Obesity is an epidemic public health problem that has progressively worsened in recent decades and is associated with low-grade chronic inflammation (LGCI) in metabolic tissues and an increased risk of several diseases. In particular, LGCI alters metabolism and increases cardiovascular risk by impairing endothelial function and altering the functions of adiponectin and high-density lipoproteins (HDLs). Adiponectin is an adipokine involved in regulating energy metabolism and body composition. Serum adiponectin levels are reduced in obese individuals and negatively correlate with chronic sub-clinical inflammatory markers. HDLs are a heterogeneous and complex class of lipoproteins that can be dysfunctional in obesity. Adiponectin and HDLs are strictly interdependent, and the maintenance of their interplay is essential for vascular function.
- low-grade chronic inflammation
- HDLs
- endothelial function
- adiponectin
- obesity
1. Adiponectin
42. High-Density Lipoproteins
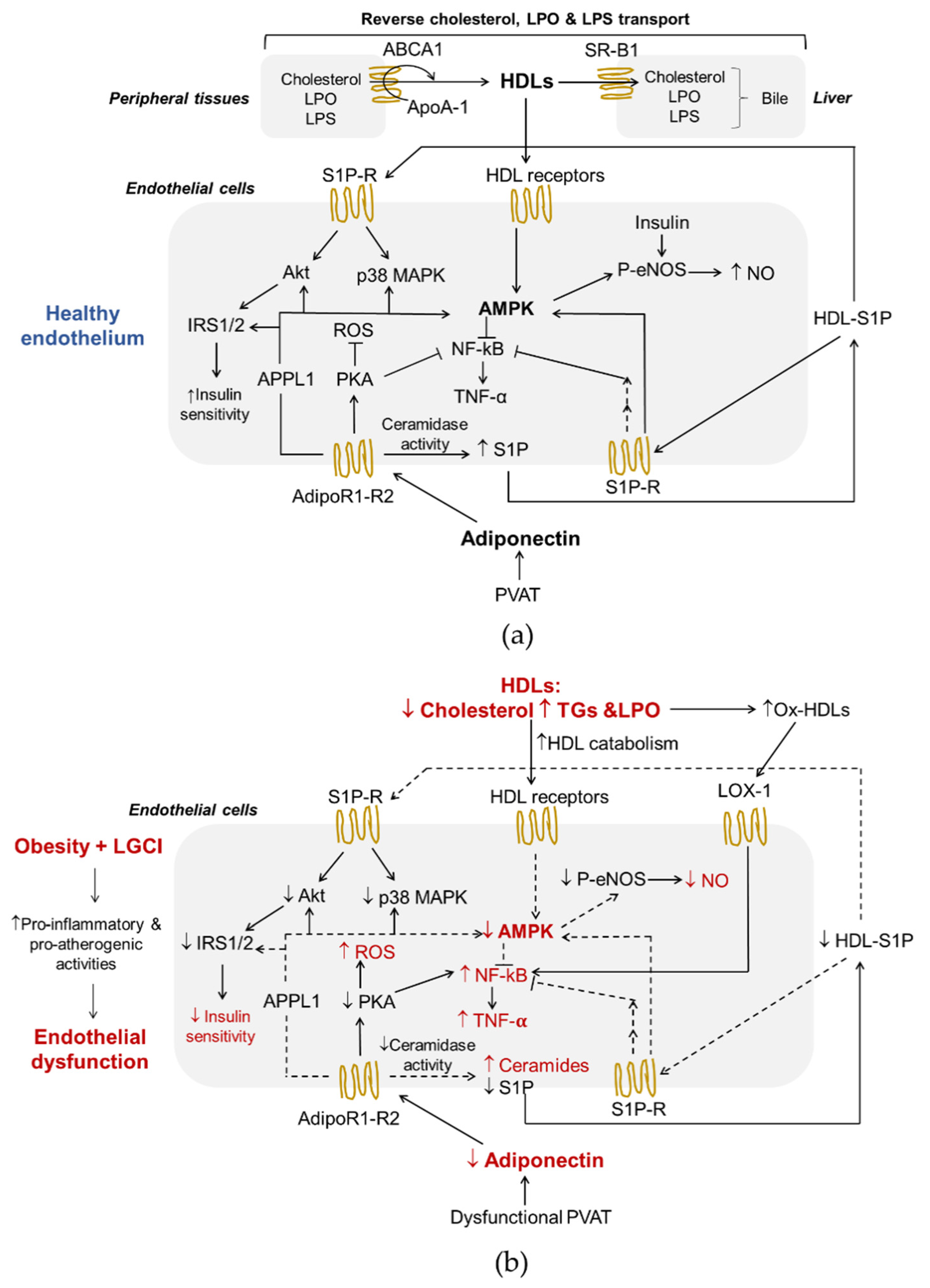
HDLs are a heterogeneous and complex class of lipoproteins with density ranging from 1.063–1.210 g/mL, considerable differences in size, shape, composition and function, produced mainly by the liver and, to a lesser extent, by the small intestine. In human plasma, the large, less dense (1.063–1.125 g/mL) lipid-enriched HDL2 and the small, dense (1.125–1.210 g/mL) protein-enriched HDL3 represent the two major sub-classes of HDLs [57][38]. HDLs contain several apolipoproteins (Apos) of which ApoA-I is quantitatively the most relevant and characterizes this lipoprotein class. Other Apos are ApoA-II, ApoA-IV, ApoC-I, ApoC-II, ApoC-III, ApoC-IV, ApoD, ApoE, ApoF, ApoH, ApoJ, ApoL-I and ApoM [57][38]. In addition, several enzymes circulate in the bloodstream associated with HDLs, including enzymes involved in lipoprotein remodeling (lecithin-cholesterol acyltransferase, LCAT, cholesterol ester transfer protein, cholesteryl ester transfer protein CETP, and phospholipid transfer protein, PLTP), paraoxonase-1 (PON-1) and lipopolysaccharide (LPS)-binding protein (LBP) [58][39]. The main lipids of HDLs are phospholipids (PLs) of which phosphatidylcholine (PC) and sphingomyelin (SM) are the main glycerophospholipids and sphingolipids, respectively. PLs modulate HDLs functions and are the precursors of a variety of regulatory molecules, including lysophospholipids and ceramides. In addition, S1P is transported in circulatory and interstitial fluids by HDLs-bound ApoM. There are several interactions between HDLs and the endothelium (Figure 1a). First of