Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by SAIFUL MALOOK.
Molecular elicitors are the key bio-elements in the detection and recognition of attacking enemies in tissue consumption. Insect oral secretion, frass, and fluid of egg deposition contain biologically active molecules called herbivore-associated elicitors (HAEs) that are recognized by pattern-recognition receptors (PRRs). Many plants distinguish insect feeding from wounding by HAEs present in their oral secretions (OS) and induce local and/or systemic responses against arthropod feeding.
- herbivore-associated elicitors
- pattern-recognition receptors
- plant defense
1. Introduction
As sessile organisms, plants cannot escape from herbivore arthropods and are substantially challenged by insect herbivores. Over millions of years of coevolution with insects, plants have evolved exquisite defense mechanisms to fend off insect herbivory on plants [1]. The recognition of herbivore attacks requires the ability of plants to detect chemical cues (Herbivore-associated elicitors; HAEs) generated by insects during infestation, and these receptors are also called receptor kinases (RKs). Plants distinguish insect feeding from wounding by recognizing specific conserved molecules present in their oral secretions (OS; shown in Figure 1) [2,3,4,5][2][3][4][5]. In literature, based on plant-insect interactions, few reports have revealed that OS constituents depend on the insect feeding of host plants and their associated microbes [6].
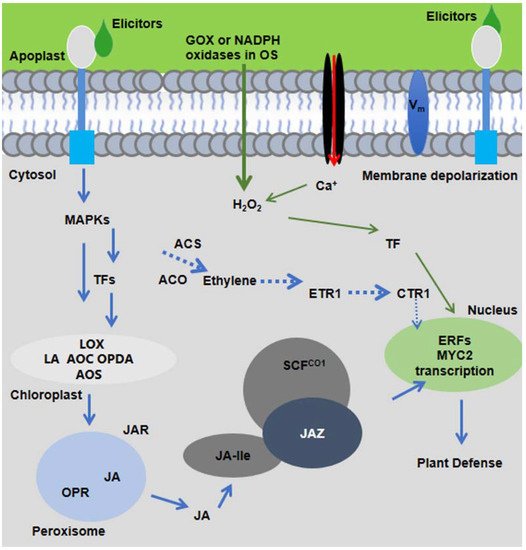
 Figure 1. The molecular signaling model of plant response to insect herbivory. HAEs from oral secretion (OS) of insect herbivores are perceived by plant receptors present in plasma membrane. Within minutes of herbivore feeding, short signaling molecules, such as ROS, Ca2+, MAPK signaling, and membrane depolarization (Vm), are activated and elicit the JA-Ile production. JA-Ile binds with SCFCOI1 and triggers the degradation of JAZ and activates downstream plant defenses.
Figure 1. The molecular signaling model of plant response to insect herbivory. HAEs from oral secretion (OS) of insect herbivores are perceived by plant receptors present in plasma membrane. Within minutes of herbivore feeding, short signaling molecules, such as ROS, Ca2+, MAPK signaling, and membrane depolarization (Vm), are activated and elicit the JA-Ile production. JA-Ile binds with SCFCOI1 and triggers the degradation of JAZ and activates downstream plant defenses.During insect herbivory, deposition of OS on the wounds causes manipulation of plant responses against insect herbivores by changing plant metabolism and gene expression [4,7][4][7]. HAEs in nature are diverse in structure and exist in the form of enzymes [e.g., glucose oxidase (GOX) and β-glucosidase], lipids [fatty acid-amino acid conjugates (FACs) such as volicitin and caeliferins], cell wall fragments (e.g., pectin and oligogalacturonides), and plant peptides (e.g., inceptin: proteolytic fragments of the chloroplastic ATP synthase subunit), but none of them were found to affect the induced defenses of tomato [4,8,9,10][4][8][9][10]. The HAEs are not general elicitors in all plants, and plant responses to insect herbivores are restricted to plant-insect associations that depend upon the specific mode of feeding style of insects [7,11,12][7][11][12]. This specificity reflects the evolutionary history of both plants and insects living and surviving together in nature, and it is important to understand the mechanism of plant-elicitors interactions in an evolutionary context [4]. Herbivore-induced defenses are mediated by signaling molecules and are employed to maintain crop resilience during insect herbivory [12,13,14][12][13][14]. Thus, despite the need for a clear understanding of induced responses, plant receptor interactions with their HAEs remain an emerging research topic in plant-insect interaction.
