Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Zeljko Todorovic.
Chimeric antigen receptor T (CAR T) cell therapy is promising for relapse/refractory chronic lymphocytic leukemia (CLL) patients. Complete and durable remission of CLL is possible in patients treated with CAR T cells but further investigations are necessary to understand and possibly predict how patient specific factors influence the outcome of this treatment.
- CAR T cells
- CAR NK cells
- CLL
1. Introduction
Chronic lymphocytic leukemia (CLL) is a chronic lymphoproliferative disease characterized by malignant transformation of mature antigen-experienced B lymphocyte and accumulation of monoclonal malignant B cells in peripheral blood, bone marrow, lymph nodes, spleen [1]. CLL is the most common leukemia in Western countries. The incidence of CLL in North America and Eastern Europe is 4.7 per 100,000 people per year, while the CLL incidence is higher than 35 in the population over 85 years of age [2]. Clinical management of CLL is challenging and depends on patients ages, comorbidities and biological features of CLL cells such as immunoglobulin heavy chain gene mutation, 17p deletion, TP53 mutation and, as recent research showed, number and type of CLL tumor clones in patient [3,4][3][4]. Treatment option varies from watch and wait approach in the asymptomatic early-stage CLL to chemo-immunotherapy and novel targeted therapies, such as Bruton’s tyrosine kinase inhibitors and inhibitors of Bcl-2 family proteins, for symptomatic and advanced disease [3]. However, despite the expansion of novel therapeutic approaches, CLL is still mostly an incurable disease.
In the last decades, cell-based immunotherapy has emerged as a novel treatment for malignant diseases. Cell based immunotherapy relies on using immune cells obtained from patients, raised in vitro, and genetically modified to increase their ability to find and kill tumor cells (Figure 1). Various T cell based treatments of malignant disorders have been developed, including tumor-infiltrating lymphocytes, T cell receptor (TCR)-modified T cells and chimeric antigen receptor T (CAR T) cells [5]. Therapy of malignancies with CAR T cells is accompanied with better and durable clinical responses compared to the treatment with tumor-infiltrating lymphocytes and TCR modified T cells [6,7][6][7]. CAR T cells are T lymphocytes with engineered synthetic receptors made to recognize and destroy the cells expressing the target antigen. CARs are artificially made proteins consisting of the single-chain variable fragment of an antibody (ScFv) that recognizes tumor antigen and the T-cell activation domain [8].