all, reverse cholesterol transport (RCT), the ability to transport cholesterol from peripheral tissues back to the liver for excretion in the bile, is the best-known function of HDLs and a process that plays a central role in preventing endothelial dysfunction and atherosclerosis. Of interest, ECs express HDLs’ scavenger receptor B type I (SR-BI), the ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1), and the ecto-F1-ATPase [59][40]. As shown in Figure 1a, upon the binding of HDLs to their receptors as well as to S1P receptors, various kinases, including Src, AMPK, p38 MAPK, PI3K and Akt, are activated [60,61][41][42]. As a result, HDLs enhance endothelial barrier-function and exert anti-inflammatory, anti-apoptotic and anti-adhesive properties [57,62,63,64][38][43][44][45]. In addition, HDLs reduce the cellular production of superoxide, an inactivator of the vasodilator NO, by decreasing the activity of endothelial nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [65][46], thus preventing ED. RCT begins with the formation of nascent HDLs particles, which consist mainly of ApoA-I (Figure 1a). Cholesterol efflux is mediated by the transporters ABCA1, ABCG1 and SR-B1. This step involves the interaction between lipid-free or lipid-free monomeric ApoA-I and ABCA1, while ABCG1 mediates the outflow of cellular cholesterol to lipidated HDL particles. The expression of the ABCA1 and ABCG1 genes is regulated at the transcriptional level by the liver X receptors (LXRs)-α and β [66][47]. Like ABCG1, SR-B1 in peripheral cells may also promote cholesterol outflow to mature HDLs particles, but its role in the RCT pathway is particularly important in the liver where it mediates the selective uptake of cholesteryl esters from HDLs [24][5]. HDLs also influence triglyceridemia because of their regulatory role on HL activity. HL binds to proteoglycans on the cell surface of hepatocytes and hepatic ECs. HDLs bind to HL and release the enzyme into the circulation where it hydrolyses TGs and PLs of plasma lipoproteins [67][48]. HDL2 is more effective in displacing proteoglycan-bound HL than HDL3. In addition, in vivo, and in vitro models suggest that HDLs promote an increase in adiponectin production from AT in a P13K-dependent manner [68][49]. Another well-known function of HDLs is their role as anti-inflammatory regulators exerted through interactions with both the vascular endothelium and circulating inflammatory cells [69][50]. As mentioned above, HDLs reduce the expression of endothelial adhesion molecules in response to inflammatory mediators and the migration