1. Introduction
Cancer, the uncontrollable growth of abnormal cells and dynamic alterations in the genome, causes cancerous features in normal cells. The progression of cancer impairs normal cell growth and processing, disrupting cellular metabolic “checkpoints”, eventually leading to what is established as “hallmarks of cancer”. These hallmarks are commonly known as universal characteristics of the pathology, such as sustained proliferative signaling and uncontrolled proliferation, evasion of growth suppressors, tissue invasion and metastasis, replicative immortality, promotion of angiogenesis and resistance to programmed cellular death
[1]. Currently, it is known that several other factors contribute to the development of the pathology, such as the tumor microenvironment and the associated microbiota
[2]. Since the discovery of the pathology, therapies and strategies for the treatment of cancer are constantly improving, with efforts in the discovery of new chemotherapies, new strategies for surgery, improved and focused radiotherapy and overall new insights and approaches to a disease as variable as the individuals that carry it.
Unfortunately, therapeutic approaches still come with risks to the patients: chemotherapies are often related to severe cytotoxicity
[3]. Cancer therapy targets cancer cells, characterized by a high basal level of proliferation. Consequently, non-cancer cells with high proliferation rates are affected by the damaging effects of ionizing radiation and chemicals of traditional therapies, such as skin, hair and gastrointestinal epithelium. Other complications, such as nephrotoxicity and neurotoxicity, often arise due to the toxic effect of chemical medications, as anticancer toxic substances alter the normal activity of the physiological systems
[4].
Even though there have been incredible advances in the last century of cancer combat, the paramount challenges remain undisputed, especially characteristics such as metastization, cancer cell stemness and pathology relapse, with cancerous cells displaying high levels of resistance to previously used chemotherapeutics
[5,6][5][6]. Indeed, cancer resistance to chemotherapeutics is responsible for most of the failures in the treatment of the pathology. This resistance can be attributed to a myriad of factors, external or internal, in which the result is the inefficiency of the drug treatment. Therefore, there is high importance in finding new compounds that can be integrated into cancer treatments to ameliorate life quality. Although there is the availability of anti-neoplastic drugs and chemotherapy, the detrimental aftermath has sparked the search for natural products for potential use as new therapeutic agents
[7]. In that context, natural products from the marine environment are considered to have enormous potential due to their bioavailability, specific and strong binding to drug targets and ability to bind to proteins with minimal entropy loss. Marine algae contain various phlorotannin derivatives, which are regarded as potent pharmacological polyphenols
[8]. The increasing interest in macroalgae phenolic content research and therapeutic development has boosted the knowledge of possible bioactive compounds of interest that can be explored and developed. Among them, phlorotannins demonstrated bioactivity to cover a wide variety of ailments.
Phlorotannins are a unique set of compounds found in high concentration in brown macroalgae and in fewer amounts on red and green macroalgae and are structurally analogous to tannins from terrestrial plants. They are polymeric chains of phloroglucinol (1,3,5-tryhidroxybenzene) residues bound through C-C or C-O-C couplings. These compounds are highly hydrophilic, with molecular weights ranging from 160 Da to 650 kDa
[5]. The biosynthetic pathway for the formation of phloroglucinol and consequently phlorotannin compounds are yet to be fully elucidated; however, it is assumed to occur via the condensation of acetate and malonate units through the shikimate or phenylpropanoid pathways. Presumably, two acetyl-CoA molecules in the presence of carbon dioxide are converted to malonyl-CoA. This process is catalyzed by a type III polyketide synthase, and the resultant chain undergoes cyclization and tautomerization, forming the end molecule phloroglucinol. Polymerization of the phloroglucinol moiety (through the above-mentioned C-C or C-O-C couplings) defines the structural variability characteristic of phlorotannins, which are further classified as fucols, phloroethols, fucophloroethol, eckols, fuhalols and carmalols
[5,7][5][7]. Specifically, eckols contain an additional hydroxyl group on the terminal monomer and are characterized by the presence of 1,4-dibenzodioxin in their structure, exemplified by eckol, dieckol and 2-phloroeckol.
