agar matrix. The effectiveness of the package was evaluated by observing the external features of packaged green apples in the agar-nanoparticle-based packaging material under ambient conditions. Two packaging films were prepared with 2% ZnO nanoparticles and 4% ZnO nanoparticles. Green grapes wrapped in plastic (polyethylene) film spoiled after 7 days due to mold development and leaking of sticky liquid, but fruits wrapped in agar-ZnO films with 2% ZnO nanoparticles remained fresh even after 14 days and for 21 days when wrapped in films with 4% ZnO nanoparticles [30]. As a result, a combination of ZnO nanoparticles and suitable coatings might be beneficial in food packaging. In another one, ZnO NPs incorporated in gelatin-based composite films demonstrated significant antibacterial activity against Gram-positive and Gram-negative food pathogens. Moreover, permeance to water and elongation at break of ZnO NPs incorporated films increased, whereas tensile strength and modulus of elasticity decreased [31].
3. Titanium Dioxide (TiO2) Nanoparticles for Food Packaging
Titanium dioxide (TiO2) shows antimicrobial activity against several food-borne pathogens, including Vibrio parahaemolyticus, Listeria monocytogenes, and Salmonella enterica even under the UV light [32]. TiO2 has great potential for controlling food hazards in food industries. Under UV irradiation, TiO2 acts as a scavenger of oxygen; therefore, it can be used to control food spoilage caused by oxygen [33]. It produces reactive oxygen species after absorbing photo energy, which is useful to kill microbes [34]. It can also absorb wavelength light, thus acting as a good UV blocking material. In addition to photostability, it also helps keep transparency in the food packages. Due to its photocatalytic activity under UVA or irradiation of black light, it has the power of self-cleaning that leads to antibacterial effects [30].
Agglomeration of TiO2 nanoparticles affects the functional property of films. This property of TiO2 nanoparticles is modified by improving its surface properties. The solvent evaporation method was used to prepare a biodegradable film using fish skin gelatin and TiO2 nanoparticles to achieve the required surface properties [35]. Different methods have also been employed during various studies focused on the application of TiO2 nanoparticles. TiO2 nanotubes were synthesized using a deposition process, where atomic layers covered electrospun PVA (polyvinyl alcohol) nanofibers at different temperature levels to obtain nanostructures with antibacterial properties and large selective area [36]. Another one used the sol-gel method to synthesize eco-friendly pectin –TiO2 nanocomposite aerogels, wherein initially pectin was dissolved in water followed by the addition of a measured amount of TiO2 colloid. In the presence of Zn ions and tert-butanol, the crosslinking reaction was initiated, and subsequently, gels were subjected to solvent exchange and supercritical CO2 drying [37].
The inclusion of TiO2-NPs in the films modifies its physical, chemical, and biological activity, emphasizing the scope of application of these nanoparticles in food packaging by developing composites. It was observed that the addition of TiO2-NPs in polyethylene-based films improved the antimicrobial activity of the film [38]. Another one revealed that integrating TiO2-NPs on polylactic acid (PLA) substrates, cellulose nanofibers, and nanocomposites coatings reduced penetrant diffusivity while having no effect on gas barrier qualities; nonetheless, it marginally lowered the optical transparency of the film [39]. When TiO2-NPs (0.5, 1, and 2 wt percent) were added to potato starch films, optical transparency and tensile strength increased somewhat, whereas water vapor transmission was significantly reduced [40]. Similarly, Goudarzi et al. (2017) found that when TiO2-NPs at different concentrations of 1, 3, and 5% were incorporated into starch films, the hydrophobicity and thermal properties increased, whereas the tensile strength, Young’s modulus, and water vapor permeability decreased with increase in the concentration of TiO2-NPs [41]. TiO2 demonstrated ethylene scavenging activity, which lowered the rate of deterioration of fresh produce. Phothisarattana et al. (2021) showed the efficiency of TiO2 integrated biodegradable films in extending the shelf life of banana [42].