of monocytes into the vascular wall, simultaneously exploiting their antioxidant activities [57][38]. HDLs prevent the induction of endothelial 32-kDa putative cysteine protease (CPP32)-like protease, resulting in a decrease in the activity of TNF-α, and, consequently, reduce the apoptotic rate of these cells [70][51]. Moreover, HDLs participate in a mechanism of intercellular communication involving the transport and delivery of specific microRNAs (miRNAs), small non-coding RNAs that post-transcriptionally regulate gene expression through translational inhibition and mRNA destabilization [71][52]. It has been shown that the transfer of miRNA-223 from HDLs into ECs reduces inflammation by suppressing the expression of intercellular adhesion molecule 1 (ICAM-1) [72][53]. The anti-inflammatory properties of HDLs may be due also to their ability to neutralize bacterial products, such as LPS (Figure 1a). LPS is a bacterial endotoxin with powerful pro-inflammatory activity that can reach the systemic circulation even during the absorption of nutrients in much smaller quantities than those associated with a bacterial infection, but sufficient to contribute to LGCI [73][54]. Moreover, LPS decreases HDL cholesterol (HDL-C) and adiponectin levels in vivo [68][49] and directly and indirectly participates in the inflammatory reaction in AT during obesity [74][55]. The exposure of ECs to this endotoxin results in endothelial activation and production of various pro-inflammatory mediators, and, ultimately, in cellular injury [75][56]. Interestingly, very recently, Han et al. have demonstrated that intestine-derived HDL3 traverses the portal vein complexed with LPS-binding protein preventing LPS activation of liver macrophages and supporting extracellular inactivation of this endotoxin [58][39]. Finally, the antioxidant activities of HDLs prevent ED via endothelial ABCG1-mediated efflux of cholesterol and 7-oxysterols [76][57] and the inhibition of lipid peroxide accumulation because of PON-1 activity. HDLs are the major carrier in the circulation of PON-1, an esterase characterized by three enzymatic activities (lactonase, arylesterase and paraoxonase) that is involved in drug metabolism, and possesses antioxidant and anti-inflammatory properties [77][58]. The esterase activities of PON-1 allow the removal of peroxidized fatty acids from PLs, limiting damage resulting from oxidative stress. PON-1 hydrolyses also lactones, including homocysteine thiolactone, a toxic metabolite of homocysteine, which, by modifying protein lysine residues, leads to cell death, altered