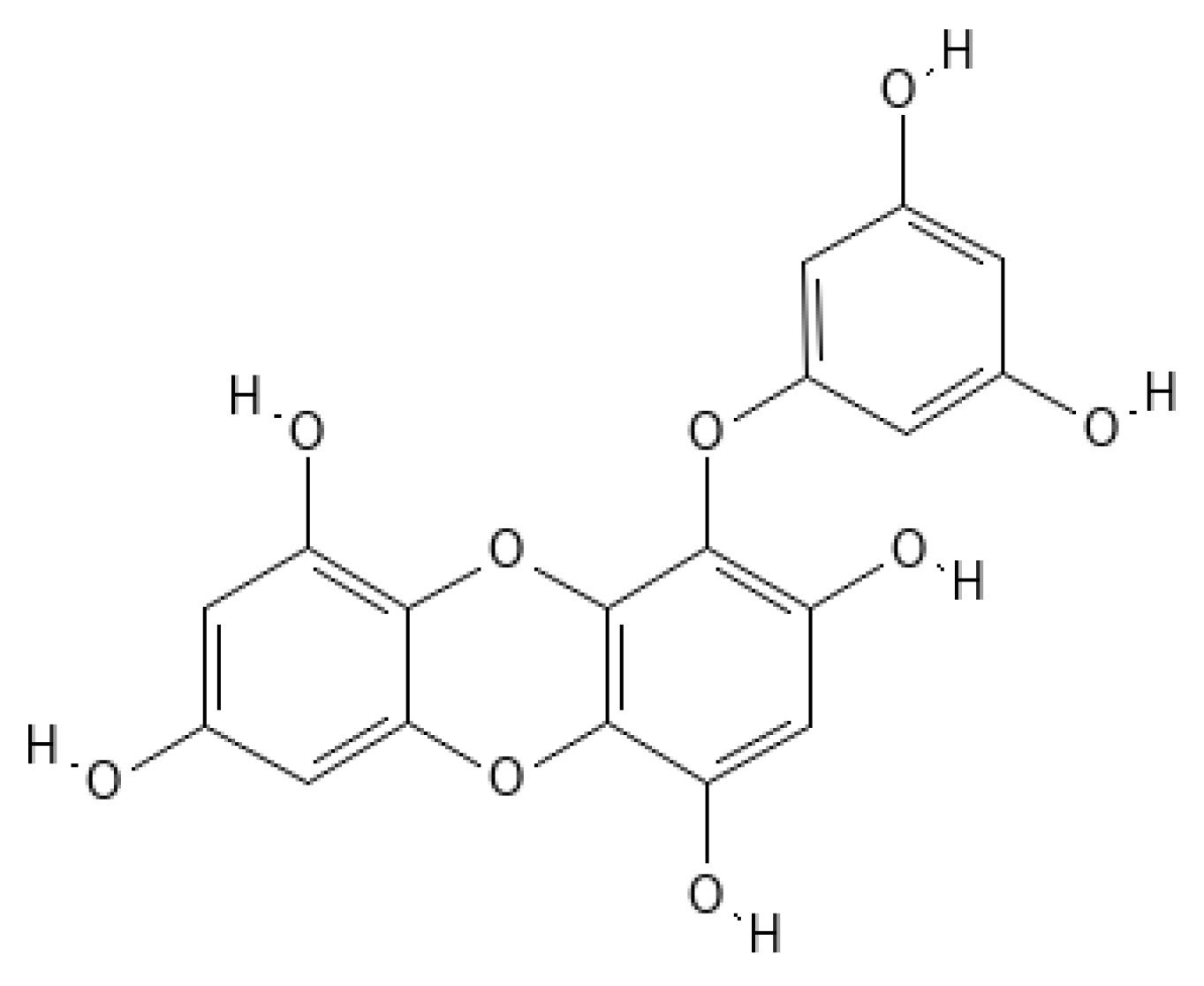
Eckol (
Figure 1) belongs to a class of phlorotannins, known to exist in high concentrations in the
Ecklonia genus
[7] but also found in other genera, such as
Fucus or
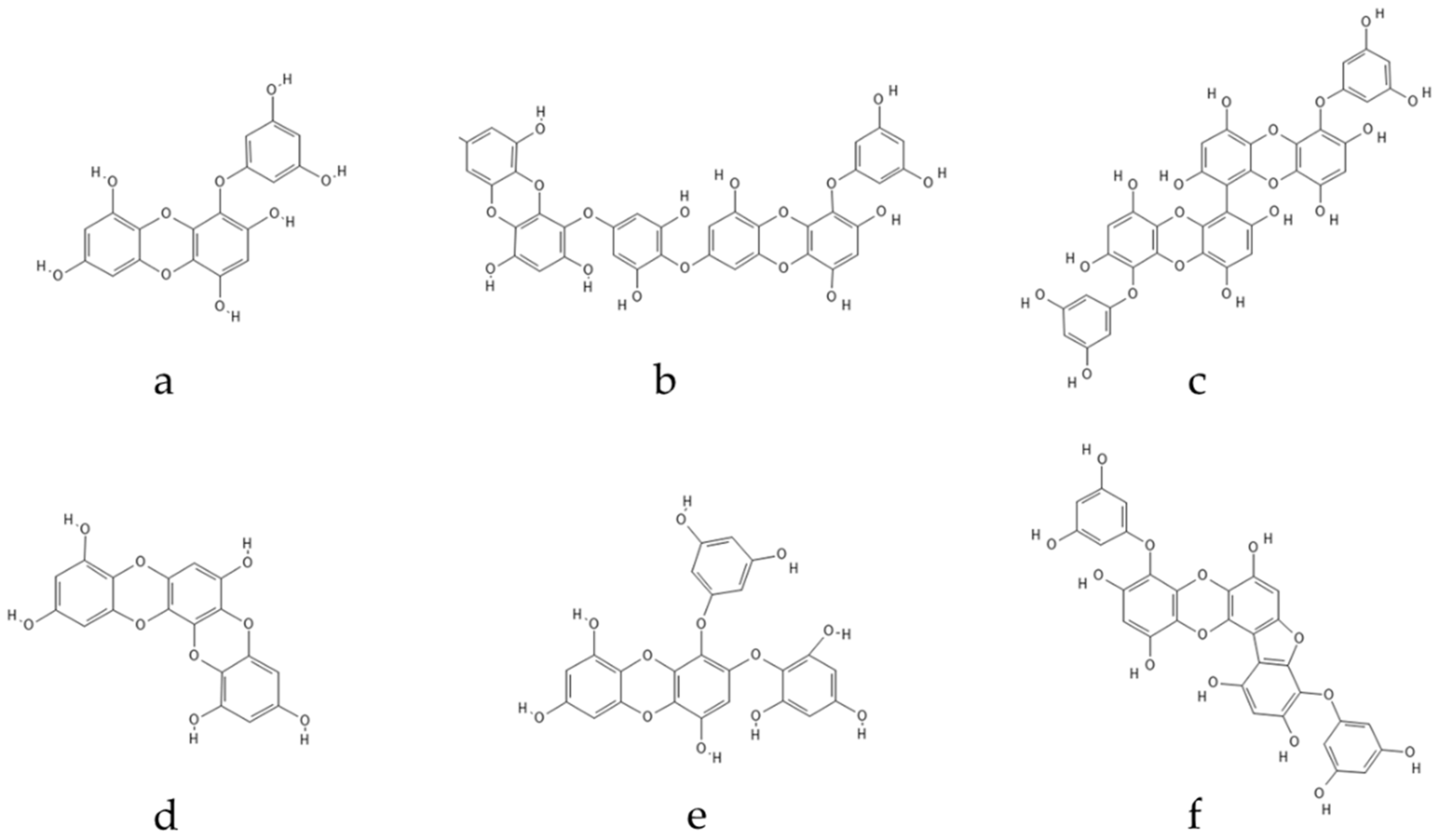
Ascophyllum. Eckol, a precursor compound illustrating the dibenzo-1,4-dioxin class of phlorotannins, contains phloroglucinol components linked to each other in multiple fashions, forming molecules such as dieckol, bieckol and phlorofurofucoeckol, among others (
Figure 2). Eckol enriched and purified extracts have been shown to exhibit antioxidant, anti-inflammatory, hepatoprotective, neuroprotective, anti-obesity, anti-hypertensive, antibacterial, antiviral, anti-cancer and radioprotective activities
[7]. Due to its numerous health properties, this compound has gathered much attention.
Figure 1. Eckol structure.
Figure 2. Eckol-class compounds: (a) Eckol; (b) Dieckol; (c) 6,6-Bieckol; (d) Dioxinodehydroeckol; (e) 2-phloroeckol; (f) Phlorofucofuroeckol.