4. Copper Oxide (CuO) Nanoparticles for Food Packaging
CuO-NPs are the most extensively used metal oxides in food packaging, attributed to their widely effective antimicrobial properties and significant potential to inhibit the growth of bacteria, viruses, and fungi [42]. The CuO-NPs are mostly used as catalysts, polymer reinforcing agents, semiconductors, solar cells, magnetic storage media, water disinfectants, gas sensors, emission devices, and food packaging materials [43]. Many methods are employed for the fabrication of CuO-NPs, such as microwave [44], auto-combustion [45], electrochemical [46], and thermal decomposition [47]. Nanocomposites have been synthesized by adding CuO-NPs, chitosan nanofibers, and bacterial nanofibers using the chemical precipitation method [48]. By Gu et al. (2018), an eco-friendly ultrasound method was used to prepare monoclinic-based CuO-NPs by utilizing extract of Cystoseira trinodis [49]. In another research, spherical CuO-NPs were made in situ under alkaline conditions using gelled cellulose II matrix as a template [50].
Copper has been found to be vital in the metabolism and electron transport mechanisms of living organisms. In the case of CuO-NP, the synthesis technique is critical in establishing its characteristics and finding applications in many fields, which subsequently depend upon biological activities. By Beigmohammadi et al. (2016) in which antimicrobial activities of packaging films, i.e., LDPE incorporated with Ag-NPs, CuO-NPs, and ZnO-NPs, against coliform in ultra-filtered cheese were determined [51]. After analysis, it was found that the coliform count decreased to 4.21 log CFU/g when stored for 4 weeks at a temperature of 4 ± 0.5 °C for each of the treatments. Another one was determined the different properties of nanocomposites, such as water vapor transmission rate, UV and thermal stability, and antimicrobial properties. It was observed that the inclusion of CuO-NPs in the film improved the properties noted above along with presenting antimicrobial effects against E. coli and Listeria monocytogenes [52]. A combined antibacterial effect was obtained when CuO-NPs were incorporated in chitosan nanofibers [48]. Similar to the other cases of metal oxides, the antimicrobial activity of CuO depends upon morphology, surface area, size, structure, and oxidation states. Moreover, the packaging material properties are also affected by doping or coupling CuO-NP with other active materials such as metal or metal oxides.
Antibacterial polymeric film (APF) was developed using a variety of combinations of sodium alginate (SA) and cellulose nanowhiskers (CNW) surrounded with copper oxide nanoparticles (CuO-NPs) [53], which was used to pack fresh cut pepper (FCP). The antimicrobial activities against different strains of pathogens were tested using the disc diffusion method. From this, it was found that the film consisting of combination CNW (0.5%)-SA (3%)-CuO-NP (5 mM) presented a significant zone of inhibition for bacteria. The zone of inhibition for S. aureus was at 27.49 ± 0.91 mm, E. coli at 12.12 ± 0.58 mm, Salmonella spp. at 25.21 ± 1.05 mm, C. albicans at 23.35 ± 0.45 mm, and Trichoderma spp. at 5.31 ± 1.16 mm. It was observed that when the combination was SA (1%)-CuO-NPs (1 mM), then the zone of inhibition was 21.65 ± 0.62 mm, whereas when the combination was SA (3%)-CuO- NPs (1 mM), the zone of inhibition became 12.25 ± 0.84 mm against S. aureus. Thus, it was concluded that with an increase in the concentration of CuO-NPs, the antimicrobial activity improved, but with the increase in the concentration of sodium alginate, diffusion of CuO-NPs in agar plates was retarded and reduced the antimicrobial action. This was attributed to the activated carbon present in the alginates, which absorbed the metal ions [54]. In this one, it was demonstrated that the presence of CuO-NP led to the antimicrobial activity of the APF; hence, the film increased the shelf life of FCP. Furthermore, the antioxidant property of the film was focused on using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) scavenging activities. It was observed that the film containing CNW (0.5%)-SA (3%)-CuO NPs (5 mM) provided the highest value for the DPPH scavenging as well as ABTS scavenging, i.e., 46.55% and 35.46%, respectively, as compared to all other films. It was due to the diffusion of CuO-NPs and CNW, wherein the CuO-NPs of the film transfer its electron density to the free radical present at the nitrogen atom in DPPH [55]. CNW contributed to the antioxidant activity by diffusion of hydrogen ions from the hydroxyl group of the glucose unit [56]. Thus, it was concluded that due to the inclusion of CuO-NP and CNW in the film, its antioxidant activity increased.