vessel structure, chronic inflammation, autoimmune response and atherosclerosis [78][59]. This PON-1 activity is probably involved in the mechanisms by which HDLs activate eNOS in an inflammatory environment [60][41]. Another important antioxidant activity of HDLs can be ascribed to their reverse transport of lipid peroxides [79][60] (Figure 1a). In fact, HDLs can acquire lipid peroxides from low-density lipoproteins (LDLs) and cell membranes holding them in an environment where they may be safely hydrolyzed and from which they may be released to the liver for elimination.63. Interplay between Adiponectin and HDLs in Endothelial Function and Obesity-Associated ED
HDLs and adiponectin reciprocally regulate their levels and metabolism (Figure 32). HDLs can enhance circulating adiponectin levels [68[49][61],108], while the amount of circulating adiponectin is an independent predictor of cellular cholesterol efflux capacity in humans [24][5]. Dias et al. [109][62] showed that elevated adiponectin levels are associated with a lower reduction in HDLs function assessed by measuring ApoA-I levels, particle size, cholesterol content and antioxidant capacity in T2DM patients. Nonetheless, the mechanisms that link the metabolism of adiponectin and lipoproteins have not been fully elucidated mainly because analytical difficulties complicate data collection and interpretation.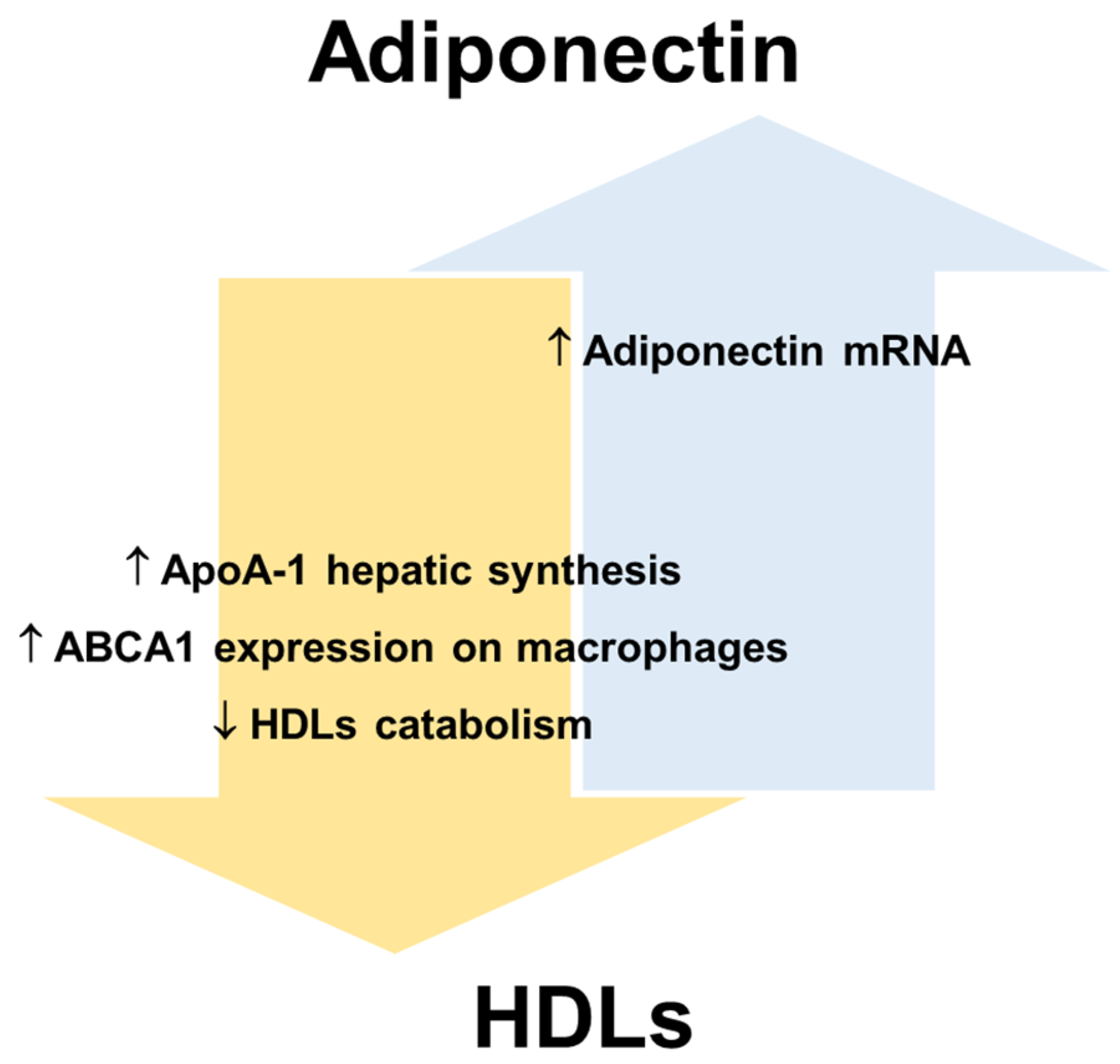
References
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321.
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783.
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New Insight into Adiponectin Role in Obesity and Obesity-Related Diseases. Biomed. Res. Int. 2014, 2014, 658913.
- Cohen, K.E.; Katunaric, B.; SenthilKumar, G.; McIntosh, J.J.; Freed, J.K. Vascular endothelial adiponectin signaling across the life span. Am. J. Physiol. Circ. Physiol. 2022, 322, H57–H65.
- Hafiane, A.; Gasbarrino, K.; Daskalopoulou, S.S. The role of adiponectin in cholesterol efflux and HDL biogenesis and metabolism. Metabolism 2019, 100, 153953.
- Astapova, O.; Leff, T. Adiponectin and PPARγ: Cooperative and Interdependent Actions of Two Key Regulators of Metabolism. Vitam. Horm. 2012, 90, 143–162.
- Kotlinowski, J.; Jozkowicz, A. PPAR Gamma and Angiogenesis: Endothelial Cells Perspective. J. Diabetes Res. 2016, 2016, 8492353.
- Chang, E.; Choi, J.M.; Kim, W.J.; Rhee, E.J.; Oh, K.W.; Lee, W.Y.; Park, S.E.; Park, S.W.; Park, C.Y. Restoration of adiponectin expression via the ERK pathway in TNFα-treated 3T3-L1 adipocytes. Mol. Med. Rep. 2014, 10, 905–910.
