Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Peter Tang and Version 2 by Peter Tang.
Cobalamin or vitamin B12 (B12) is a cofactor for methionine synthase and methylmalonyl-CoA mutase, two enzymes implicated in key pathways for cell proliferation: methylation, purine synthesis, succinylation and ATP production. Ensuring these functions in cancer cells therefore requires important cobalamin needs and its uptake through the transcobalamin II receptor (TCII-R).
- neoplasms
- vitamin B12
- transcobalamins
- metabolism
1. Cobalamin: from absorption to intracellular metabolism
Cobalamin, or vitamin B12 (B12), is only produced by certain bacteria and archaea, and is composed of a corrin ring centered by a cobalt atom in mono, bi or trivalent form, which links a variable residue by either adenosyl-, methyl-, hydroxyl- or cyano- group [1]. Cobalamin is an essential vitamin for human beings as human cells cannot synthetize it, and its daily dose comes from ruminants and fish meat as the main food source [2][3]. Cobalamin is released from food cobalamin-binding proteins by the acidity of gastric juice, then carried by salivary haptocorrin, which is later degraded by pancreatic enzymes. Then, cobalamin is linked to the intrinsic factor produced by gastric parietal cells and uptaken via endocytosis by the cubam receptor, a complex formed by the cubulin and the amnionless transmembrane protein, into the terminal ileum [2]. A small cobalamin part is also absorbed via passive diffusion throughout the intestine [4][5]. Once internalized into the enterocytes, the cobalamin is transferred into the lysosome and then transported into the blood [6].
Circulating cobalamin is carried by two proteins: transcobalamin I (TCI, also called haptocorrin) and transcobalamin II (TCII, Figure 1). TCI belongs to R-binder proteins and is encoded by the TCN1 gene. TCI is present in various body fluids and is secreted by many cell types including glandular cells and granulocytes [7][8]. TCI carries most of the blood circulating cobalamin [6][9][10]. Salivary TCI is also