Flavonoids are small molecules, produced de novo by plants as secondary metabolites in response to diverse biotic and abiotic factors. These chemical compounds have a broad spectrum of established health-promoting effects. They are due to their antioxidative, anti-inflammatory, anti-mutagenic, and anti-carcinogenic properties coupled with their capacity to modulate key cellular enzyme functions. Flavonoids are widely distributed chemical compounds in the plant kingdom. Plants are therefore an inexhaustible source of flavonoids.
- flavonoids
- natural sources
1. Flavonoids—Classification
- Flavonoids—Classification
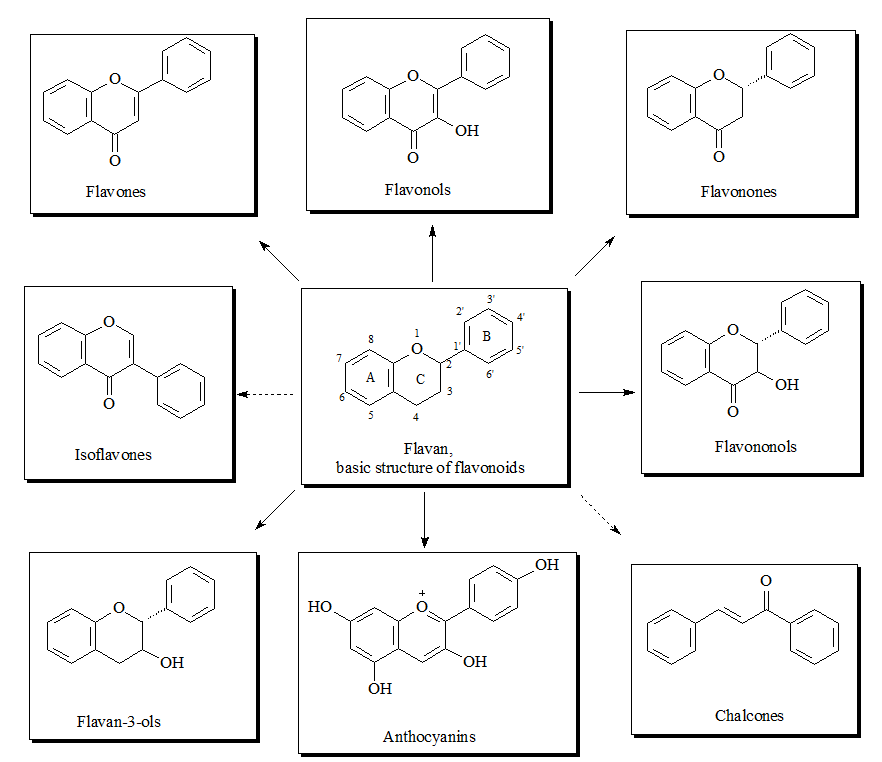
The flavonoid family includes more than 6000 low-molecular-weight phenolic compounds [1], derivatives of flavan. The main subgroups are flavones, flavonols, flavonones, flavononols, flavan-3-ols, anthocyanines, isoflavones, and chalcones (Figure 1).
Figure 1. Structures of flavonoid subgroups.
Structures of flavonoid subgroups.
The flavan core is recognized in every single flavonoid structure. It consists of 15 carbon atoms which build two aromatic rings (commonly denoted as A and B) linked by a three-carbon chain. The connecting carbon chain is a part of a heterocyclic central ring (designed as C) and is available in the most flavonoids with one exception: in the structure of the chalcones the carbon chain between the A and B rings is linear [2]. For that reason the chalcones can be referred to as open-chain flavonoids.
Depending on the position of the linked B-ring to the benzopyrano (called also chromano) structure the flavones, flavonols, flavonones, flavononols, flavon-3-ols and anthocyanines, are 2-phenylbenzopyrans and the isoflavonoids are 3-phynylbenzopyrans, which makes this structures positional isomers. The flavonoids of the 2-phenylbenzopyran-subgroup differ in the degree of oxidation and saturation of the heterocyclic C-ring [3].