Upon the recognition of insect elicitors, plants activate defense responses by triggering calcium ion influx (Ca2+), plasma membrane depolarization (Vm), mitogen-activated protein kinases (MAPKs), NADPH oxidase, production of reactive oxygen species (ROS), and activation of nitrogen species (NO) [15]. The molecular signaling cascades elicit the production of defense hormones, mainly jasmonic acid (JA), ethylene, and salicylic acid (SA) as well as transcription factors (TFs). Defense hormone, especially JA, is the central component to regulate downstream defense metabolites including but not limited to glucosinolates, benzoxazinoids (Bxs), cyanogenic glucosides, alkaloids, phenolics, and proteinase inhibitors in damaged and systemic leaves, as shown in Figure 1 [13].
2. Plant and Insect Origin Elicitors
Plants are exposed to biotic stresses by microbes, insects, and animal feeding. To fend off insect herbivory, plants have adapted responses and recognition systems that depend on specific HAEs. HAEs take part in signaling pathways and can activate the defense reaction system in plants. Apart from components of OS, HAEs originate in bacteria, caterpillar frass, the oviposition fluid, and some insect pheromone compounds that can either disrupt or induce plant defenses [55][16]. In other words, many molecules in OS can cause the plant to manipulate its defense response, involving enzymes such as glucose oxidase and β-glycosidase, peptides such as inceptin, and fatty acid conjugates such as volicitin (Table 1) [4,6,56][4][6][17]. However, as time passes, some plants can overcome this inhibition when they have adapted themselves to recognize the molecules from the insect [57][18]. Therefore, fatty acid-amino acid conjugates (FACs), or fatty acid amides, were one of the first types identified as an elicitor in the saliva of insects [10]. A two-pronged methodology to study FACs in M. sexta exhibited increased indirect defense response in a host plant by the inducing the volatiles organic compounds (VOCs) and attracting the predators [58][19]. Since the initial discovery, other types of elicitors have been identified, with their specific molecular activity varying greatly between plant species [46][20]. Furthermore, inceptins and caeliferins in oral secretions activate insect defensive pathways [59][21]. Moreover, in previous studies, the induction of defense signaling has been reported in response to the presence of glucose oxidase (GOX) in insect saliva, for example, the Proteinase Inhibitor 2 (PIN2) produced by the salivary component of Ostrinia nubilalis induces in maize and tomato [60,61][22][23]. However, some OS inhibit the defense pathway in plants. According to the literature, it has been observed in the larval stages of S. littoralis and P. brassicae, where salivary secretions inhibited defense to allow larvae to grow [62][24]. As a result, depending on which organism oversees the evolutionary process at that time, the plant or the insect, these molecules can either activate or repress plant defense responses, respectively. Table 1. The list of HAEs and their known receptors against insect herbivory.
