Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Li-Fen Huang and Version 2 by Peter Tang.
A plant cell-based recombinant glucocerebrosidase was approved by the FDA in 2012 for the treatment of human inherited Gaucher disease, indicating that plant suspension cells have advantages in biosafety and a low production cost as a commercial pharmaceutical recombinant protein expression system. A low allergenic rice suspension cell-based recombinant protein expression system controlled by the αAmy3/RAmy3D promoter has been shown to result in relatively high protein yields in plant cell-based systems.
- αAmy3 promoter
- recombinant protein
- rice suspension cell
- signal peptide
1. Introduction
The production of valuable recombinant proteins in bacteria, yeasts, insect cells, mammalian cells and even plant cells by genetic engineering has become an important and common biotechnology in medical uses, with an estimated market value of approximately USD 400 billion by 2025 [1]. Each type of host cell has its own characteristics; thus, even the same recombinant proteins often show differences in biological activity and stability among various host cell protein expression systems. More than 50% of human proteins and 40% of commercial recombinant pharmaceutical proteins are glycoproteins; therefore, eukaryotic cells are often required as host cells to perform glycosylation for these recombinant proteins to have their best performances [2]. Although the current commercial biosimilars are mostly produced by Chinese hamster ovary (CHO) cells [3], the susceptibility to feedback inhibition of animal host cells limits the production of pharmaceutical recombinant proteins. In addition, production costs by expensive serum or required growth factors and biosafety concerns from common animal pathogens have pushed scientists to seek nonanimal-derived eukaryotic cell expression systems. Plant cells have advantages in protein post-modification, low production cost and high biosafety, and they are therefore applied to produce pharmaceutical recombinant proteins [4][5][6][4,5,6]. Many biopharmaceutical proteins, including antibodies, antigens and therapeutic proteins, have been successfully expressed in plant cells, and some are in clinical trials [7]; therefore, plant cell expression systems have attracted global attention.
Plant suspension cells are commonly cultured in relatively simple and inexpensive liquid medium compared to microbial and animal cells [1][4][5][6][7][1,4,5,6,7]. The recombinant protein can be fused to a signal peptide and then secreted into cell culture liquid medium. Secretory recombinant proteins can be obtained easily without breaking plant host cells, and purification from medium proteins is much easier than purification from cellular proteins. Recombinant protein expression platforms based on suspension cell culture have been generated from several plant species, such as rice, tobacco and carrot. The first plant recombinant pharmaceutical protein, human beta-glucocerebrosidase for Gaucher disease patients, was produced from carrot suspension cells and approved by the FDA in 2012 [8]. This commercial case shows that the plant suspension cell expression system can fit the requirements of GMP and government regulation. It also proves that plant suspension cell expression systems have the potential to compete with the two major commercial protein expression systems, microbial E. coli and mammalian CHO cells.
2. Advantages of Plant Suspension Culture Cells
Among the current well-developed foreign protein expression platforms, plant cells have many advantages compared with microbial fermentation and animal and insect cell culture. Microbial systems perform limited post-translational modification for recombinant genes derived from eukaryotic cells. Recombinant proteins expressed in the E. coli system commonly have incorrect protein folding and form insoluble inclusion bodies. These inclusion bodies require extra solubilization and refolding steps to allow recombinant proteins to fold into the correct structure and therefore increase the protein purification cost [9][10][9,10]. In addition, the cost of recombinant protein production is further increased to completely remove possible endotoxins produced during the bacterial culture process. On the other hand, it is more expensive to produce recombinant proteins in mammalian and insect cells than in plant cells because of artificial culture medium supplemented with a variety of expensive growth factors and cytokines to meet the needs of host cell growth, coupled with cell sensitivity to mechanical stress in the bioreactor [1][4][5][6][7][1,4,5,6,7]. Well-established plant cell culture technology has been applied for the production of valuable metabolites, such as artemisinin, chlorogenic acid and ginsenosides [11][12][13][14][11,12,13,14]. Simons et al. were the first to use potato and tobacco cells to produce medically valuable recombinant human serum albumin in liquid culture medium [15]. Plant cells cultivated in a sterile cell culture environment have more advantages than plants grown in greenhouses or fields. For example, agricultural fertilizers and pesticides are not used in the plant suspension cell culture expression system, and they therefore do not cause environmental pollution. There is no potential transgene contamination in plant suspension cells. In addition, the proliferation rate of plant suspension cells is high as compared to solid cultured cells, and the