- Kusminski, C.M.; McTernan, P.G.; Schraw, T.; Kos, K.; O’Hare, J.P.; Ahima, R.; Kumar, S.; Scherer, P.E. Adiponectin complexes in human cerebrospinal fluid: Distinct complex distribution from serum. Diabetologia 2007, 50, 634–642.
- Kaser, S.; Tatarczyk, T.; Stadlmayr, A.; Ciardi, C.; Ress, C.; Tschoner, A.; Sandhofer, A.; Paulweber, B.; Ebenbichler, C.F.; Patsch, J.R. Effect of obesity and insulin sensitivity on adiponectin isoform distribution. Eur. J. Clin. Investig. 2008, 38, 827–834.
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23.
- Ishtiaq, S.M.; Rashid, H.; Hussain, Z.; Arshad, M.I.; Khan, J.A. Adiponectin and PPAR: A setup for intricate crosstalk between obesity and non-alcoholic fatty liver disease. Rev. Endocr. Metab. Disord. 2019, 20, 253–261.
- Diep Adiponectin: Role in physiology and pathophysiology. Int. J. Prev. Med. 2020, 11, 136.
- Hug, C.; Wang, J.; Ahmad, N.S.; Bogan, J.S.; Tsao, T.S.; Lodish, H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 2004, 101, 10308–10313.
- Sternberg, J.; Wankell, M.; Subramaniam, V.N.; Hebbard, L.W.; Sternberg, J.; Wankell, M.; Subramaniam, V.N.; Hebbard, L.W. The functional roles of T-cadherin in mammalian biology. AIMS Mol. Sci. 2017, 4, 62–81.
- Kalkman, H.O. An Explanation for the Adiponectin Paradox. Pharmaceuticals 2021, 14, 1266.
- Sabaratnam, R.; Svenningsen, P. Adipocyte-Endothelium Crosstalk in Obesity. Front. Endocrinol. 2021, 12, 681290.
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100.
- Sharma, A.X.; Holland, W.L. Adiponectin and its Hydrolase-Activated Receptors. J. Nat. Sci. 2017, 3, e396.
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625.
- Nègre-Salvayre, A.; Augé, N.; Camaré, C.; Bacchetti, T.; Ferretti, G.; Salvayre, R. Dual signaling evoked by oxidized LDLs in vascular cells. Free Radic. Biol. Med. 2017, 106, 118–133.
- Aburasayn, H.; Al Batran, R.; Ussher, J.R. Targeting ceramide metabolism in obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E423–E435.
- Fang, H.; Judd, R.L. Adiponectin regulation and function. Compr. Physiol. 2018, 8, 1031–1063.
- Ahima, R.S. Adipose tissue as an endocrine organ. Obesity 2006, 14 (Suppl. S5), 242S–249S.
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180.
- Kim, J.Y.; Barua, S.; Jeong, Y.J.; Lee, J.E. Adiponectin: The Potential Regulator and Therapeutic Target of Obesity and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6419.
- Ahima, R.S. Metabolic actions of adipocyte hormones: Focus on adiponectin. Obesity 2006, 14 (Suppl. S1), 9S–15S.
- Kubota, N.; Terauchi, Y.; Yamauchi, T.; Kubota, T.; Moroi, M.; Matsui, J.; Eto, K.; Yamashita, T.; Kamon, J.; Satoh, H.; et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 2002, 277, 25863–25866.
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2017, 19, 121–135.
- Wu, J.H.Y.; Marklund, M.; Imamura, F.; Tintle, N.; Ardisson Korat, A.V.; de Goede, J.; Zhou, X.; Yang, W.S.; de Oliveira Otto, M.C.; Kröger, J.; et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974.
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219.
- Wang, Q.; Liu, S.; Zhai, A.; Zhang, B.; Tian, G. AMPK-Mediated Regulation of Lipid Metabolism by Phosphorylation. Biol. Pharm. Bull. 2018, 41, 985–993.
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245.
- Myeong, J.Y.; Gha, Y.L.; Chung, J.J.; Young, H.A.; Seung, H.H.; Jae, B.K. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 2006, 55, 2562–2570.
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18.
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766.
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213.
- Bonizzi, A.; Piuri, G.; Corsi, F.; Cazzola, R.; Mazzucchelli, S. HDL Dysfunctionality: Clinical Relevance of Quality Rather Than Quantity. Biomedicines 2021, 9, 729.
- Han, Y.H.; Onufer, E.J.; Huang, L.H.; Sprung, R.W.; Davidson, W.S.; Czepielewski, R.S.; Wohltmann, M.; Sorci-Thomas, M.G.; Warner, B.W.; Randolph, G.J. Enterically derived high-density lipoprotein restrains liver injury through the portal vein. Science 2021, 373, eabe6729.