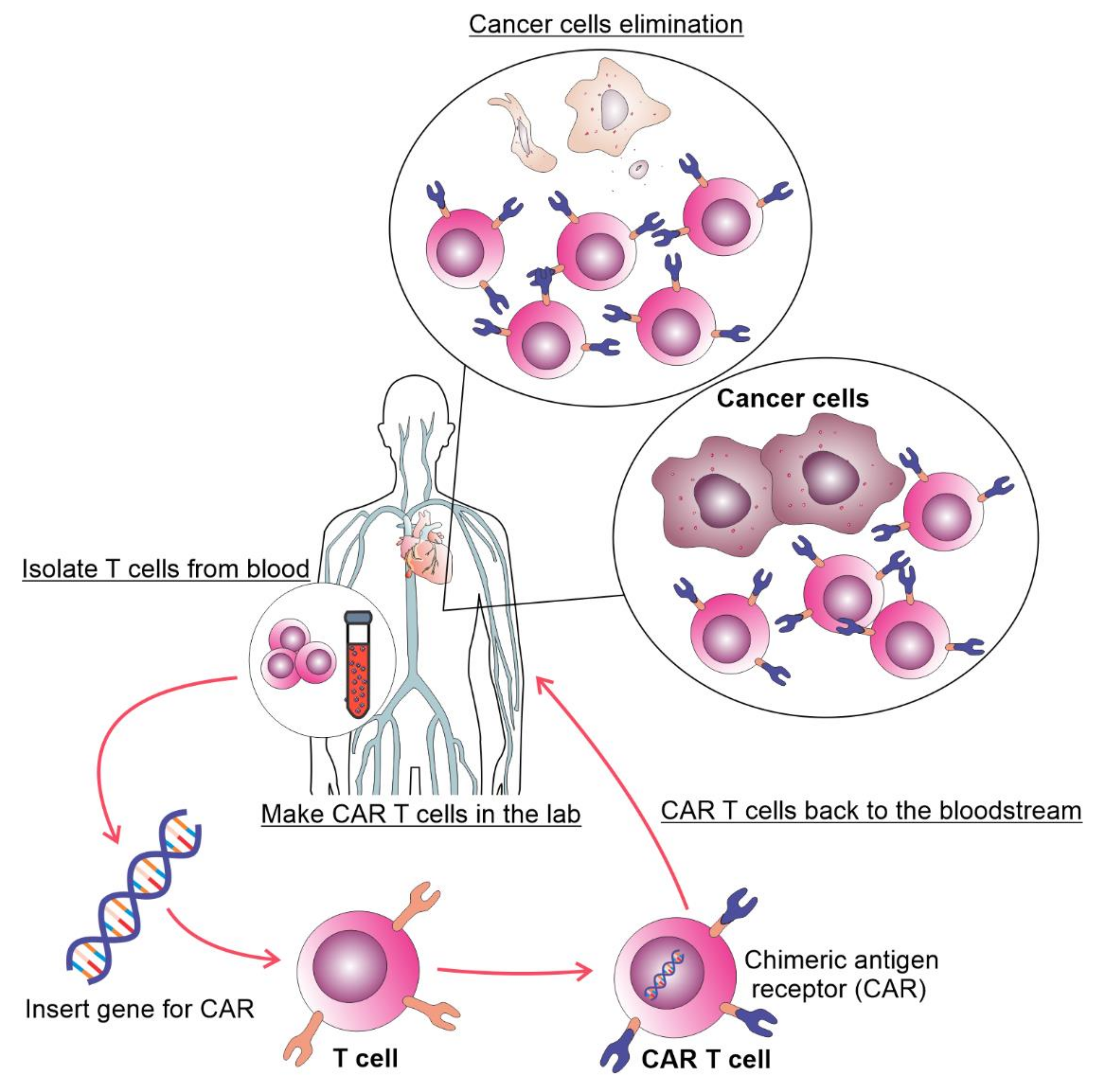
Figure 1. The process of autologous CAR T cell therapy. T cells are collected via apheresis, genetically reengineered in a laboratory by introducing DNA that encodes CAR, multiplied, and then infused into the patient. The CAR T cells may eliminate all of the cancer cells and may remain in the body months after the infusion.
According to the T-cell activation domain, which is part of the hybrid receptor, CAR T cells are classified into four generations. The first generation of CAR T cells contains only CD3 zeta domain in fusion with receptors specific for a specified tumor antigen. These first generation CAR T cells showed limited clinical effects. Hybrid receptors of the second generation of CAR T cells in addition to CD3 contain one of the additional costimulatory molecules such as CD28, ICOS, CD-137/4-1BB or OX40, while coupling two or more costimulatory molecules into the receptor sequence led to the creation of the third generation CAR T cells [8]. The fourth CAR T cells’ generation is engineered with the inducible expression of cytokines that potentiate antitumor immunity and a self-withdrawal mechanism, with a suicide gene that can be activated after the achievement of the anti-tumor effect whose product rapidly withdraws CAR T cells [9]. The good clinical responses to CAR T cell therapy in some malignancies are frequently accompanied by several obstacles (Figure 2). Since most of the patients are highly medicated resulting in a low number and quality of T cells, manufacturing and expanding autologous CAR T cells from such patients can be difficult [10]. The manufacture of allogenic CAR T cells from healthy donors is promising but allogenic CAR T cells can cause a serious graft-versus-host disease (GvHD) [11]. However, the fact that allogenic NK cells have reduced the risk for induction of GvDH led to the construction of CAR Natural Killer (NK) cells. CAR constructs in CAR NK cells play the role in the NK cell activation and also improve the efficiency of innate ability of NK cells to kill malignant cells [12].