important for the enteral cobalamin absorption, and plasma TCI allows liver storage of cobalamin via the asialoglycoprotein receptor (ASGPR) [11]. The ASGPR is highly present in the hepatocytes’ membrane, but the ASGPR has also been found with a lower expression in other normal tissues (salivary glands, small intestine, testes, thyroid, kidneys, brain, lung) [12][13][14][15]. Hepatocellular carcinoma cells have variable levels of ASGPR expression [16][17], but to the researchers' knowledge, no study has demonstrated the absorption of TCI-bound cobalamin through the ASGPR in non-liver malignant cells. Some authors suggested specific roles for TCI: the large proportion of cobalamin bound to TCI prevents the loss of free Cbl, and the relatively low specificity of TCI for cobalamin allows TCI to link corrin-like compounds, called corrinoids, to limit their cell uptake for metabolic use via the TCII-receptor pathway [8].
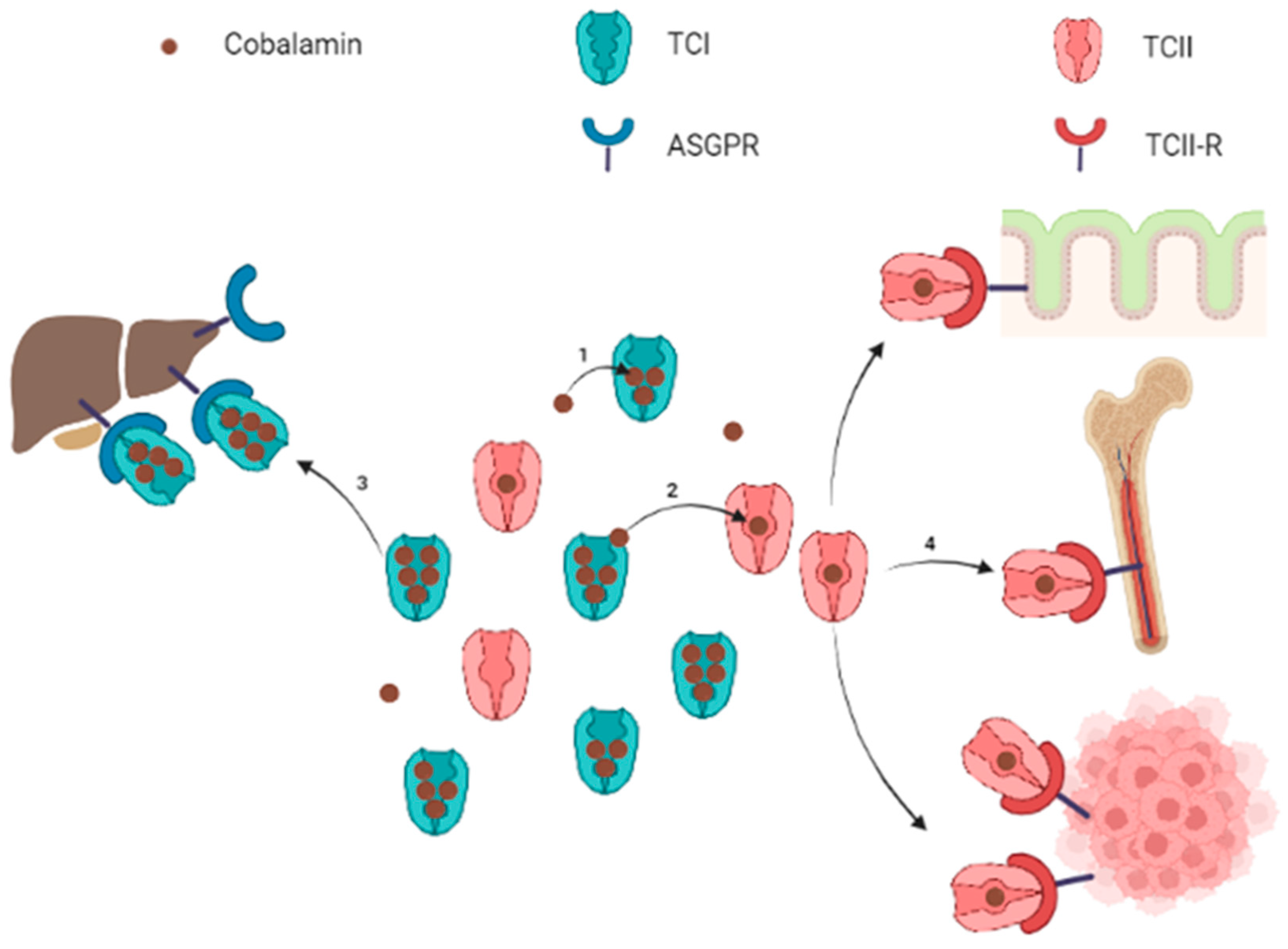 TCII is encoded by the TCN2 gene and binds 10 to 30% of the plasma cobalamin [6][8][9][10]. Circulating TCII is mainly synthetized by endothelial cells, although many kinds of cells may produce faint TCII levels [8][18][19][20][21][22]. TCII is highly specific for cobalamin and is more selective, binding cobalamin rather than corrinoids, compared to TCI. The cobalamin-TCII complex, called holotranscobalamin, binds to the membrane TCII-receptor (TCII-R) in order to provide cobalamin to all cells. The expression of TCII-R on the cell surface is increased in actively dividing cells, whereas it is decreased in quiescent cells [23]. This regulation combined to the efflux of a cellular excess of cobalamin promotes its availability to cells most needing it.
Inside cells, the cobalamin-TCII complex is dissociated in the lysosome and TCII-R is recycled to the plasma membrane, while cobalamin is released into the cytoplasm [8]. Ultimately, cytoplasmic cobalamin either remains in the cytoplasm or is transferred into the mitochondria to form methylcobalamin or adenosylcobalamin, respectively [2].