2. Natural Sources of Flavonoids
- Natural Sources of Flavonoids
Flavonoids are small molecules, produced de novo by plants as secondary metabolites in response to diverse biotic and abiotic factors. They are widely distributed chemical compounds in the plant kingdom. Plants are therefore an inexhaustible source of flavonoids.
In 1936, the Hungarian scientist Szent-Gyorgyi (the discoverer of vitamin C) isolated a new substance from lemons. He called it “citrin” [3]. The structure of citrin was determined later: it is composed of the flavonoids hesperidin and eriodictyol [4]. Citrin was called also vitamin P. But, in 1950 was concluded that flavonoids did not meet the strict definition of a vitamin and the name vitamin P was taken off [5].
Their biochemical roles of the flavonoids in the plant are multifarious: from flower pigmentation to taking part in the growing processes, and the defense against diseases [6]. Each flavonoid group plays a unique biochemical role and has a particular distribution in plants [3].
The most popular edible plants rich in flavonoids, categorized by Tzanova et al. [7] in subgroups, are systematized in Tables 1.
Table 1. Food plants rich in flavonoids
in flavonoids
|
Flavonoid subgroup |
Flavonoids |
Food |
References |
|
|
Flavonols and Flavan-3-ols |
Kaempferol; quercetin; myrecitin; (−)-epicatechin |
Black berries; wine |
[8] [9] [10] |
|
|
Kaempferol; quercetin |
Tomato |
[11] [12] |
|
|
|
(+)-Catechin; (−)-epicatechin; epigallocatechin; chrysin; apigenin; quercetin; kaempferol |
Tea |
[13] [14] [15] |
|
|
|
(+)-Catechin; (−)-epicatechin; quercetin |
Coffee; cocoa; apple |
[9] [16] [17] [18] [19] [20] |
|
|
|
Kaempferol; quercetin; myricetin; tamarixetin |
Onion; red wine; olive oil; berries; grapefruit; orange |
[21] [22] |
|
|
|
(+)-Catechin; (−)-epicatechin; quercetin; kaempferol |
Red berries; strawberries |
[9] [23] [24] [25] |
|
|
|
Quercetin |
Lemon; olive; aspargus |
[22] [26] [27] |
|
|
|
Kaempferol |
Saffron spice |
[28] |
|
|
|
Kaempferol; quercetin |
Broccoli; brussel sprouts |
[15] [22] |
|
|
|
(+)-Catechin; (−)-epicatechin |
Apricot; nectarine; peach; plum; fig; banana; kiwi; hazelnut |
[9] [10] [29] [30] [31] |
|
|
|
(+)-Catechin; (−)-epicatechin; quercetin; isorhamnetin; kaempferol |
Almond |
[31] [32] |
|
|
|
Flavones |
Luteolin |
Fruit skins; red wine; buckwheat; red pepper; tomato skin; lemon; watermelon; brussel sprouts; pumpkin |
[22] [31] [33] [34] |
|
|
Luteolin; apigenin; isorhoifolin |
Olive |
[26] [35] |
|
|
|
Flavonones |
Naringin; eriodictyol |
Almond |
[32] |
|
|
Naringin; maringenin; taxifolin; hesperitin; eriodictyol |
Citrus fruits; grapefruit; lemon; orange |
[22] [36] [37] |
|
|
|
Anthocyanins |
Apigenidin; cyanidin |
Cherry; easberry; strawberry |
[21] [22] [25] |
|
|
Cyanidin |
Olive |
[26] [35] |
|
|
|
Isoflavones |
Daidzin; genistein; glycitin; sissotrin; ononin |
Soya bean |
[38] [39] [40] [41] |
|
|
Biochanin A; formononetin |
Red clover |
[41] |
|
|
|
Genistin; daidzin; biochanin A |
Peanut |
[42] |
|
|
Flavonoid subgroup |
Flavonoids |
Food |
References |
|
|
Flavonols and Flavan-3-ols |
Kaempferol; quercetin; myrecitin; (−)-epicatechin |
Black berries; wine |
|
|
|
Kaempferol; quercetin |
Tomato |
|
||
|