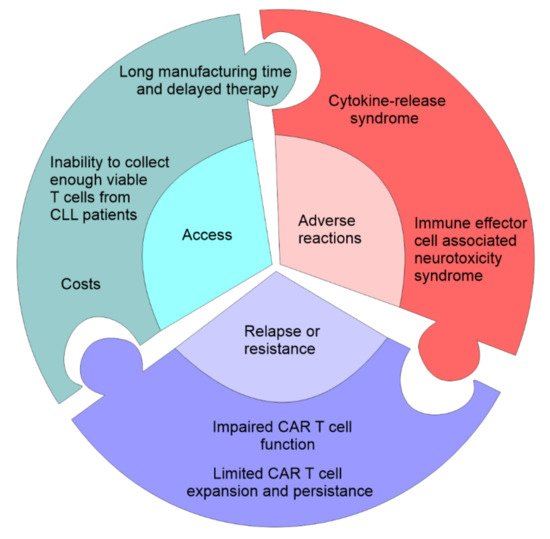
Figure 2. Challenges in CAR T-cell therapy. The first problem associated with CAR T cell therapy is access which can be limited by the cost of the manufacture of CAR T cells, time needed to manufacture and expand CAR T cells, failure to produce autologous CAR T cells, and eligibility for the clinical trial. The second problem associated with CAR T cell therapy are serious adverse event: cytokine-release storm (widespread pyroptosis of tumor cells is followed by the release of different factors that activate macrophages to produce inflammatory cytokines that induce systemic inflammatory response), and neurotoxicity syndrome (toxic encephalopathy caused by the disruption of the blood-brain-barrier). CAR T cell therapy may be accompanied by the primary resistance due to the impaired CAR T cell function and the inability to induce remission and limited CAR T cell expansion.
Although the use of cellular immunotherapy in many hematologic malignancies is already approved outside clinical trials [13,14[13][14][15][16],15,16], the benefit of CAR T and CAR NK cells in CLL treatment is still not clear.
2. CAR-T as Promising CLL Therapy
In the early stage of development of CAR-T cells for B cell leukemia, CD19 has become attractive target antigen because it is expressed on the most B-cell malignancies [17]. Results of preclinical studies indicate better in vivo expansion and far greater effectiveness of the second generation of CAR-T cells, with CARs constructed to target CD19 coupled with CD137 signaling or CD28 costimulatory domain, in comparison to the first generation of CAR T cells [18,19][18][19]. The first use of CAR T cells in the therapy of CLL was reported in 2011 [20]. Porter et al. reported the treatment of two patients with refractory/relapsed CLL with reinfusion of approximately 1.5 × 105 autologous CAR T cells per kilogram of body weight that expanded to a level that was more than 1000 times higher than the initial engraftment level in vivo [20]. After treatment, patients achieved complete remission (CR) [20]. Further, it has been recently reported that both patients sustained remission for more than ten years after the therapy and that CAR T cells are still detectable in their blood [21]. These long-persisting CAR T cells are CD4+ cells with cytotoxic capabilities, but also an expanded population of gamma delta CAR T cells has been found in one patient concomitant with CD8+ CAR T cells during the initial response phase [21].
So far, over 100 CLL patients have been treated with anti-CD19 CAR-T cells (Table 1). The majority of studies included relapsed patients or patients refractory to conventional therapy regimens, except for one which enrolled patients with only a partial response (PR) to the first line therapy [22]. Overall response rate (ORR; sum of CR and PR) varies between studies. Brentjens et al. suggested that chemotherapy that induced the depletion of lymphocytes prior to CAR T infusion enhanced the efficacy of CAR T cells [23]. In line with this are the results of the studies reporting the lowest ORR in patients without lymphodepletion therapy prior to CAR T infusion [23,24,25,26][23][24][25][26]. It is considered that lymphodepletion chemotherapy reduced tumor mass but also the number of regulatory cells, which may attenuate the antitumor activity of the infused CAR T cells.