Within the one-carbon cycle, methylcobalamin is a cofactor for the methionine synthase, which catalyzes the methyl transfer from 5-methyltetrahydrofolate to homocysteine, to produce methionine and tetrahydrofolate [24]. Methionine synthase is a key enzyme of the one-carbon metabolism and methylation process, straddling the folate and methionine cycles (Figure 2). Indeed, methionine is thereafter transformed into S-adenosylmethionine (SAM), which is the universal methyl donor by transformation into S-adenosylhomocysteine (SAH) [25]. Methylcobalamin and methionine synthase are therefore directly involved in the methylation process. Salvage methionine synthesis pathways exist independently of folate and cobalamin, based on the polyamine pathway and the betaine-homocysteine methyltransferase [26], but these pathways are of minor importance and the inhibition of the methionine synthase results in a severe decrease in methylation reactions [27]. In addition, the methionine cycle is involved in reactive oxygen species (ROS) clearance via the transsulfuration pathway, and SAM is also itself an allosteric activator of the transsulfuration pathway through the cystathionine beta synthase (CBS). The activation of CBS is associated with the production of H2S to stimulate angiogenesis [28][29]. Finally, the methionine cycle is also involved in purine bases synthesis through the adenosine generation from SAH.
TCII is encoded by the TCN2 gene and binds 10 to 30% of the plasma cobalamin [6][8][9][10]. Circulating TCII is mainly synthetized by endothelial cells, although many kinds of cells may produce faint TCII levels [8][18][19][20][21][22]. TCII is highly specific for cobalamin and is more selective, binding cobalamin rather than corrinoids, compared to TCI. The cobalamin-TCII complex, called holotranscobalamin, binds to the membrane TCII-receptor (TCII-R) in order to provide cobalamin to all cells. The expression of TCII-R on the cell surface is increased in actively dividing cells, whereas it is decreased in quiescent cells [23]. This regulation combined to the efflux of a cellular excess of cobalamin promotes its availability to cells most needing it.
Inside cells, the cobalamin-TCII complex is dissociated in the lysosome and TCII-R is recycled to the plasma membrane, while cobalamin is released into the cytoplasm [8]. Ultimately, cytoplasmic cobalamin either remains in the cytoplasm or is transferred into the mitochondria to form methylcobalamin or adenosylcobalamin, respectively [2].
Within the one-carbon cycle, methylcobalamin is a cofactor for the methionine synthase, which catalyzes the methyl transfer from 5-methyltetrahydrofolate to homocysteine, to produce methionine and tetrahydrofolate [24]. Methionine synthase is a key enzyme of the one-carbon metabolism and methylation process, straddling the folate and methionine cycles (Figure 2). Indeed, methionine is thereafter transformed into S-adenosylmethionine (SAM), which is the universal methyl donor by transformation into S-adenosylhomocysteine (SAH) [25]. Methylcobalamin and methionine synthase are therefore directly involved in the methylation process. Salvage methionine synthesis pathways exist independently of folate and cobalamin, based on the polyamine pathway and the betaine-homocysteine methyltransferase [26], but these pathways are of minor importance and the inhibition of the methionine synthase results in a severe decrease in methylation reactions [27]. In addition, the methionine cycle is involved in reactive oxygen species (ROS) clearance via the transsulfuration pathway, and SAM is also itself an allosteric activator of the transsulfuration pathway through the cystathionine beta synthase (CBS). The activation of CBS is associated with the production of H2S to stimulate angiogenesis [28][29]. Finally, the methionine cycle is also involved in purine bases synthesis through the adenosine generation from SAH.