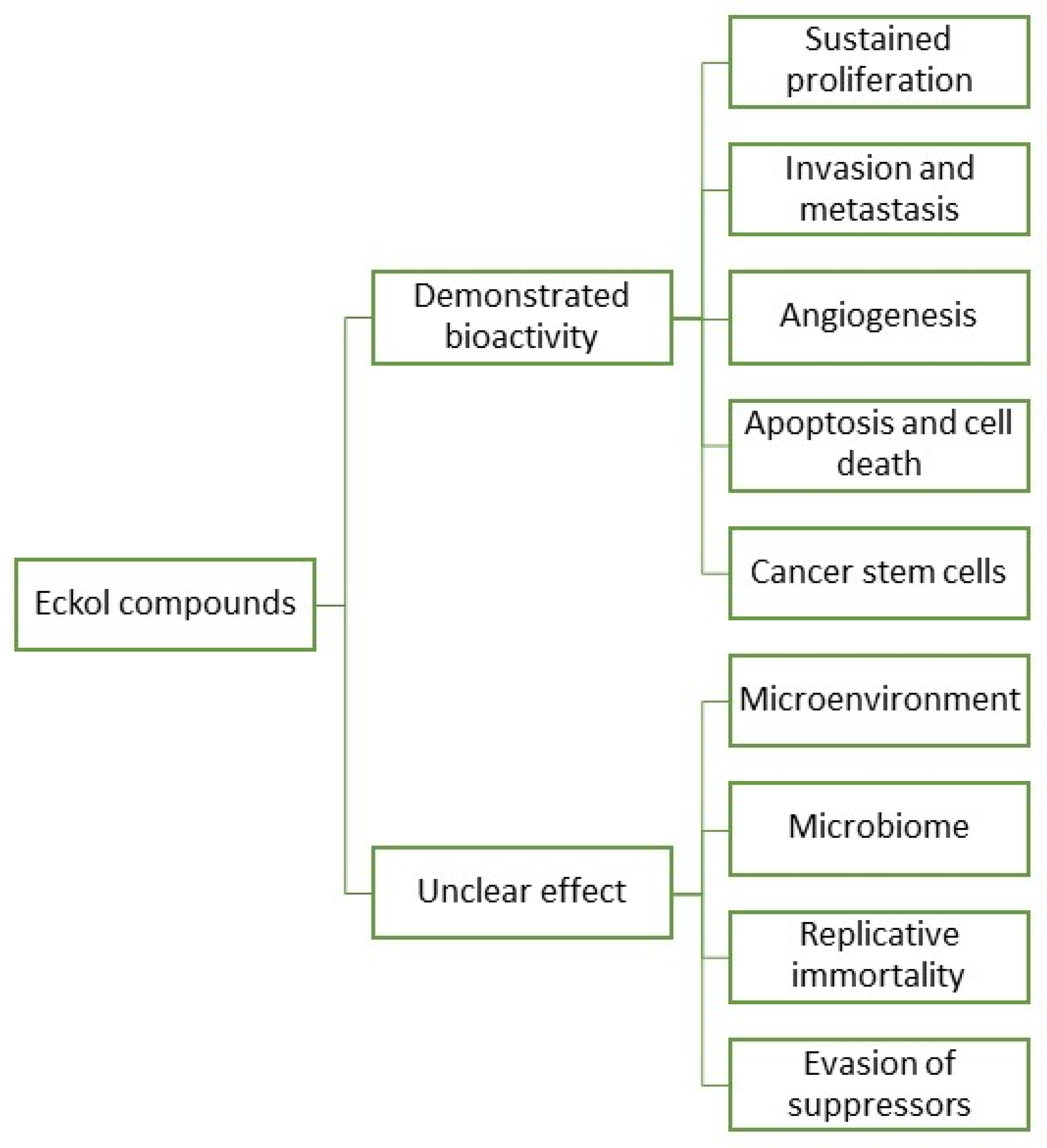
In fact, research and development for applications of eckols are gaining momentum, with a growing body of literature targeting the biotechnical applications of these compounds. However, information regarding eckols anti-cancer compounds is loose and spread, which makes it hard to infer their range of applications. As such, it is the objective of this review to o cluster together the available information regarding eckols in the known hallmarks of cancer in order to enlighten the mechanisms of action by which these compounds exert their bioactive properties. Additionally, the future prospects of this class of compounds are also assessed, with insight into how other cancer hallmarks can be explored as targets for these compounds (Figure 3). There is mention of the interesting radioprotective effects of eckols with potential as therapeutic adjuvants, and finally, challenges in the extraction, purification and biological properties of eckols, such as their bioavailability and toxicity, are addressed.
Figure 3. Eckol compounds demonstrated an unclear bioactivity against cancer hallmarks.
2. Eckols as Potential Agents against Cancer Hallmarks
2.1. Eckols in Sustained Proliferation and Signaling
One of the most fundamental traits of cancer is sustained unregulated proliferation. In contrast to the careful regulation of growth and division in normal cells, cancer cells have unchecked production of growth signals, over-activating growth pathways. Generally, these alterations into prolific phenotypes occur through mutations in specific regulatory genes
[1]. Cancer cells can sustain proliferative signaling in several ways. They produce growth factors themselves, to which they produce cognate receptors denominated autocrine proliferative stimulation, as well as signals to stimulate normal cells in the environment (tumor-associated stroma), which reciprocates through the production of growth factors for the cancer cell. Overproduction of surface receptors, conferring hypersensitivity of the cancer cell to a somewhat limiting factor of the milieu, was also reported
[1,8,9][1][8][9]. There are several signaling pathways for growth and proliferation that can be altered into oncogenic mutations. Mitogen-activated protein kinase (MAPK) pathways are a conserved family of kinase modules that connect extracellular signals to intracellular machinery that regulates fundamental processes such as growth, proliferation, differentiation, migration and apoptosis
[9]. The phosphatidylinositol 3-kinase/protein kinase-B/mechanistic target of the rapamycin (PI3K/AKT/mTOR) signaling pathway is a key intracellular pathway that regulates the survival, cell growth, differentiation, cellular metabolism and cytoskeletal reorganization of cells in reaction to a wide range of signals, including growth receptor tyrosine kinases (RTK) and G-protein coupled receptors
[10]. NF-κB transcription factors are responsible for regulating the expression of key genes for innate and adaptive immunity, cell proliferation and survival and lymphoid organ development. NF-κB is found to be activated in many cancers by several stimuli, including pro-inflammatory cytokines such as IL-1β, epidermal growth factor (EGF), T-cells and B-cell mitogens, among others
[11].