- Tran-Dinh, A.; Diallo, D.; Delbosc, S.; Varela-Perez, L.M.; Dang, Q.B.; Lapergue, B.; Burillo, E.; Michel, J.B.; Levoye, A.; Martin-Ventura, J.L.; et al. HDL and endothelial protection. Br. J. Pharmacol. 2013, 169, 493.
- Marín, M.; Moya, C.; Máñez, S. Mutual Influences between Nitric Oxide and Paraoxonase 1. Antioxidants 2019, 8, 619.
- Nofer, J.R. Signal transduction by HDL: Agonists, receptors, and signaling cascades. Handb. Exp. Pharmacol. 2015, 224, 229–256.
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006, 98, 1352–1364.
- Rohrer, L.; Hersberger, M.; Von Eckardstein, A. High density lipoproteins in the intersection of diabetes mellitus, inflammation and cardiovascular disease. Curr. Opin. Lipidol. 2004, 15, 269–278.
- Afonso, C.B.; Spickett, C.M. Lipoproteins as targets and markers of lipoxidation. Redox Biol. 2019, 23, 101066.
- Stadler, J.T.; Marsche, G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. Int. J. Mol. Sci. 2020, 21, 8985.
- Frambach, S.J.C.M.; de Haas, R.; Smeitink, J.A.M.; Rongen, G.A.; Russel, F.G.M.; Schirris, T.J.J. Brothers in Arms: ABCA1- and ABCG1-Mediated Cholesterol Efflux as Promising Targets in Cardiovascular Disease Treatment. Pharmacol. Rev. 2020, 72, 152–190.
- Santamarina-Fojo, S.; González-Navarro, H.; Freeman, L.; Wagner, E.; Nong, Z. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1750–1754.
- Van Linthout, S.; Foryst-Ludwig, A.; Spillmann, F.; Peng, J.; Feng, Y.; Meloni, M.; Van Craeyveld, E.; Kintscher, U.; Schultheiss, H.P.; De Geest, B.; et al. Impact of HDL on adipose tissue metabolism and adiponectin expression. Atherosclerosis 2010, 210, 438–444.
- Jia, C.; Anderson, J.L.C.; Gruppen, E.G.; Lei, Y.; Bakker, S.J.L.; Dullaart, R.P.F.; Tietge, U.J.F. High-Density Lipoprotein Anti-Inflammatory Capacity and Incident Cardiovascular Events. Circulation 2021, 143, 1935–1945.
- Sugano, M.; Tsuchida, K.; Makino, N. High-density lipoproteins protect endothelial cells from tumor necrosis factor-alpha-induced apoptosis. Biochem. Biophys. Res. Commun. 2000, 272, 872–876.
- Vickers, K.C.; Michell, D.L. HDL-small RNA Export, Transport, and Functional Delivery in Atherosclerosis. Curr. Atheroscler. Rep. 2021, 23, 1–10.
- Tabet, F.; Vickers, K.C.; Cuesta Torres, L.F.; Wiese, C.B.; Shoucri, B.M.; Lambert, G.; Catherinet, C.; Prado-Lourenco, L.; Levin, M.G.; Thacker, S.; et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 2014, 5, 1–14.
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 3379.
- Hersoug, L.G.; Møller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163.
- Bohannon, J.K.; Hernandez, A.; Enkhbaatar, P.; Adams, W.L.; Sherwood, E.R. The Immunobiology of TLR4 Agonists: From Endotoxin Tolerance to Immunoadjuvants. Shock 2013, 40, 451.
- Terasaka, N.; Yu, S.; Yvan-Charvet, L.; Wang, N.; Mzhavia, N.; Langlois, R.; Pagler, T.; Li, R.; Welch, C.L.; Goldberg, I.J.; et al. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J. Clin. Investig. 2008, 118, 3701–3713.
- Kotur-Stevuljević, J.; Vekić, J.; Stefanović, A.; Zeljković, A.; Ninić, A.; Ivanišević, J.; Miljković, M.; Sopić, M.; Munjas, J.; Mihajlović, M.; et al. Paraoxonase 1 and atherosclerosis-related diseases. Biofactors 2020, 46, 193–205.
- Levy, D.; Reichert, C.O.; Bydlowski, S.P. Paraoxonases Activities and Polymorphisms in Elderly and Old-Age Diseases: An Overview. Antioxidants 2019, 8, 118.