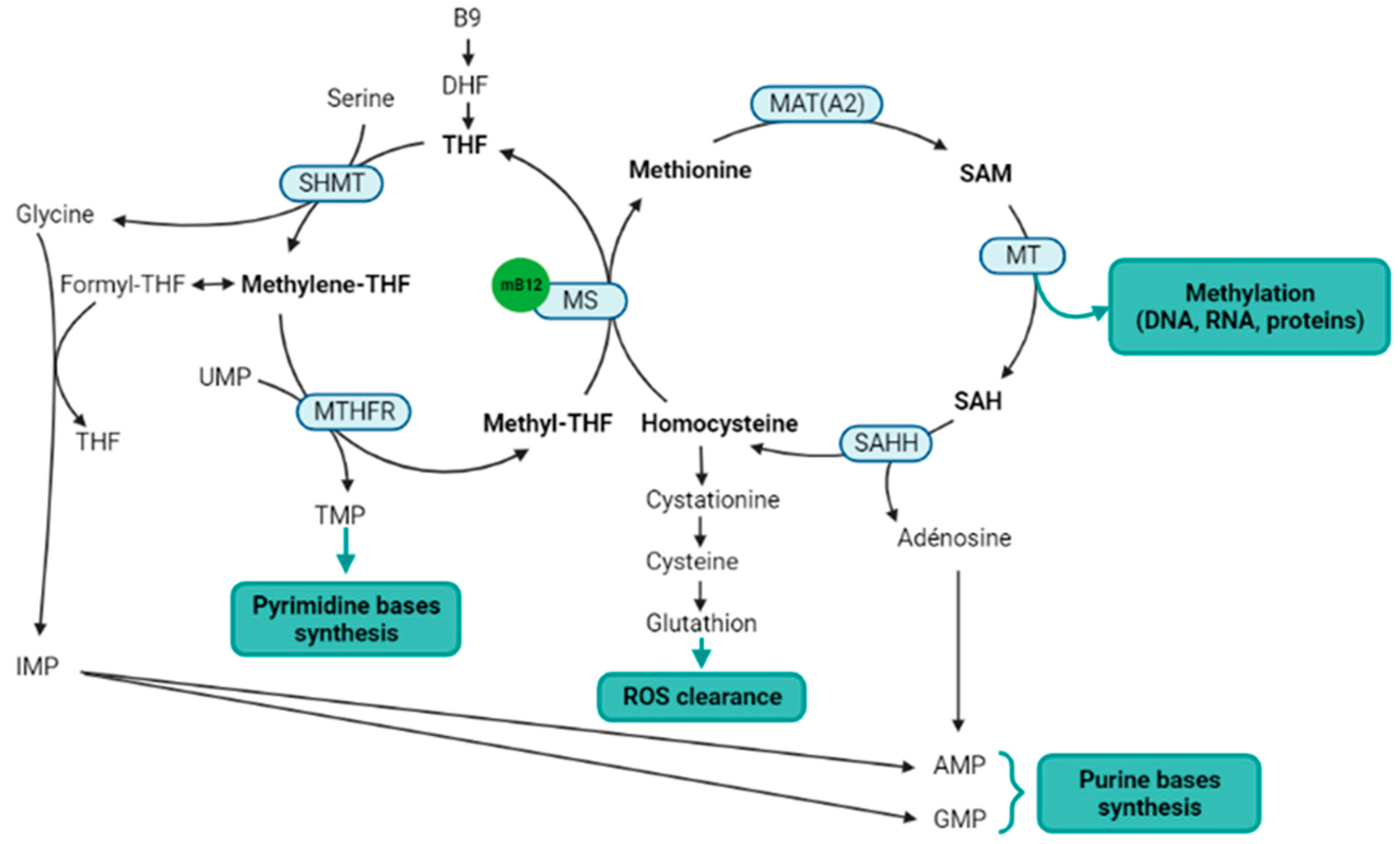
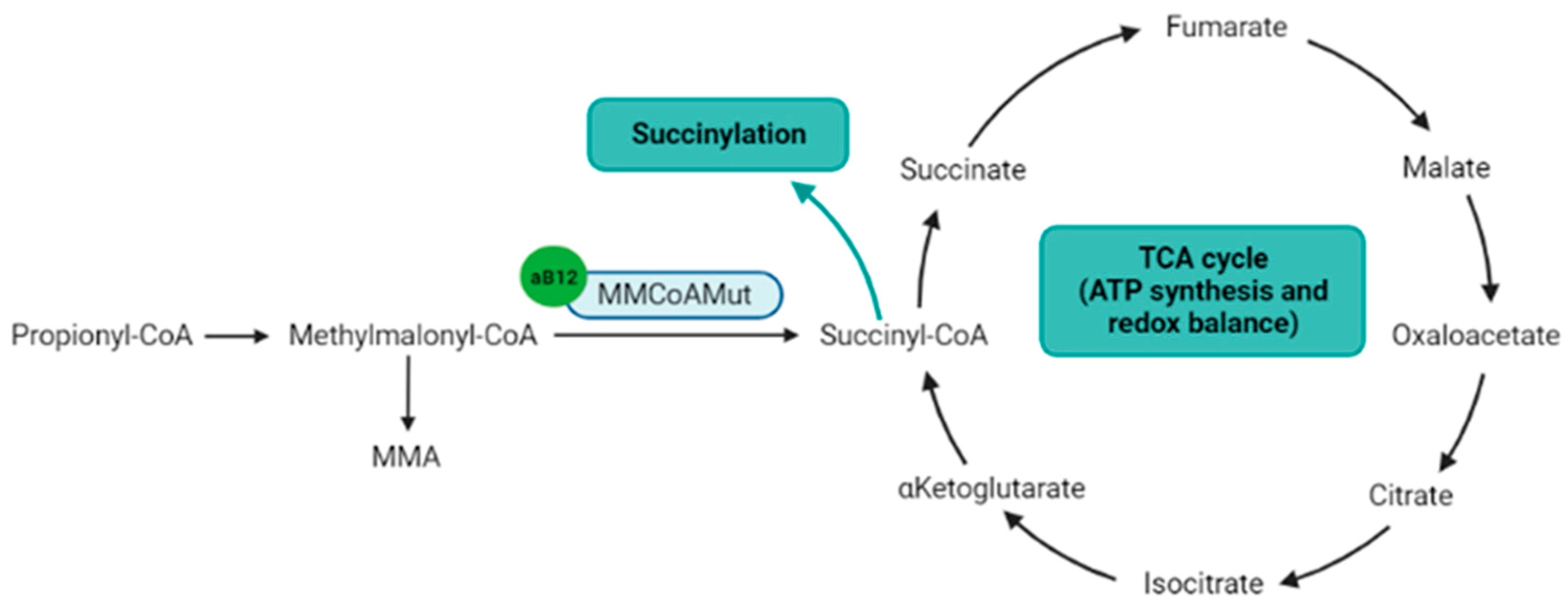

Figure 1. Schematic representation of circulating cobalamin bound to transcobalamins and uptake of transcobalamin-bound cobalamin by target organs. Note: Free cobalamin represents a small part of the total circulating cobalamin. Cobalamin is mostly carried by TCI (1), which constitutes a circulating pool of quickly available cobalamin. Cobalamin carried by TCI can be either transferred to TCII (2) or internalized into liver cells for storage throughout the ASGPR (3). The TCII-bound cobalamin is picked up by cells expressing the TCII-R when cobalamin is required for metabolism (4). The TCII-R is particularly expressed by epithelial cells, bone marrow and in physiological or pathological highly proliferating cells. ASGPR: asialoglycoprotein receptor; TCI: transcobalamin I (haptocorrin); TCII: transcobalamin II; TCII-R: transcobalamin II receptor. Figure created with BioRender.com