Some studies have demonstrated the potential effects of eckols as therapeutic agents against sustained proliferation (
Figure 3 and
Table 1). In a study by Zhang et al.
[12], eckol was reported to inhibit the gene protein Reg3A, which induces the initiation, survival, growth and proliferation of pancreatic cancer cells. The human PaC cell line, SW1990, was used to test the antiproliferative effect of eckol. Cells were treated with 5, 10 or 20 µg/mL of eckol for 72 h. The treatment did not result in a significant change in cell viability for any concentration, but when cells were treated with Reg3A and eckol, results suggested that eckol did not have a significant direct cytotoxic effect on human SW1990 PaC cells but attenuated the Reg3A-mediated increase in SW1990 cell survival. Additionally, it appears that eckol reverted the Reg3A-mediated upregulation of JAK2, STAT3, NF-κB and cyclin D1, proteins involved in signaling pathways related to the proliferation, migration and apoptosis of cells, promoting a reduced proliferation of pancreatic cancer cells
[12]. A reduced proliferation of cancer cell lines has been tested on the viability of three cellular lines (HeLa, H157 and MCF7) with compounds extracted from
Ecklonia maxima (Phaeophyceae)
[13]. Pretreated cells with eckol revealed a decrease in viability correlated with increasing eckol concentration, confirming the strong antiproliferative activity of eckol on HeLa, H157 and MCF7 cell lines. From the results, the authors believe that the high proliferation activity was due to cytotoxic activity of eckol against the cell lines, as the compound possesses functional hydroxyl groups that are well-positioned in the dibenzodioxin moiety that is effectively exposed to the cells and exert antiproliferative action. Dioxinodehydroeckol from
E. cava has also been investigated against human breast cancer cells; the analysis confirmed the antiproliferative action through reduced expression of Bcl-2 anti-apoptotic proteins and NF-κB, triggered by the presence of dioxinodehydroeckol in tumoral cells. These pre-clinical studies suggest eckol class phlorotannins as potential candidates to perform clinical tests for breast cancer therapy
[14]. Dieckol extracted from
Ecklonia cava (Phaeophyceae) inhibited 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced human hepatocellular carcinoma SK-Hep1 cell motility, and dieckol inhibited extracellular signal-regulated kinases 1/2 and c-Jun N-terminal kinases (JNKs), but not p38 Mitogen-Activated Protein Kinase (MAPK). TPA-induced activator protein-1 transcriptional activity was reduced by dieckol. Furthermore, dieckol administration significantly inhibited TPA-induced matrix metalloproteinase-9 (MMP-9) activity in SK-Hep1 cells. These findings demonstrate that dieckol acts as a powerful inhibitor of tumor promoter-mediated MAPK signaling pathways, resulting in the activation of Activator Protein 1 (AP-1) and MMP-9, and hence govern cancer cell motility
[15]. In a study by Wang et al.
[16], dieckol inhibited the proliferative and migratory properties of non-small–cell lung cancer cell line A549, interfering with Pi3K/AKT/mTOR signaling and caspases levels. It also induced cell apoptosis, mediating the increase of the tumor suppressor protein E-cadherin. Moreover, eckol assisted in the treatment of spheroid-forming glioma cells. Eckol successfully decreased sphere formation as well as CD133 cell population, blocking both Pi3K-AKT and Ras-Raf-1-Erk signaling pathways. It additionally suppressed the expression of glioma cell markers without causing cell death and significantly attenuated anchorage-independent growth on soft agar and tumor formation in xenograft mice. Importantly, eckol reduced the resistance of glioma-stem cells to ionizing radiation and temozolomide
[17]. Furthermore, eckol suppresses stemness and malignancies, sensitizing glioma-stem-like cells to anticancer treatments, which can provide an alternative to current cancer treatments
[18]. Eckol demonstrated the highest activity against HeLa cells, H157 and MCF7, which makes eckol a promising therapeutic candidate
[13]. Overall, and concerning the traditional proliferative pathways that are known to be altered in cancer, eckols demonstrated potential activity as antiproliferative compounds, acting to some degree in signalization pathways, consequently halting the characteristic uncontrolled growth. It would be interesting to assess how and if eckols have any impact on the Wnt/β-catenin pathway. The Wnt/β-catenin signaling pathways are another cascade of signalization important in regulating cell proliferation, differentiation and migration. Reports demonstrate how this pathway is involved in several cancers (colorectal, breast), and reportedly, most alterations involve the stabilization of β-catenin, which is presumed to be correlated with tumor aggressiveness
[11,19][11][19].