(+)-Catechin; (−)-epicatechin; epigallocatechin; chrysin; apigenin; quercetin; kaempferol |
Tea |
|
||
|
(+)-Catechin; (−)-epicatechin; quercetin |
Coffee; cocoa; apple |
|
||
|
Kaempferol; quercetin; myricetin; tamarixetin |
Onion; red wine; olive oil; berries; grapefruit; orange |
|
||
|
(+)-Catechin; (−)-epicatechin; quercetin; kaempferol |
Red berries; strawberries |
|
||
|
Quercetin |
Lemon; olive; aspargus |
|
||
|
Kaempferol |
Saffron spice |
[28] |
|
|
|
Kaempferol; quercetin |
Broccoli; brussel sprouts |
|
||
|
(+)-Catechin; (−)-epicatechin |
Apricot; nectarine; peach; plum; fig; banana; kiwi; hazelnut |
|
||
|
(+)-Catechin; (−)-epicatechin; quercetin; isorhamnetin; kaempferol |
Almond |
|
||
|
Flavones |
Luteolin |
Fruit skins; red wine; buckwheat; red pepper; tomato skin; lemon; watermelon; brussel sprouts; pumpkin |
||
|
Luteolin; apigenin; isorhoifolin |
Olive |
|
||
|
Flavonones |
Naringin; eriodictyol |
Almond |
[32] |
|
|
Naringin; maringenin; taxifolin; hesperitin; eriodictyol |
Citrus fruits; grapefruit; lemon; orange |
|
||
|
Anthocyanins |
Apigenidin; cyanidin |
Cherry; easberry; strawberry |
|
|
|
Cyanidin |
Olive |
|
||
|
Isoflavones |
Daidzin; genistein; glycitin; sissotrin; ononin |
Soya bean |
|
|
|
Biochanin A; formononetin |
Red clover |
[41] |
|
|
|
Genistin; daidzin; biochanin A |
Peanut |
[42] |
|
. Food plants rich
Fruits and vegetables are a rich diet source of flavonols and flavan-3-ols. Kaempferol, quercetin and myricetin are most studied flavonols, and catechin and epicatechin – the most studied flavan-3-ols. Because of their similar solubility in the polar alcoholic solvents, flavonols and flavan-3-ols were simultaneously extracted and detected, e.g. in black berries and wines [8][9][10][8-10]; in red berries [9][23][24][9, 23, 24]; in tee [13][15][13-15]; or coffee [9][16][17][18][19][20][9, 16-20]. Onions, tomatoes, broccoli, apples and grapes are rich sources of flavonols [21][22][21, 22].
Flavones are widely present in leaves, flowers and fruits as glucosides and their major sources are green leafy spices like parsley [43]. Luteolin is the most found flavone [22][26][31][32][33][34][35].
[22, 26, 31 – 35].
Flavanones are generally present in all citrus fruits [22][36][37][22, 36, 37]. Hesperitin, naringenin and eriodictyol are examples of this class of flavonoids. These compounds are responsible for the bitter taste of the juice and peel of citrus fruits. Almonds are rich also in naringin and eriodictyol [32].
Anthocyanins are pigments responsible for the coloring of flowers and fruits. Cyanidin is the most commonly studied anthocyanin. Various red and blue fruits are a rich diet sources of anthocyanins, which are concentrated in the fruit skins [21][22][25][26][35].
[21, 22, 25, 26, 35].
Isoflavones are found in legumes predominantly, e.g. soybean [38][39][40][41][38 – 41], but also in red clover [41] and in peanuts [42].
Major examples of chalcones include phloridzin, arbutin, phloretin, and chalconaringenin. Chalcones occur in significant amounts in tomatoes, pears, strawberries, bearberries, and certain wheat products [43].
Tzanova et al. [7] systemized the flavonoids detected in medicinal plants rich in their subgroups (Table 2).
Table 2.