Some of the research also showed that many properties of packaging materials, including oxygen and water barrier properties, optical properties, antimicrobial properties, and bactericidal effects against Gram-positive and Gram-negative bacteria, can be modified with the hybridization of nanoparticles such as Cu/CuO-NPs, CuO-NPs/Ag-NPs and so on. It was carried out in which the antibacterial effects of Cu-ONPs and ZnO-NPs against Gram-positive (E. coli) and Gram-negative (S. aureus) bacteria were evaluated using the Kirby Bauer Disk diffusion method, where the CuO-NPs and ZnO-NPs were developed using the wet chemical method [57]. Antibacterial activity was focused at different concentrations from 5 mg/mL to 0.01 mg/mL. It was found that CuO-NP, at a concentration lower than 1 mg/mL, did not show any antibacterial effect against E. coli. As the concentration increased to 1 mg/mL, it showed marginal antibacterial activity on E. coli. It presented good antibacterial activity at a concentration of 5 mg/mL and 2 mg/mL. Similarly, when the antibacterial activity of CuO was tested against S. aureus, it showed significant activity at concentrations from 5 mg/mL to 0.25 g/mL. In contrast, ZnO was not effective against E. coli, but it showed a significant effect against S. aureus. Several were also revealed that antimicrobial properties incorporated into polymers effectively compensated for poor barrier qualities of biodegradable packaging films [58][59].
5. Silicon Dioxide (SiO2) Nanoparticles for Food Packaging
Silicon is one of the major solid elements found on the earth and is available in silica and silicate. According to many researches, SiO2–NPs, when added into different polymers, increased the mechanical strength and thermal stability [60]. It has also been observed that SiO2–NPs can be applied on jars, bags, and bottles as a non-sticky coating [61]. By Li et al. (2016), when polypropylene (used in many packaging for proper printing) was mixed with Nano-SiO2 (0.09 wt%) and modified with ethylene/vinyl acetate (EVA), adsorption of ink on the polymer decreased along with an increase in the tensile strength and decrease in gas permeability [62].
SiO2–NPs can also be included in different coatings that are further used to prepare nanocomposites. In an experiment using the sol-gel process, a PLA-based coating was developed with the incorporation of SiO2–NPs using tetraethoxysilane as a precursor and 3-iso-cyanatopropyl-triethoxysilane as a coupling agent to produce a biodegradable packaging system. The biodegradable packaging film thus formed maintained good transparency and a high gas barrier property, which was 70% more than the film made from pure PLA [63]. The addition of SiO2–NPs in the packaging material increased corresponding antimicrobial activity. It was observed that the shelf life of shrimp packaged using LDPE-SiO2 packaging increased by eight days compared to other samples stored otherwise [64]. A decreased oxygen permeability reduced oxygen levels in the package headspace that accelerated quality deterioration in packaged shrimp. Improving barrier properties would increase the utilization of biodegradable packaging to preserve quality of packaged products [65].
References
- Wahid, F.; Zhong, C.; Wang, H.S.; Hu, X.H.; Chu, L.Q. Recent advances in antimicrobial hydrogels containing metal ions and metals/metal oxide nanoparticles. Polymers 2017, 9, 636.
- Fan, Z.; Lu, J.G. Zinc oxide nanostructures: Synthesis and properties. J. Nanosci. Nanotechnol. 2005, 5, 1561–1573.
- Zanet, V.; Vidic, J.; Auger, S.; Vizzini, P.; Lippe, G.; Iacumin, L.; Comi, G.; Manzano, M. Activity evaluation of pure and doped zinc oxide nanoparticles against bacterial pathogens and Saccharomyces cerevisiae. J. Appl. Microbiol. 2019, 127, 1391–1402.
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781.
- Song, Z.; Kelf, T.A.; Sanchez, W.H.; Roberts, M.S.; Rička, J.; Frenz, M.; Zvyagin, A.V. Characterization of optical properties of ZnO nanoparticles for quantitative imaging of transdermal transport. Biomed. Opt. Express 2011, 2, 3321.
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489.
- Yu, J.; Yang, J.; Liu, B.; Ma, X. Preparation and characterization of glycerol plasticized-pea starch/ZnO-carboxymethylcellulose sodium nanocomposites. Bioresour. Technol. 2009, 100, 2832–2841.
- Sawai, J.; Kawada, E.; Kanou, F.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Japan 1996, 29, 627–633.