Table 1. Anticancer activity of eckols.
2.2. Eckols in Invasion and Metastasis
Cancer cells develop morphological alterations, as well as attachment modifications to other cells in the extracellular matrix (ECM). The best-characterized alteration in carcinoma cells is the loss of E-cadherin, a key adhesion molecule that forms adherens junction with neighboring cells. Increased expression of E-cadherin is well established as an antagonist of invasion and metastasis, and the reduction of E-cadherin expression levels is known to potentiate these phenotypes. The expression of genes encoding cell-to-ECM adhesion molecules is altered in aggressive carcinomas, while genes promoting cytostasis are often downregulated
[32]. This stepwise invasion program is believed to be regulated by the epithelial-mesenchymal transition (EMT), prominently implicated as a means by which transformed epithelial cells can acquire the ability to invade, resist apoptosis and disseminate into other tissues
[33,34][33][34]. This EMT program can be activated transiently or stably during the course of invasion and metastasis
[7,20][7][20]. A set of transcription factors is believed to regulate EMT, such as Snail, Slug and Zeb1/2
[13]. Several of these factors can directly repress E-cadherin gene expression, allowing for the motility and invasiveness of cancer cells.
Dieckol derived from
E. cava has been investigated in vitro against human fibrosarcoma cell invasion. Dieckol interfered with the migration and invasion of the HT1080 cell line by scavenging intracellular reactive oxygen species (ROS), which are also responsible for increasing migration and invasion of tumoral cells
[25]. The inhibition of non-small–cell lung cancer cells migration and proliferation has also been detected in vitro using 6,6-bieckol from
Ecklonia cava [20]. The compound inhibited the migration and induced apoptosis of tumoral cells, increasing the expression of E-cadherin and down-regulated Snail1 and Twist1 transcriptional levels, whose high expression is associated with lower survival rates in patients with cancer
[35]. Lee et al.
[21] tested both dieckol and phlorofucofuroeckol from
Ecklonia cava to determine their antiproliferative and anti-invasive activity on human breast cancer MCF-7 and MDA-MB-231 cells. Dieckol and phlorofucofuroeckol reduced the migration and invasion of tumoral cells, as well as decreased the expression of receptor 4 (TLR-4) and NF-κB promoter-driven transcriptional activity, which is essential for cells proliferation, migration, tumor growth and inflammation. Dieckol reduced CoCl2-induced EMT in HT29 cells. Furthermore, dieckol therapy reduced ROS production, EMT marker protein expression and intracellular localization, cell motility and cell invasion. Dieckol may suppress hypoxia-induced EMT in HT29 cells via modulating cellular ROS and protein expression levels downstream of the HIF-1α signaling pathway
, according to the findings of this study. Therefore, dieckol has the potential to be a promising therapeutic agent in the treatment of colorectal cancer
[22]. Dieckol’s anticancer property against the non-small–cell lung cancer (NSCLC) cell line A549 is mediated by the inhibition of the invasive and migratory properties of A549 cells, as well as induction of apoptosis via inhibition of Pi3K/AKT/mTOR signaling and activation of the tumor suppressor protein E-cadherin, indicating that dieckol is a potent natural anticancer drug to treat NSCLC
[36,37][36][37]. Dieckol extracted from
Ecklonia cava inhibits Wiskott–Aldrich syndrome protein (WASP)-family verprolin-homologous protein 2 (WAVE2)-mediated invasive migration of B16 murine melanoma cells. The steady-state intracellular ROS levels in malignant B16F10 cells were greater than in parental, nonmetastatic B16F0 cells. H
2O
2 treatment boosted B16F0 cell migration and invasion capacity to levels comparable to B16F10 cells, suggesting that intracellular ROS signaling underlies the pro-metastatic features of B16 murine melanoma cells. ROS levels, as well as B16 melanoma cell motility and invasion capacity, were shown to be connected to Rac1 activation and WAVE2 expression. Overexpression of dominant negative Rac1 and siRNA-mediated WAVE2 depletion inhibited H
2O
2-induced cell invasion in B16F0 and B16F10 cells. Similarly, dieckol inhibits ROS-mediated Rac1 activation and WAVE2 expression, resulting in reduced B16 melanoma cell motility and invasion. Furthermore,
wresearche
rs discovered that dieckol inhibits the interaction of WAVE2 with the NADPH oxidase component p47phox. As a result, the WAVE2 functions to link intracellular Rac1/ROS signaling to B16 melanoma cell invasive migration is blocked by dieckol
[24]. Additionally, dieckol reduced MMP-2 and -9 expression in a dose-dependent manner, as well as cell invasion and cytomorphology in a 3D culture system on HT1080 cells. Furthermore, dieckol may influence the NF-κB route while having no effect on the activator protein-1 (AP-1) pathway or the tissue inhibitor of metalloproteinases (TIMPs). Finally, dieckol was able to drastically decrease MMP-2 and -9 expression as well as change the cytomorphology of the HT1080 cell line via the NF-κB pathway
[38].