Medicinal plants rich in flavonoids
|
Flavonoid subgroup |
Flavonoids |
Medicinal Plant (Family) |
References |
|
|
Flavonols and Flavan-ols |
(+)-Catechin |
Brysonima crassa (Compositae) |
[44] |
|
|
Isorhamnetin |
Calendula officinalis (Compositae) |
[45] |
|
|
|
Kaempferol |
Acalypha indica (Euphorbiaceae); Clitoria ternatea (Fabaceae); Pteris vittata L (Pteridaceae) |
[46] [47] [48] |
|
|
|
Quercetin |
Betula pendula (Betulaceae) Bauhinia monandra (Fabaceae); Pteris vittata L (Pteridaceae) Cannabis sativa (Compositae); Azadirachta indica (Meliaceae); Angelica L. (Apiaceae); |
[45] [48] [49] [50] [51] |
|
|
|
Hyperoside |
Tilia cordata (Tiliaceae) |
[45] |
|
|
|
Isoquercetin |
Mimosa pudica (Mimosoideae) |
[52] |
|
|
|
Pongaflavonol |
Pongamia pinnata (Fabaceae) |
[53] |
|
|
|
2-(3, 4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one |
Chenopodium album L. (Chenopodiaceae) |
[54] |
|
|
|
2-(3,4-dihydroxy-5-methoxy-phenyl)-3,5-dihydroxy-6,7-dimethoxychromen-4-one |
Euphorbia neriifolia (Euphorbiaceae) |
[55] |
|
|
|
Flavones |
Pectolinarigenin |
Clerodendrum phlomidis (Verbenaceae) |
[49] |
|
|
Luteolin |
Aloe vera (Asphodelaceae); Momordica charantia (Curcurbitaceae); Bacopa moneirra (Scrophulariaceae); Angelica L. (Apiaceae); Mentha longifolia (Lamiaceae) |
[46] [50] [56] [57] |
|
|
|
Hispidulin; apigenin; cirsimaritin |
Rosmarinus officinalis L. (Lamiaceae) |
[58] [59] |
|
|
|
Luteolin; hispidulin; apigenin; cirsimaritin |
Salvia officinalis L. (Lamiaceae) |
[58] |
|
|
|
Luteolin; hispidulin |
Thymus L. (Lamiaceae) |
[58] [60] |
|
|
|
Apigenin; hispidulin |
Verbena officinalis L. (Verbenaceae) |
[60] |
|
|
|
5-hydroxy-7,8-dimethoxyflavone |
Andrographis paniculata (Acanthaceae) |
[45] |
|
|
|
3,4-methlenedioxyflavone |
Limnophila indica (Scrophulariaceae) |
[52] |
|
|
|
Chrysin |
Oroxylum indicum (Bignoniaceaea) |
[52] |
|
|
|
Vitexin |
Passiflora incarnate (Passifloraceae) |
[45] |
|
|
|
Flavonones |
Narginin |
Rosmarinus officinalis L. (Lamiaceae) |
[58] [59] |
|
|
Hesperidin |
Citrus medica (Rutaceae) |
[46] |
|
|
|
Liquiritin |
Glyccheriza glabra (Leguminosae) |
[45] |
|
|
|
Kurarinol; kurarinone |
Sophora flavescens Ait. (Fabaceae) |
[61] |
|
|
|
Flavononols |
Kushenol I; kushenol N |
Sophora flavescens Ait. (Fabaceae) |
[61] |
|
|
Isoflavones |
Genistein |
Calopogonium muconoides (Fabaceae) Butea monospermea (Fabaceae); Andira macrothyrsa (Fabaceae); |
[41] [51] |
|
|
Biochanin A |
Cratylia argentea (Fabaceae); A. macrothyrsa (Melastomataceae) |
[41] |
|
|
Flavonoid subgroup |
Flavonoids |
Medicinal Plant (Family) |
References |
|
|
Flavonols and Flavan-ols |
(+)-Catechin |
Brysonima crassa (Compositae) |
[44] |
|
|
Isorhamnetin |
Calendula officinalis (Compositae) |
[45] |
|
|
|
Kaempferol |
Acalypha indica (Euphorbiaceae); Clitoria ternatea (Fabaceae); Pteris vittata L (Pteridaceae) |