production time required to obtain recombinant protein is shorter than that of transgenic plants. Compared with the whole plant system, the plant suspension cell system produces recombinant proteins under a controlled aseptic culture environment and therefore improves the biological safety of recombinant proteins [16][17][16,17]. Purification of recombinant proteins remains the major cost of commercial recombinant protein products; thus, protein purification is an expensive and challenging step in the biotechnology industry [18][19][18,19]. Plant suspension cells can easily reduce the cost of recombinant protein purification by fusing the signal peptide sequence with the target gene, and the recombinant protein can be directly secreted into the extracellular culture medium. The strategy simplifies the process of recombinant protein purification by avoiding interference from a large amount of intracellular proteins. Therefore, using plant suspension cells to produce valuable recombinant proteins is a good, economical, safe and simple strategy, with the following advantages: (1) plant cells are eukaryotic, similar to animal cells, and can perform complex post-translational modification; (2) recombinant proteins produced by plants can be expressed in particular subcellular organelles, such as the endoplasmic reticulum, chloroplasts and vacuole [20][21][20,21]; and (3) the recombinant human proteins produced by plants can avoid cross-contamination of human or animal pathogens and have less interference from other family members, which share similar protein structures, to make protein purification easy and safe. Since the first successful case was reported in 1990, recombinant proteins, including medical vaccines, antibodies, cytokines, other protein drugs, industrial enzymes and food additives, have been successfully expressed in plant cells [9][22][23][24][25][26][9,22,23,24,25,26].3. Rice Suspension Cell Recombinant Protein Expression System
Tobacco and rice cells are commonly used in suspension culture to express recombinant proteins. In general, the constitutively active cauliflower mosaic virus CaMV 35S promoter is used to produce foreign proteins in tobacco cells. The yield of recombinant human antibody in tobacco cells in 200 L bioreactor was approximately 20 mg/L [27], and tobacco cells are known for the production of toxic alkaloids and proteolytic enzymes. On the other hand, the recombinant protein production platform of rice suspension cells generally has a protein yield more than 10 times that of tobacco cells [9]. Rice suspension cell protein expression systems have been established on a large scale to produce various recombinant proteins in several biotech companies, such as Natural Bio-Materials at Korea [28]. Several valuable pharmaceutical recombinant proteins produced from rice cells are under clinical trials, such as Vibrio cholera CTB (cholera toxin B subunit) for cholera [29]. Various growth factors, enzymes, interferons and antibodies have been successfully produced in rice cell suspension culture systems [22][30][31][32][33][22,30,31,32,33]. The recombinant protein expression system of rice suspension cells often uses a transgenic cell line containing the inducible αAmy3 (also called RAmy3D) promoter and its signal peptide to secrete the recombinant protein into the culture medium under sugar starvation conditions [34][35][34,35]. Diagrammatic representation of the rice suspension cell expression system and process for various recombinant protein production under the control of αAmy3 promoter and its signal peptide is shown in Figure 1. Briefly, a gene of interest, which may be modified with codon optimized sequence in rice, is cloned downstream of the αAmy3 promoter-signal peptide sequence either by Gateway or restriction enzyme cloning technology. The high yield of stable transgenic rice cell lines can be screened after an expression cassette is transferred into the rice nucleus by Agrobacterium- or particle bombardment-mediated transformation. αAmy3 is a member of the α-amylase gene family, and rice contains 10 α-amylase genes. When cereal seeds are in the germination stage, α-amylase genes in embryos and aleurone cells begin to express and secrete α-amylase into the endosperm to break down starch into small molecular sugars by cutting the α-1,4 glucose linkage in amylose and amylopectin molecules. The main α-amylase genes expressed in rice suspension cells are αAmy3, αAmy7/RAmy1A and αAmy8/RAmy3E, and their promoters are regulated by gibberellic acid, abscisic acid and carbohydrates. αAmy3 has the highest expression level induced by glucose deficiency among the whole rice α-amylase gene family in rice suspension cells [36], and the expression level can be continuously enhanced for up to 48 h [37]. The signal peptide of αAmy3 can be applied to allow the fusion of recombinant proteins secreted into the extracellular medium to simplify the protein purification steps.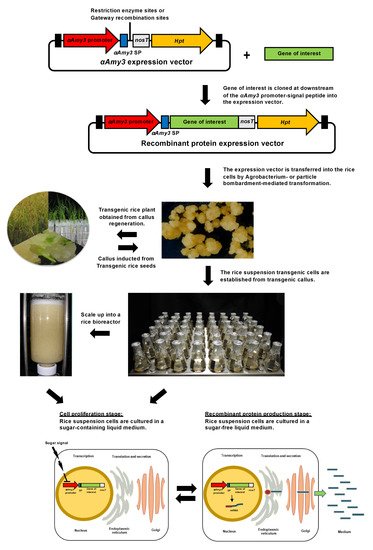
Figure 1. Schematic diagram of the αAmy3 promoter-signal peptide-based recombinant protein production system. The expression vector contained the rice αAmy3 promoter-signal peptide sequence, nos terminator (nosT), and the hygromycin selection marker (Hpt). The αAmy3 promoter-signal peptide-gene of interest expression cassette is transferred into rice nucleus. The recombinant proteins are secreted into sugar-free liquid medium from rice suspension cells under recombinant protein production stage.