2.3. Eckols in Angiogenesis
During tumor progression, an angiogenic switch, which is turned transiently in adult individuals, is almost permanently activated, which causes vasculature to continuously sprout new blood vessels and sustain neoplasic growths. These angiogenic switches are regulated by a balance of factors that induce or suppress angiogenesis. A well-known angiogenic inducer is the vascular endothelial growth factor A (VEGF-A)
[1,2][1][2].
The VEGF-A gene encodes ligands involved in forming new blood vessel growth during embryonic and postnatal development. VEGF-A signaling is modulated by three tyrosine kinases (VEGFR 1-3) and can be regulated both by hypoxia and oncogene signaling, thus sustaining tumor masses. Hypoxic conditions promote the expression of hypoxia-induced factors (HIF-1α) that lead to the expression of angiopoietin, matrix metalloproteinases (MMP’s) and VEGF, as well as other pro-angiogenic signals such as members of the fibroblast growth factor (FGF), that have been revealed to play a role in sustaining tumor angiogenesis when their expression is upregulated
[39,40][39][40]. In some tumors, dominating oncogenes such as Ras and Myc can upregulate the expression of angiogenic factors, while in other cases, these factors are produced indirectly by immune cells.
A recent study by Shengtao Yang et al.
[41] described how 7-phloro-eckol from
E. cava demonstrated inhibition in pro-angiogenic factors in liver cancer, through analysis of the secretion of MMP-1, MMP-9 and VEGF proteins and HIF-1α under hypoxic conditions, in HepG2 and HUVEC cells. The results obtained demonstrated that 7PE could inhibit the production of HIF-1α under hypoxic conditions through the regulation of the MAPK and Pi3K pathways, consequently blocking the expression of VEGF. Saadeesh Kumar et al.
[30] explored how the administration of dieckol would modulate the expression of pro-angiogenic factors (MMP, VEGF) in rats with N-nitrosodiethylmine (NDEA)-induced hepatocarcinogenesis. Their results demonstrated a reduction in the expression of MMP-2, MMP-9 and VEGF in NDEA rats treated with dieckol by more than 15% when compared to the expression in NDEA rats.
Overall, the literature supports the anti-angiogenic potential of eckols in hepatocellular cancer through the modulation of signaling pathways and factors in the development of new vasculature. It would be interesting to verify if the reduction in angiogenesis, demonstrated in hepatocarcinogenic models, is reflected in other cellular lines. One interesting observation could be that the direct regulation of angiogenesis by oncogenes that also drive proliferative signaling presumes that distinct tumor characteristics can be co-regulated by the same transforming agents
[39].
2.4. Eckols in Resisting Programmed Cell Death (Apoptosis)
Programmed cell death by apoptosis serves as a natural barrier to cancer development. Apoptosis is triggered in response to physiological stresses cancer cells suffer during tumorigenesis and anticancer therapy
[42]. The apoptotic “trigger” that regulates signals between regulators and effectors is controlled through a counterbalance between pro and anti-apoptotic members of the Bcl-2 family of regulatory proteins. Bcl-2 and its closest relatives (Bcl-xl, Bcl-2, Mcl-1 and A1) are apoptosis inhibitors that act through binding and suppressing two pro-apoptotic triggering proteins, Bax and Bak, that are embedded in the mitochondrial outer membrane. When these two pro-apoptotic proteins are relieved of inhibition by the Bcl-2 family, Bax and Bak disrupt the mitochondrial outer membrane, consequently releasing pro-apoptotic signaling proteins, in which the major player is cytochrome
c. The released cytochrome
c activates a cascade of caspases that, through their proteolytic activities, induce cellular changes associated with apoptosis
[42].