|
||
|
Quercetin |
Betula pendula (Betulaceae) Bauhinia monandra (Fabaceae); Pteris vittata L (Pteridaceae) Cannabis sativa (Compositae); Azadirachta indica (Meliaceae); Angelica L. (Apiaceae); |
|
||
|
Hyperoside |
Tilia cordata (Tiliaceae) |
[45] |
|
|
|
Isoquercetin |
Mimosa pudica (Mimosoideae) |
[52] |
|
|
|
Pongaflavonol |
Pongamia pinnata (Fabaceae) |
[53] |
|
|
|
2-(3, 4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one |
Chenopodium album L. (Chenopodiaceae) |
[54] |
|
|
|
2-(3,4-dihydroxy-5-methoxy-phenyl)-3,5-dihydroxy-6,7-dimethoxychromen-4-one |
Euphorbia neriifolia (Euphorbiaceae) |
[55] |
|
|
|
Flavones |
Pectolinarigenin |
Clerodendrum phlomidis (Verbenaceae) |
[49] |
|
|
Luteolin |
Aloe vera (Asphodelaceae); Momordica charantia (Curcurbitaceae); Bacopa moneirra (Scrophulariaceae); Angelica L. (Apiaceae); Mentha longifolia (Lamiaceae) |
|
||
|
Hispidulin; apigenin; cirsimaritin |
Rosmarinus officinalis L. (Lamiaceae) |
|
||
|
Luteolin; hispidulin; apigenin; cirsimaritin |
Salvia officinalis L. (Lamiaceae) |
[58] |
|
|
|
Luteolin; hispidulin |
Thymus L. (Lamiaceae) |
|
||
|
Apigenin; hispidulin |
Verbena officinalis L. (Verbenaceae) |
[60] |
|
|
|
5-hydroxy-7,8-dimethoxyflavone |
Andrographis paniculata (Acanthaceae) |
[45] |
|
|
|
3,4-methlenedioxyflavone |
Limnophila indica (Scrophulariaceae) |
[52] |
|
|
|
Chrysin |
Oroxylum indicum (Bignoniaceaea) |
[52] |
|
|
|
Vitexin |
Passiflora incarnate (Passifloraceae) |
[45] |
|
|
|
Flavonones |
Narginin |
Rosmarinus officinalis L. (Lamiaceae) |
|
|
|
Hesperidin |
Citrus medica (Rutaceae) |
[46] |
|
|
|
Liquiritin |
Glyccheriza glabra (Leguminosae) |
[45] |
|
|
|
Kurarinol; kurarinone |
Sophora flavescens Ait. (Fabaceae) |
[61] |
|
|
|
Flavononols |
Kushenol I; kushenol N |
Sophora flavescens Ait. (Fabaceae) |
[61] |
|
|
Isoflavones |
Genistein |
Calopogonium muconoides (Fabaceae) Butea monospermea (Fabaceae); Andira macrothyrsa (Fabaceae); |
[41] [51] |
|
|
Biochanin A |
Cratylia argentea (Fabaceae); A. macrothyrsa (Melastomataceae) |
[41] |
|
Among the medicinal plants the Fabaceae family are mostly investigated: flavonoles are found in Bauhinia monandra [45], Clitoria ternatea [47], and Pongamia pinnata [53]; flavonones and flavononols – in Sophora flavescens Ait [51]. Best represented are of course the isoflavones – in Calopogonium muconoides, Butea monospermea, Andira macrothyrsa, and Cratylia argentea [41][51][41, 51].
Lamiaceae family is also good presented: plants from this family are rich source of flavones and flavonones, for example Rosmarinus officinalis L. [58][59][58, 59]. From other members of this family are isolated flavones: Salvia officinalis L. [58], Thymus L. [58][60][58, 60], and Mentha longifolia [57]. Flavonoles and flavones are the wide distributed in the medicinal plants flavonoids, just like in the food plants.