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646.
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78.
- Jeng, H.A.; Swanson, J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Heal.—Part A Toxic/Hazardous Subst. Environ. Eng. 2006, 41, 2699–2711.
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76.
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surf. B Biointerfaces 2013, 102, 21–28.
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331.
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028.
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316.
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192.
- Sevinç, B.A.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2010, 94, 22–31.
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res.—Part A 2006, 78, 595–604.
- Karami, H.; Fakoori, E. Synthesis and characterization of ZnO nanorods based on a new gel pyrolysis method. J. Nanomater. 2011, 2011, 628203.
- Amna, T.; Yang, J.; Ryu, K.S.; Hwang, I.H. Electrospun antimicrobial hybrid mats: Innovative packaging material for meat and meat-products. J. Food Sci. Technol. 2015, 52, 4600–4606.
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, 829–858.
- Mahmud, S. One-dimensional growth of zinc oxide nanostructures from large micro-particles in a highly rapid synthesis. J. Alloys Compd. 2011, 509, 4035–4040.
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66–73.
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Seo, H.K.; Kim, G.S.; Khang, G.; Shin, H.S. Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater. Res. Bull. 2007, 42, 1640–1648.
- Zhang, J.; Sun, L.; Yin, J.; Su, H.; Liao, C.; Yan, C. Control of ZnO morphology via a simple solution route. Chem. Mater. 2002, 14, 4172–4177.
- Wahab, R.; Mishra, A.; Yun, S., Il; Kim, Y.S.; Shin, H.S. Antibacterial activity of ZnO nanoparticles prepared via non-hydrolytic solution route. Appl. Microbiol. Biotechnol. 2010, 87, 1917–1925.
- Wahab, R.; Khan, F.; Lutfullah; Singh, R.B.; Khan, A. Enhance antimicrobial activity of ZnO nanomaterial’s (QDs and NPs) and their analytical applications. Phys. E Low-Dimens. Syst. Nanostructures 2014, 62, 111–117.
- Wu, G.; Cheng, Y.; Xie, Q.; Jia, Z.; Xiang, F.; Wu, H. Facile synthesis of urchin-like ZnO hollow spheres with enhanced electromagnetic wave absorption properties. Mater. Lett. 2015, 144, 157–160.
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867.
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.W. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271.
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24.
- Xiao-e, L.; Green, A.N.M.; Haque, S.A.; Mills, A.; Durrant, J.R. Light-driven oxygen scavenging by titania/polymer nanocomposite films. J. Photochem. Photobiol. A Chem. 2004, 162, 253–259.
- Zhu, Z.; Cai, H.; Sun, D.W. Titanium dioxide (TiO2) photocatalysis technology for nonthermal inactivation of microorganisms in foods. Trends Food Sci. Technol. 2018, 75, 23–35.
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of gelatin-TiO2 nanocomposite film and its structural, antibacterial and physical properties. Int. J. Biol. Macromol. 2016, 84, 153–160.
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26.
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-based nanocomposite aerogels for potential insulated food packaging application. Carbohydr. Polym. 2018, 195, 128–135.
- Noori Hashemabad, Z.; Shabanpour, B.; Azizi, H.; Ojagh, S.M.; Alishahi, A. Effect of Tio2 Nanoparticles on the Antibacterial and Physical Properties of Low-Density Polyethylene Film. Polym.—Plast. Technol. Eng. 2017, 56, 1516–1527.
- Roilo, D.; Maestri, C.A.; Scarpa, M.; Bettotti, P.; Checchetto, R. Gas barrier and optical properties of cellulose nanofiber coatings with dispersed TiO2 nanoparticles. Surf. Coatings Technol. 2018, 343, 131–137.
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264.
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313.
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly (Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192.
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals 2016, 9, 75.
- Karunakaran, C.; Manikandan, G.; Gomathisankar, P. Microwave, sonochemical and combustion synthesized CuO nanostructures and their electrical and bactericidal properties. J. Alloys Compd. 2013, 580, 570–577.
- Kamble, S.P.; Mote, V.D. Structural, optical and magnetic properties of Co doped CuO nano-particles by sol-gel auto combustion technique. Solid State Sci. 2019, 95, 105936.