Table 1. CAR T cells trials in CLL. Abbreviation: ORR—overall response rate, CR—complete response, CLL—chronic lymphocytic leukemia.
| Study (Reff. Number) | Number of CLL Patients | Target Antigen | Costimulatory Domain | ORR (%) | CR (%) |
|---|---|---|---|---|---|
| CAR-T | |||||
| [23] | 8 | CD19 | CD28 | 0 | 0 |
| [27] | 3 | CD19 | 4–1BB | 100 | 67 |
| [28] | 4 | CD19 | CD28 | 75 | 25 |
| [24] | 4 | CD19 | CD28 | 25 | 0 |
| [29] | 5 | CD19 | CD28 | 100 | 60 |
| [30] | 14 | CD19 | 4–1BB | 57 | 29 |
| [25] | 5 | CD19 | CD28 | 40 | 20 |
| [26] | 2 | IgKappa | CD28 | 0 | 0 |
| [31] | 32 | CD19 | 4–1BB | 44 | 28 |
| [13] | 24 | CD19 | 4–1BB | 71 | 17 |
| [22] | 8 | CD19 | CD28 | 75 | 25 |
| [32] | 19 | CD19 | 4–1BB | 53 | 53 |
| [33] | 10 | CD19 | 4–1BB | 60 | 40 |
| [34] | 19 | CD19 | 4–1BB | 79 | 21 |
| [35] | 3 | CD19 | 4–1BB | 100 | 67 |
Another factor that could influence response to therapy is a number of CLL T applied to patients. One recent study has shown that a higher dose of anti-CD19 CAR T cells (5.0 × 108 vs. 5.0 × 107) produces higher rates of ORR (55% vs. 31%) and CR (36% vs. 8%) [31].
References
- Hodgson, K.; Ferrer, G.; Montserrat, E.; Moreno, C. Chronic lymphocytic leukemia and autoimmunity: A systematic review. Haematologica 2011, 96, 752–761.
- Noone, A.M.; Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2015; Based on November 2017 SEER Data Submission, Posted to the SEERWeb Site; National Cancer Institute: Bethesda, MD, USA, 2018.
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. ESMO Guidelines Committee. Electronic address: . Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 23–33.
- Mimmi, S.; Maisano, D.; Nisticò, N.; Vecchio, E.; Chiurazzi, F.; Ferrara, K.; Iannalfo, M.; D’Ambrosio, A.; Fiume, G.; Iaccino, E.; et al. Detection of chronic lymphocytic leukemia subpopulations in peripheral blood by phage ligands of tumor immunoglobulin B cell receptors. Leukemia 2021, 35, 610–614.
- Barrett, D.M.; Grupp, S.A.; June, C.H. Chimeric antigen receptor-and TCR-modified T cells enter main street and wall street. J. Immunol. 2015, 195, 755–761.
- Fujiwara, H. Adoptive immunotherapy for hematological malignancies using T cells gene-modified to express tumor antigen-specific receptors. Pharmaceuticals 2014, 7, 1049–1068.
- Chmielewski, M.; Hombach, A.A.; Abken, H. Antigen-specific T-cell activation independently of the MHC: Chimeric antigen receptor-redirected T cells. Front. Immunol. 2013, 4, 371.
- Srivastava, S.; Riddell, S.R. Engineering CAR-T cells: Design concepts. Trends Immunol. 2015, 36, 494–502.
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154.
- Mancikova, V.; Smida, M. Current State of CAR T-Cell Therapy in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2021, 22, 5536.
- Frey, N.V.; Porter, D.L. Graft-versus-host disease after donor leukocyte infusions: Presentation and management. Best Pract. Res. Clin. Haematol. 2008, 21, 205–222.
- Corral Sánchez, M.D.; Fernández Casanova, L.; Pérez-Martínez, A. Beyond CAR-T cells: Natural killer cells immunotherapy. Med. Clin. 2020, 154, 134–141.
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 449–459.
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42.
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2019, 380, 45–56.
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342.
- Scheuermann, R.H.; Racila, E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma 1995, 18, 385–397.
- Carpenito, C.; Milone, M.C.; Hassan, R.; Simonet, J.C.; Lakhal, M.; Suhoski, M.M.; Varela-Rohena, A.; Haines, K.M.; Heitjan, D.F.; Albelda, S.M.; et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA 2009, 106, 3360–3365.
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Campana, D.; et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009, 17, 1453–1464.