Several abnormality sensors that play key roles in tumor development have been identified, notably the DNA-damage sensor that functions via the TP53 tumor suppressor. TP53 induces apoptosis by upregulating the expression of Noxa and Puma BH3-only proteins through response to the number of DNA damage and other chromosomal abnormalities. In contrast, insufficient factor signaling (e.g., low levels of interleukin-3 in lymphocytes or low insulin-like growth factor 1/2 [Igf1/2] in epithelial cells) can elicit apoptosis through a BH3-only protein called Bim. Furthermore, hyperactive signaling by oncoproteins such as Myc can trigger apoptosis, in part via Bim and other BH3 proteins
[43].
Tumor cells acquire a variety of strategies to limit or evade apoptosis. Loss of the TP53 tumor suppressing function eliminates the damage-sensor from the apoptosis circuitry, and these ends can also be achieved by increasing the expression of anti-apoptotic regulators (Bcl-2) and survival signals (Igf 1/2), by downregulation of pro-apoptotic factors such as Bax, Bim and Puma, or by short-circuiting the extrinsic ligand-induced death pathway. The variety of apoptosis evading mechanisms can reflect the diversity of apoptosis-inducing signals that cancer-cell populations encounter in the evolution to the malignant state.
The therapeutic potential of dieckol against 7,12-dimethylbenz(a)anthracene (DMBA)-induced skin carcinogenesis (in mice) studies indicated that 30 mg/kg of dieckol supplementation significantly restored body and liver weight and reduced tumor incidence in DMBA-incited animals. In DMBA-induced rats, dieckol administration increased antioxidants (SOD, CAT, GPx and GSH) while decreasing phase-I enzymes Cyt-p450 and Cyt-b5. Dieckol also reduced pro-inflammatory modulators such as IL-6, IL-1 and TNF-α. Furthermore, the data showed that dieckol blocked the IB/NF-κB signaling pathway. Furthermore, dieckol increased the production of a pro-apoptotic protein (p53, Bax, caspase-3 and -9). Dieckol inhibited DMBA-induced skin cancer in mice, suggesting that it might be a promising agent
[23]. Molecular pathways on dieckol have been explored for ovarian cancer cells (SKOV3 and A2780) subcutaneously inoculated in a xenograft mice model. Results evidenced that mice treated with dieckol have cytotoxic effects on A2780 and SKOV3 ovarian cancer cells, activate the expression of apoptotic proteins caspase-8, caspase-9 and caspase-3 and decrease the expression of AKT and p38, which are involved in cell apoptosis
[29]. Dieckol also suppressed in vivo NDEA-initiated hepatocarcinogenesis by modulating xenobiotic-metabolizing enzymes, inducing apoptosis cascade and inhibiting proliferation, invasion and angiogenesis signaling
[30]. Yoon et al.
[28] investigated dieckol from
Ecklonia cava subsp.
stolonifera (formerly
Ecklonia stolonifera) (Phaeophyceae) on human hepatocellular carcinoma (HCC) Hep3B cells. Results showed that cells treated with dieckol had reduced viability in a dose-dependent way; the presence of dieckol increased the permeability of mitochondrial membranes and the release of cytochrome
c from mitochondria into the cytosol, which triggered apoptosis along with the proteins Bid, Bim, BAK, caspases-3, 7, 8 and 9 and cleaved poly (ADP-ribose) polymerase, all found in high expression in cells treated with dieckol. Thus, eckols demonstrate induction of apoptosis via the activation of both death receptor and mitochondrial-dependent pathways in tumoral Hep3B cells
[28]. In vivo anti-tumoral effect of eckol has been examined in the development and progression of transplanted sarcoma in sarcoma 180 (S180) xenografts-bearing mice. Compared with the model control group, cells treated with eckol showed inhibitory tumoral effect at dosages of 0.5 and 1.0 mg/kg, revealing an inhibition by 36% and 52%, respectively, through apoptosis and antiproliferative activity, through modulation of the protein expression levels of Caspase-3 and Caspase-9 (two key apoptotic proteins) and Bcl-2 and Bax (two key anti-apoptosis-related genes), as well as epidermal growth factor receptor (EGFR, a proliferation stimulating protein). Results confirmed that eckol had no inhibitory effect on the weights of the thymus and spleen, whereas the spleen index of S180-bearing mice was significantly enhanced by 1.0 mg/kg eckol compared with the model control. It might suggest that eckol might have the potential to activate the anti-tumor immune response in vivo. A significant result is the stimulation of innate and adaptive immune responses in treated mice; activation of the mononuclear phagocytic system has been detected, along with major recruitment and activation of dendritic cells, which promoted the type 1 helper T cells anti-tumor response, increased the CD4+/CD8+ T lymphocyte ratio and enhanced cytotoxic T lymphocyte responses
[26,27][26][27]. This pro-apoptotic capacity demonstrated by dieckol has also been detected in phlorofucofuroeckol A and dioxinodehydroeckol.