- Ahotupa, M.; Suomela, J.P.; Vuorimaa, T.; Vasankari, T. Lipoprotein-specific transport of circulating lipid peroxides. Ann. Med. 2010, 42, 521–529.
- Christou, G.A.; Kiortsis, D.N. Adiponectin and lipoprotein metabolism. Obes. Rev. 2013, 14, 939–949.
- Dias, G.D.; Cartolano, F.C.; Freitas, M.C.P.; Santa-Helena, E.; Markus, M.R.P.; Santos, R.D.; Damasceno, N.R.T. Adiponectin predicts the antioxidant capacity and size of high-density lipoprotein (HDL) in individuals with diabetes mellitus. J. Diabetes Complicat. 2021, 35, 107856.
- Vergès, B.; Petit, J.M.; Duvillard, L.; Dautin, G.; Florentin, E.; Galland, F.; Gambert, P. Adiponectin is an important determinant of apoA-I catabolism. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1364–1369.
- Marsche, G.; Zelzer, S.; Meinitzer, A.; Kern, S.; Meissl, S.; Pregartner, G.; Weghuber, D.; Almer, G.; Mangge, H. Adiponectin Predicts High-Density Lipoprotein Cholesterol Efflux Capacity in Adults Irrespective of Body Mass Index and Fat Distribution. J. Clin. Endocrinol. Metab. 2017, 102, 4117–4123.
- Thakkar, H.; Vincent, V.; Sukhla, S.; Sra, M.; Kanga, U.; Aggarwal, S.; Singh, A. Improvements in cholesterol efflux capacity of HDL and adiponectin contribute to mitigation in cardiovascular disease risk after bariatric surgery in a cohort with morbid obesity. Diabetol. Metab. Syndr. 2021, 13, 1–11.
- Von Eynatten, M.; Schneider, J.G.; Humpert, P.M.; Rudofsky, G.; Schmidt, N.; Barosch, P.; Hamann, A.; Morcos, M.; Kreuzer, J.; Bierhaus, A.; et al. Decreased plasma lipoprotein lipase in hypoadiponectinemia: An association independent of systemic inflammation and insulin resistance. Diabetes Care 2004, 27, 2925–2929.
- Terazawa-Watanabe, M.; Tsuboi, A.; Fukuo, K.; Kazumi, T. Association of adiponectin with serum preheparin lipoprotein lipase mass in women independent of fat mass and distribution, insulin resistance, and inflammation. Metab. Syndr. Relat. Disord. 2014, 12, 416–421.
- Tsutsumi, K. Lipoprotein lipase and atherosclerosis. Curr. Vasc. Pharmacol. 2003, 1, 11–17.
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2021, 477, 15–38.
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Hussain, M.M. Sphingolipids and Lipoproteins in Health and Metabolic Disorders. Trends Endocrinol. Metab. 2017, 28, 506.
- Camerer, E.; Regard, J.B.; Cornelissen, I.; Srinivasan, Y.; Duong, D.N.; Palmer, D.; Pham, T.H.; Wong, J.S.; Pappu, R.; Coughlin, S.R. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Investig. 2009, 119, 1871–1879.
- Garcia, J.G.N.; Liu, F.; Verin, A.D.; Birukova, A.; Dechert, M.A.; Gerthoffer, W.T.; Bamburg, J.R.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Investig. 2001, 108, 689–701.
- Lee, M.J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular Endothelial Cell Adherens Junction Assembly and Morphogenesis Induced by Sphingosine-1-Phosphate. Cell 1999, 99, 301–312.
- Salvia, R.; Halbac-Cotoara-zamfir, R.; Cividino, S.; Gutterman, D.D.; Quaranta, G. Manipulation of the Sphingolipid Rheostat Influences the Mediator of Flow-Induced Dilation in the Human Microvasculature. J. Am. Heart Assoc. 2019, 8, 1–12.
- Obata, Y.; Kita, S.; Koyama, Y.; Fukuda, S.; Takeda, H.; Takahashi, M.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 2018, 3, e99680.
- Gradinaru, D.; Margina, D.; Borsa, C.; Ionescu, C.; Ilie, M.; Costache, M.; Dinischiotu, A.; Prada, G.I. Adiponectin: Possible link between metabolic stress and oxidative stress in the elderly. Aging Clin. Exp. Res. 2017, 29, 621–629.