| Elicitors | Receptors | Source of Elicitors | Host Plant | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| DNA | n.d. | These elicitors are of plant source | Bean, maize | [25] | |||||
| Pep | Pep receptor (PEPR) | Maize | [26,27] | [26][27] | |||||
| ATP | ATP receptors (DORN1/P2K1) | Arabidopsis | [28] | ||||||
| Systemin | Systemin receptor (SYR1) | Tomato | [29] | ||||||
| FACs (volicitin) | Unknown membrane proteins | Spodoptera exigua | Maize | [10] | |||||
| β | -Glucosidase | n.d. | Pieris brassicae | Maize | [30] | ||||
| Caeliferins | n.d. | Schistocerca americana | Maize | [31] | |||||
| Inceptin | Inceptin receptor (INR) | Spodoptera frugiperda | Maize | [9] | |||||
| Lipase | n.d. | Schistocerca gregaria | Arabidopsis | [32] | |||||
| Porin-like proteins | n.d. | Spodoptera littoralis | Arabidopsis | [33] | |||||
| β | -Galactofuranose polysaccharide | HAK/PBL27 | Spodoptera | spp. | Arabidopsis | [34] | |||
| Bruchins | n.d. | Bruchus pisorum | , | Nilaparvata lugens | Cowpea, pea | [35] | |||
| Glucose oxidase | n.d. | Helicoverpa zea | , | Spodoptera exigua | , | Helicoverpa armigera | Nicotiana | [36,37] | [36][37] |
| Mucin-like protein | n.d. | Callosobruchus maculatus | Rice | [38] | |||||
| Oligouronides | n.d. | Produced by breakdown of plant cell walls by insect feeding | Tomato | [39] |
n.d. = not detected.
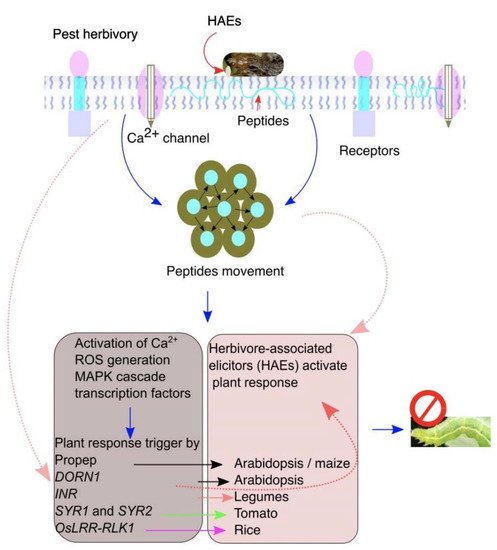
 Figure 2. The plant pattern-recognition receptors (PRRs) recognize herbivore-associated elicitors (HAEs) to elicit plant responses against insect herbivory. A plant perceives and detects herbivory by recognizing HAEs on insect feeding. Damaged cells release intracellular molecules that move into the apoplast and to the undamaged neighboring cells to induce the plant responses. Insect feeding induces endogenous peptides that bind with HAEs to elicit the downstream plant defenses.
Figure 2. The plant pattern-recognition receptors (PRRs) recognize herbivore-associated elicitors (HAEs) to elicit plant responses against insect herbivory. A plant perceives and detects herbivory by recognizing HAEs on insect feeding. Damaged cells release intracellular molecules that move into the apoplast and to the undamaged neighboring cells to induce the plant responses. Insect feeding induces endogenous peptides that bind with HAEs to elicit the downstream plant defenses.
3. Elicitors of Plants’' Intracellular Products
The simplest form of plant elicitors is the intracellular products released upon leaf damage by insect feeding [63][40]. The intracellular liquid moves to the apoplast and is recognized by DORN1/P2K1 in neighboring, undamaged cells and activates ATP-induced Ca2+ defenses (Figure 2 and Table 1) [28]. In tomatoes, degradation activity of adenosine-5-triphosphate (ATP) was detected in Helicoverpa zea OS assay with tomato leaf fluid. On the other hand, salivary glands of H. zea secrete apyrase and ATP-hydrolyzing enzymes that interfere with ATP signaling and suppress defense-related genes in tomatoes [64][41]. During insect feeding, regurgitation on leaves provides signaling cues recognized by plants. For example, lignocellulose deposited in the herbivore gut during feeding and digested products can be recognized by receptors in Arabidopsis upon e digestion, functioning as elicitors. However, it is not clear whether these compounds are gut-derived or are plant cell wall degradation products [2,65][2][42]. In maize and lima bean, insect feeding produces extracellular self-DNA (esDNA), and plants exposed to esDNA and extracellular heterologous DNA increased plasma membrane potential (Vm) and calcium flux (Ca2+), confirming that esDNA trigger plant responses [25]. Whether the perception of esDNA requires specific receptors other than insect herbivory is another interesting research question to answer.