- Porter, D.L.; Levine, B.L.; Kalos, M.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011, 365, 725–733.
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022, 602, 503–509.
- Geyer, M.B.; Rivière, I.; Sénéchal, B.; Wang, X.; Wang, Y.; Purdon, T.J.; Hsu, M.; Devlin, S.M.; Halton, E.; Lamanna, N.; et al. Autologous CD19-targeted CAR T cells in patients with residual CLL following initial purine analog-based therapy. Mol. Ther. 2018, 26, 1896–1905.
- Brentjens, R.J.; Rivière, I.; Park, J.H.; Wang, X.; Wang, Y.; Purdon, T.J.; Hsu, M.; Devlin, S.M.; Halton, E.; Lamanna, N.; et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011, 118, 4817–4828.
- Cruz, C.R.Y.; Micklethwaite, K.P.; Savoldo, B.; Ramos, C.A.; Lam, S.; Ku, S.; Diouf, O.; Liu, E.; Barrett, A.J.; Ito, S.; et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: A phase 1 study. Blood 2013, 122, 2965–2973.
- Brudno, J.N.; Somerville, R.P.; Shi, V.; Rose, J.J.; Halverson, D.C.; Fowler, D.H.; Gea-Banacloche, J.C.; Pavletic, S.Z.; Hickstein, D.D.; Lu, T.; et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 2016, 34, 1112–1121.
- Ramos, C.A.; Savoldo, B.; Torrano, V.; Ballard, B.; Zhang, H.; Dakhova, O.; Liu, E.; Carrum, G.; Kamble, R.T.; Gee, A.P.; et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J. Clin. Investig. 2016, 126, 2588–2596.
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.; Grupp, S.A.; Bagg, A.; June, C.H. T Cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011, 3, 95ra73.
- Kochenderfer, J.N.; Dudley, M.E.; Feldman, S.A.; Wilson, W.H.; Spaner, D.E.; Maric, I.; Stetler-Stevenson, M.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood 2012, 119, 2709–2720.
- Kochenderfer, J.N.; Dudley, M.E.; Kassim, S.H.; Somerville, R.P.; Carpenter, R.O.; Stetler-Stevenson, M.; Yang, J.C.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. Chemotherapy-refractory diffuse large B-Cell lymphoma and indolent b-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015, 33, 540–549.
- Porter, D.L.; Hwang, W.T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015, 7, 303ra139.
- Frey, N.V.; Gill, S.; Hexner, E.O.; Schuster, S.; Nasta, S.; Loren, A.; Svoboda, J.; Stadtmauer, E.; Landsburg, D.J.; Mato, A.; et al. Long-term outcomes from a randomized dose optimization study of chimeric antigen receptor modified T cells in relapsed chronic lymphocytic leukemia. J. Clin. Oncol. 2020, 38, 2862–2871.
- Gill, M.S.I.; Vides, B.V.; Frey, N.V.; Metzger, S.; O’Brien, M.; Hexner, E.; Mato, A.R.; Lacey, S.F.; Melenhorst, J.; Pequignot, E.; et al. Prospective clinical trial of anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia shows a high response rate. Blood 2018, 132, 298.
- Siddiqi, T.; Soumerai, J.D.; Wierda, W.G.; Dubovsky, J.A.; Gillenwater, H.H.; Gong, L.; Mitchell, A.; Thorpe, J.; Yang, L.; Dorritie, K.A. Rapid MRD-negative responses in patients with relapsed/refractory CLL Treated with Liso-Cel, a CD19-directed CAR T-cell product: Preliminary results from transcend CLL 004, a phase 1/2 study including patients with high-risk disease previously treated with ibrutinib. Blood 2018, 132, 300.
- Gauthier, J.; Hirayama, A.V.; Purushe, J.; Hay, K.A.; Lymp, J.; Li, D.H.; Yeung, C.C.S.; Sheih, A.; Pender, B.S.; Hawkins, R.; et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood 2020, 135, 1650–1660.
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; Krueger, W.; Worden, A.A.; Kadan, M.J.; Yim, S.; et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial. Nat. Med. 2020, 26, 1569–1575.
More