- Kupczyk, D.; Bilski, R.; Sokołowski, K.; Pawłowska, M.; Woźniak, A.; Szewczyk-Golec, K. Paraoxonase 1: The lectin-like oxidized ldl receptor type i and oxidative stress in the blood of men with type ii obesity. Dis. Markers 2019, 2019, 6178017.
- Ru, D.; Zhiqing, H.; Lin, Z.; Feng, W.; Feng, Z.; Jiayou, Z.; Yusheng, R.; Min, F.; Chun, L.; Zonggui, W. Oxidized high-density lipoprotein accelerates atherosclerosis progression by inducing the imbalance between treg and teff in LDLR knockout mice. APMIS 2015, 123, 410–421.
- Kontush, A.; Lhomme, M.; Chapman, M.J. Thematic review series: High density lipoprotein structure, function, and metabolism: Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963.
- Feingold, K.R.; Grunfeld, C. Effect of inflammation on HDL structure and function. Curr. Opin. Lipidol. 2016, 27, 521–530.
- Fritz, K.S.; Petersen, D.R. An Overview of the Chemistry and Biology of Reactive Aldehydes. Free Radic. Biol. Med. 2013, 59, 85.
- Holvoet, P.; Collen, D. Oxidation of low density lipoproteins in the pathogenesis of atherosclerosis. Atherosclerosis 1998, 137 (Suppl. S1), S33–S38.
- Pérez, L.; Vallejos, A.; Echeverria, C.; Varela, D.; Cabello-Verrugio, C.; Simon, F. OxHDL controls LOX-1 expression and plasma membrane localization through a mechanism dependent on NOX/ROS/NF-κB pathway on endothelial cells. Lab. Investig. 2019, 99, 421–437.
- Kattoor, A.J.; Kanuri, S.H.; Mehta, J.L. Role of Ox-LDL and LOX-1 in Atherogenesis. Curr. Med. Chem. 2019, 26, 1693–1700.
- Besler, C.; Heinrich, K.; Rohrer, L.; Doerries, C.; Riwanto, M.; Shih, D.M.; Chroni, A.; Yonekawa, K.; Stein, S.; Schaefer, N.; et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Investig. 2011, 121, 2693–2708.
- Kakino, A.; Fujita, Y.; Ke, L.Y.; Chan, H.C.; Tsai, M.H.; Dai, C.Y.; Chen, C.H.; Sawamura, T. Adiponectin forms a complex with atherogenic LDL and inhibits its downstream effects. J. Lipid Res. 2021, 62, 100001.
- Erdbruegger, U.; Dhaygude, A.; Haubitz, M.; Woywodt, A. Circulating endothelial cells: Markers and mediators of vascular damage. Curr. Stem Cell Res. Ther. 2010, 5, 294–302.
- Peterson, S.J.; Shapiro, J.I.; Thompson, E.; Singh, S.; Liu, L.; Weingarten, J.A.; O’Hanlon, K.; Bialczak, A.; Bhesania, S.R.; Abraham, N.G. Oxidized HDL, Adipokines, and Endothelial Dysfunction: A Potential Biomarker Profile for Cardiovascular Risk in Women with Obesity. Obesity 2019, 27, 87–93.
- Parhami, F.; Basseri, B.; Hwang, J.; Tintut, Y.; Demer, L.L. High-density lipoprotein regulates calcification of vascular cells. Circ. Res. 2002, 91, 570–576.
- Harun, N.H.; Anisah Froemming, G.R.; Nawawi, H.M.; Muid, S.A. Inflammation and Vascular Calcification Causing Effects of Oxidized HDL are Attenuated by Adiponectin in Human Vascular Smooth Muscle Cells. Int. J. Mol. Cell. Med. 2019, 8, 39–54.
- Son, B.K.; Akishita, M.; Iijima, K.; Kozaki, K.; Maemura, K.; Eto, M.; Ouchi, Y. Adiponectin Antagonizes Stimulatory Effect of Tumor Necrosis Factor-α on Vascular Smooth Muscle Cell Calcification: Regulation of Growth Arrest-Specific Gene 6-Mediated Survival Pathway by Adenosine 5′-Monophosphate-Activated Protein Kinase. Endocrinology 2008, 149, 1646–1653.
- Lu, Y.; Ma, Y.; Wang, R.; Sun, J.; Guo, B.; Wei, R.; Jia, Y. Adiponectin inhibits vascular smooth muscle cell calcification induced by beta-glycerophosphate through JAK2/STAT3 signaling pathway. J. Biosci. 2019, 44, 1–9.