 Figure 2. The plant pattern-recognition receptors (PRRs) recognize herbivore-associated elicitors (HAEs) to elicit plant responses against insect herbivory. A plant perceives and detects herbivory by recognizing HAEs on insect feeding. Damaged cells release intracellular molecules that move into the apoplast and to the undamaged neighboring cells to induce the plant responses. Insect feeding induces endogenous peptides that bind with HAEs to elicit the downstream plant defenses.
Figure 2. The plant pattern-recognition receptors (PRRs) recognize herbivore-associated elicitors (HAEs) to elicit plant responses against insect herbivory. A plant perceives and detects herbivory by recognizing HAEs on insect feeding. Damaged cells release intracellular molecules that move into the apoplast and to the undamaged neighboring cells to induce the plant responses. Insect feeding induces endogenous peptides that bind with HAEs to elicit the downstream plant defenses.4. Elicitors in OS of Insects
Among well-known HAEs, fatty acid–amino acid conjugates (FACs) are a best-studied group of elicitors, which trigger defense responses upon herbivore feeding in many plant species, including maize, soybean, eggplant, and tobacco [4,10,46][4][10][20]. A maize elicitor, volicitin, a hydroxyl FAC [N-(17-hydroxylinolenoyl)-L-glutamine], was isolated from the OS of S. exigua larvae by Alborn and his colleagues in 1997. Applying the volicitin onto the wounds of maize leaves elicits the emission of an increased level of volatiles and attracts parasitic wasps, natural enemies of S. exigua. Wounding without the application of volicitin did not emit the blend of volatiles that attract the natural enemies of herbivores [10]. Since the discovery of volicitin, several FACs have been found in OS of lepidopteran species, and their biological functions are well studied in N. attenuate. Manduca sexta larvae induced increased accumulation of MAPKs, JA, ethylene biosynthesis genes, metabolome, and transcriptome reprogramming in infested and systemic leaves [72,73,74,75,76,77][43][44][45][46][47][48]. Wounding elicits the increased transcript level of transcription factor (TF) WRKY3, and applying the FACs into the wounds of nicotiana leaves caused increased WRKY6 transcript accumulation. Importantly, WRKY3 is required for the elicitation of WRKY6, and silencing of either gene made plants susceptible to herbivores [75][46]. Manduca sexta herbivory induced high levels of salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK). Silencing of SIPK and WIPK showed decreased defense levels, but M. sexta larvae grown on transgenic plants did not show a difference compared to wild-type plants.
However, green leaf volatiles (GLVs) were attenuated in both transgenic plants and wild-type plants, and the addition of synthetic GLVs restore the increased M. sexta performance in transgenic plants [74][45]. Glucose oxidase (GOX) is the main component of OS in H. zea, which acts as a salivary protein to suppress the herbivore-induced plant responses in Nicotiana tabacum (tobacco) [36]. In response to larval herbivory, Medicago trancatula (alfalfa) responds by saponins and terpenoid production. Spodoptera exigua (Beat armyworm) suppressed the transcript accumulation of saponins biosynthetic genes for terpenoid production. Researchers hypothesized that GOX may involve in the suppression of gene expression following insect feeding. Further experiments by comparing wounding, the addition of GOX into wounds, and insect feeding confirmed the function of GOX in suppressing defense responses in herbivore attacks [78][49].
Chemical analysis of the OS in S. exigua indicated that FACs are composed of fatty acid (linoleic acid (LA)/linolenic acid), which is plant originated and insect-derived amino acid (Glu/Gln). Interestingly, 17 hydroxylation and conjugation occur in the midgut of insects, which is important for the biological activity that emits plant volatiles to attract natural enemies of herbivores [79][50]. Feeding experiments with radio-labeled glutamine, glutamic acid, and linolenic acid to S. litura caterpillars revealed that FACs are involved in nitrogen assimilation and function as glutamine storage in the insect. Glutamine is the main component in insect nitrogen metabolism, and hence, it is not possible for caterpillars to stop production of FACs when feeding on plants, even though plants perceive caterpillar feeding in the presence of FACs [80][51].