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper Oxide Nanoparticles: Synthesis, Characterization and Their Antibacterial Activity. J. Clust. Sci. 2011, 22, 121–129.
- Ibrahim, E.M.M.; Abdel-Rahman, L.H.; Abu-Dief, A.M.; Elshafaie, A.; Hamdan, S.K.; Ahmed, A.M. The synthesis of CuO and NiO nanoparticles by facile thermal decomposition of metal-Schiff base complexes and an examination of their electric, thermoelectric and magnetic Properties. Mater. Res. Bull. 2018, 107, 492–497.
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties. Carbohydr. Polym. 2018, 186, 273–281.
- Gu, H.; Chen, X.; Chen, F.; Zhou, X.; Parsaee, Z. Ultrasound-assisted biosynthesis of CuO-NPs using brown alga Cystoseira trinodis: Characterization, photocatalytic AOP, DPPH scavenging and antibacterial investigations. Ultrason. Sonochem. 2018, 41, 109–119.
- Eivazihollagh, A.; Bäckström, J.; Dahlström, C.; Carlsson, F.; Ibrahem, I.; Lindman, B.; Edlund, H.; Norgren, M. One-pot synthesis of cellulose-templated copper nanoparticles with antibacterial properties. Mater. Lett. 2017, 187, 170–172.
- Beigmohammadi, F.; Peighambardoust, S.H.; Hesari, J.; Azadmard-Damirchi, S.; Peighambardoust, S.J.; Khosrowshahi, N.K. Antibacterial properties of LDPE nanocomposite films in packaging of UF cheese. LWT—Food Sci. Technol. 2016, 65, 106–111.
- Shankar, S.; Wang, L.F.; Rhim, J.W. Preparation and properties of carbohydrate-based composite films incorporated with CuO nanoparticles. Carbohydr. Polym. 2017, 169, 264–271.
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214.
- Goksungur, Y.; Uren, S.; Guvenc, U. Biosorption of Copper Ions by Caustic Treated Waste Baker’s Yeast Biomass. Turkish J. Biol. 2003, 27, 23–29.
- Tran, T.H.; Nguyen, V.T. Copper Oxide Nanomaterials Prepared by Solution Methods, Some Properties, and Potential Applications: A Brief Review. Int. Sch. Res. Not. 2014, 2014, 856592.
- Zimoch-Korzycka, A.; Bobak, Ł.; Jarmoluk, A. Antimicrobial and antioxidant activity of chitosan/hydroxypropyl methylcellulose film-forming hydrosols hydrolyzed by cellulase. Int. J. Mol. Sci. 2016, 17, 1436.
- Asamoah, R.B.; Annan, E.; Mensah, B.; Nbelayim, P.; Apalangya, V.; Onwona-Agyeman, B.; Yaya, A. A Comparative Study of Antibacterial Activity of CuO/Ag and ZnO/Ag Nanocomposites. Adv. Mater. Sci. Eng. 2020, 2020, 7814324.
- Khumkomgool, A.; Saneluksana, T.; Harnkarnsujarit, N. Active meat packaging from thermoplastic cassava starch containing sappan and cinnamon herbal extracts via LLDPE blown-film extrusion. Food Packag. Shelf Life 2020, 26, 100557.
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal packaging of sorbate and benzoate incorporated biodegradable films for fresh noodles. Food Control 2021, 123, 107763.
- Toǧrul, H.; Arslan, N. Moisture sorption isotherms and thermodynamic properties of walnut kernels. J. Stored Prod. Res. 2007, 43, 252–264.
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923.
- Li, D.; Zhang, J.; Xu, W.; Fu, Y. Effect of SiO2/EVA on the mechanical properties, permeability, and residual solvent of polypropylene packaging films. Polym. Compos. 2016, 37, 101–107.
- Bang, G.; Kim, S.W. Biodegradable poly(lactic acid)-based hybrid coating materials for food packaging films with gas barrier properties. J. Ind. Eng. Chem. 2012, 18, 1063–1068.
- Luo, Z.; Xu, Y.; Ye, Q. Effect of nano-SiO2-LDPE packaging on biochemical, sensory, and microbiological quality of Pacific white shrimp Penaeus vannamei during chilled storage. Fish. Sci. 2015, 81, 983–993.
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and applicability of starch-based films: An eco-friendly approach. Foods 2021, 10, 2181.
More