In addition to FAC, there are several other elicitors reported in insect OS. Inceptin, a proteolytic fragment of the chloroplastic ATP synthase γ-subunit, an elicitor isolated in OS of S. frugiperda, perceives the insect herbivory and enhances production of ethylene as well as increases accumulation of phenylpropanoid, VOCs, and protease inhibitor in Vigna unguiculata (cowpea) [9]. Comparing treatment with FAW, OS of Anticarsia gemmatalis, a legume specialist herbivore (Velvetbean caterpillar; VBC), did not induce large production of ethylene and direct herbivory to induce a smaller level of predominant volatile (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT). The examination of OS in VBC for the discovery of truncated form of inceptin in VBC suggested that truncated form of inceptin may be recognized by PPRs and thus suppress the plant defense [81][52].
References
- Wu, J.Q.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24.
- Arimura, G.I. Making Sense of the Way Plants Sense Herbivores. Trends Plant Sci. 2021, 26, 288–298.
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329.
- Bonaventure, G.; VanDoorn, A.; Baldwin, I.T. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011, 16, 294–299.
- Reymond, P. Receptor kinases in plant responses to herbivory. Curr. Opin. Biotechnol. 2021, 70, 143–150.
- Kallure, G.S.; Kumari, A.; Shinde, B.A.; Giri, A.P. Characterized constituents of insect herbivore oral secretions and their influence on the regulation of plant defenses. Phytochemistry 2022, 193, 113008.
- Felton, G.W.; Tumlinson, J.H. Plant-insect dialogs: Complex interactions at the plant-insect interface. Curr. Opin. Plant Biol. 2008, 11, 457–463.
- Mattiacci, L.; Dicke, M.; Maartin, A.P. β-Glucosidase- an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 2036–2040.
- Schmelz, E.A.; Carroll, M.J.; LeClere, S.; Phipps, S.M.; Meredith, J.; Chourey, P.S.; Alborn, H.T.; Teal, P.E. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. USA 2006, 103, 8894–8899.
- Alborn, H.T.; Turlings, T.C.J.; Jones, T.H.; Stenhagen, G.; Loughrin, J.H.; Tumlinson, J.H. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–949.
- Acevedo, F.E.; Rivera-Vega, L.J.; Chung, S.H.; Ray, S.; Felton, G.W. Cues from chewing insects—The intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 2015, 26, 80–86.
- Schmelz, E.A. Impacts of insect oral secretions on defoliation-induced plant defense. Curr. Opin. Insect Sci. 2015, 9, 7–15.
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557.
- Chen, C.-Y.; Mao, Y.-B. Research advances in plant-insect molecular interaction. F1000Research 2020, 9.
- Zebelo, S.A.; Maffei, M.E. Role of early signalling events in plant-insect interactions. J. Exp. Bot. 2015, 66, 435–448.
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963.
- Wu, J.; Baldwin, I.T. Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 2009, 32, 1161–1174.
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428.
- Gaquerel, E.; Weinhold, A.; Baldwin, I.T. Molecular Interactions between the Specialist Herbivore Manduca sexta (Lepidoptera, Sphigidae) and Its Natural Host Nicotiana attenuata. VIII. An Unbiased GCxGC-ToFMS Analysis of the Plant’s Elicited Volatile Emissions. Plant Physiol. 2009, 149, 1408–1423.
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; Tumlinson, J.H.; Teal, P.E.A. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. USA 2009, 106, 653–657.
- Rodriguez, P.A.; Bos, J.I. Toward understanding the role of aphid effectors in plant infestation. Mol. Plant Microbe Interact. 2013, 26, 25–30.
- Tian, D.; Peiffer, M.; Shoemaker, E.; Tooker, J.; Haubruge, E.; Francis, F.; Luthe, D.S.; Felton, G.W. Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS ONE 2012, 7, e36168.
- Louis, J.; Shah, J. Arabidopsis thaliana–Myzus persicae interaction: Shaping the understanding of plant defense against phloem-feeding aphids. Front. Plant Sci. 2013, 4, 213.
- Consales, F.; Schweizer, F.; Erb, M.; Gouhier-Darimont, C.; Bodenhausen, N.; Bruessow, F.; Sobhy, I.; Reymond, P. Insect oral secretions suppress wound-induced responses in Arabidopsis. J. Exp. Bot. 2011, 63, 727–737.
- Barbero, F.; Guglielmotto, M.; Capuzzo, A.; Maffei, M.E. Extracellular Self-DNA (esDNA), but Not Heterologous Plant or Insect DNA (etDNA), Induces Plasma Membrane Depolarization and Calcium Signaling in Lima Bean (Phaseolus lunatus) and Maize (Zea mays). Int. J. Mol. Sci. 2016, 17, 1659.
- Huffaker, A.; Pearce, G.; Veyrat, N.; Erb, M.; Turlings, T.C.J.; Sartor, R.; Shen, Z.X.; Briggs, S.P.; Vaughan, M.M.; Alborn, H.T.; et al. Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense. Proc. Natl. Acad. Sci. USA 2013, 110, 5707–5712.
- Yamaguchi, Y.; Huffaker, A.; Bryan, A.C.; Tax, F.E.; Ryan, C.A. PEPR2 Is a Second Receptor for the Pep1 and Pep2 Peptides and Contributes to Defense Responses in Arabidopsis. Plant Cell 2010, 22, 508–522.
- Choi, J.; Tanaka, K.; Yangrong, C.; Yue, Q.; Qiu, J.; Liang, Y.; Yeol Lee, S.; Stacey, G. Identification of a Plant Receptor for Extracellular ATP. Science 2014, 343, 290–294.
- Wang, L.; Einig, E.; Almeida-Trapp, M.; Albert, M.; Fliegmann, J.; Mithofer, A.; Kalbacher, H.; Felix, G. The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat. Plants 2018, 4, 152–156.
- Hopke, J.; Donatha, J.; Blechertb, S.; Boland, W. Herbivore-induced volatiles—The emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a b-glucosidase and jasmonic acid. FEBS Lett. 1994, 352, 146–150.
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H.; Schmelz, E.A.; Teal, P.E.A. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA 2007, 104, 12976–12981.
- Schafer, M.; Fischer, C.; Meldau, S.; Seebald, E.; Oelmuller, R.; Baldwin, I.T. Lipase Activity in Insect Oral Secretions Mediates Defense Responses in Arabidopsis. Plant Physiol. 2011, 156, 1520–1534.
- Guo, H.J.; Wielsch, N.; Hafke, J.B.; Svatos, A.; Mithofer, A.; Boland, W. A porin-like protein from oral secretions of Spodoptera littoralis larvae induces defense-related early events in plant leaves. Insect Biochem. Mol. Biol. 2013, 43, 849–858.
- Bricchi, I.; Occhipinti, A.; Bertea, C.M.; Zebelo, S.A.; Brillada, C.; Verrillo, F.; De Castro, C.; Molinaro, A.; Faulkner, C.; Maule, A.J.; et al. Separation of early and late responses to herbivory in Arabidopsis by changing plasmodesmal function. Plant J. 2013, 73, 14–25.
- Doss, R.P.; Oliver, J.E.; Proebsting, W.M.; Potter, S.W.; Kuy, S.R.; Clement, S.L.; Williamson, R.T.; Carney, J.R.; DeVilbiss, E.D. Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc. Natl. Acad. Sci. USA 2000, 97, 6218–6223.
- Musser, R.O.; Hum-Musser, S.M.; Eichenseer, H.; Peiffer, M.; Ervin, G.; Murphy, J.B.; Felton, G.W. Caterpillar saliva beats plant defences. Nature 2002, 416, 599–600.
- Diezel, C.; von Dahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different Lepidopteran Elicitors Account for Cross-Talk in Herbivory-Induced Phytohormone Signaling. Plant Physiol. 2009, 150, 1576–1586.
- Shangguan, X.X.; Zhang, J.; Liu, B.F.; Zhao, Y.; Wang, H.Y.; Wang, Z.Z.; Guo, J.P.; Rao, W.W.; Jing, S.L.; Guan, W.; et al. A Mucin-Like Protein of Planthopper Is Required for Feeding and Induces Immunity Response in Plants. Plant Physiol. 2018, 176, 552–565.
- Doares, S.H.; Syrovets, T.; Weiler, E.W.; Ryan, C.A. Oligogalacturonides and Chitosan Activate Plant Defensive Genes through the Octadecanoid Pathway. Proc. Natl. Acad. Sci. USA 1995, 92, 4095–4098.
- Jeter, C.R.; Tang, W.Q.; Henaff, E.; Butterfield, T.; Roux, S.J. Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 2004, 16, 2652–2664.
- Wu, S.; Peiffer, M.; Luthe, D.S.; Felton, G.W. ATP Hydrolyzing Salivary Enzymes of Caterpillars Suppress Plant Defenses. PLoS ONE 2012, 7, e41947.
- Vadassery, J.; Reichelt, M.; Mithofer, A. Direct Proof of Ingested Food Regurgitation by Spodoptera littoralis Caterpillars during Feeding on Arabidopsis. J. Chem. Ecol. 2012, 38, 865–872.
- Giri, A.P.; Wunsche, H.; Mitra, S.; Zavala, J.A.; Muck, A.; Svatos, A.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant’s proteome. Plant Physiol. 2006, 142, 1621–1641.
- Halitschke, R. Molecular Interactions between the Specialist Herbivore Manduca sexta (Lepidoptera, Sphingidae) and Its Natural Host Nicotiana attenuata. III. Fatty Acid-Amino Acid Conjugates in Herbivore Oral Secretions Are Necessary and Sufficient for Herbivore-Specific Plant Responses. Plant Physiol. 2001, 125, 711–717.
- Meldau, S.; Wu, J.; Baldwin, I.T. Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata’s susceptibility to herbivores in the glasshouse and in nature. New Phytol. 2009, 181, 161–173.
- Skibbe, M.; Qu, N.; Galis, I.; Baldwin, I.T. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell 2008, 20, 1984–2000.
- Wu, J.; Hettenhausen, C.; Meldau, S.; Baldwin, I.T. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 2007, 19, 1096–1122.
- Pohnert, G.; Jung, V.; Haukioja, E.; Lempa, K.; Boland, W. New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron 1999, 55, 11275–11280.
- Bede, J.C.; Musser, R.O.; Felton, G.W.; Korth, K.L. Caterpillar herbivory and salivary enzymes decrease transcript levels of Medicago truncatula genes encoding early enzymes in terpenoid biosynthesis. Plant Mol. Biol. 2006, 60, 519–531.
- Pare, P.W.; Alborn, H.T.; Tumlinson, J.H. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc. Natl. Acad. Sci. USA 1998, 95, 13971–13975.
- Yoshinaga, N.; Aboshi, T.; Abe, H.; Nishida, R.; Alborn, H.T.; Tumlinson, J.H.; Mori, N. Active role of fatty acid amino acid conjugates in nitrogen metabolism in Spodoptera litura larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 18058–18063.
- Schmelz, E.A.; Huffaker, A.; Carroll, M.J.; Alborn, H.T.; Ali, J.G.; Teal, P.E.A. An Amino Acid Substitution Inhibits Specialist Herbivore Production of an Antagonist Effector and Recovers Insect-Induced Plant Defenses. Plant Physiol. 2012, 160, 1468–1478.
More