Figure 2. Metabolic pathways directly or indirectly related to the methylcobalamin. Notes: Methylcobalamin is a cofactor for methionine synthase, which is the central enzyme of the one-carbon metabolism (metabolites in bold) that includes the folate cycle (left) and the methionine cycle (right). Two methionine salvage pathways are not represented in this figure: a first salvage pathway allows methionine synthesis from homocysteine and betaine thanks to the cobalamin independent betaine-homocysteine methyltransferase, and a second salvage pathway allows the methionine synthesis from SAM via the polyamine pathway. B9: vitamin B9; AMP: adenosine monophosphate; DHF: dihydrofolate; GMP: guanosine monophosphate; IMP: inosine monophosphate; MAT(A2): methionine adenosyltransferase (notably MATA2); mB12: methylcobalamin; MS: methionine synthase; MT: methyltransferases; MTHFR: methylenetetrahydrofolate reductase; SAH: S-adenosylhomocysteine; SAHH: SAH hydrolase; SAM: S-adenosylmethionine; THF: tetrahydrofolate; TMP: thymidine monophosphate; UMP: uridine monophosphate. Figure created with BioRender.com.
The second biologically active form of Cobalamin is the adenosylcobalamin. The adenosylcobalamin is a cofactor for the methylmalonyl-CoenzymeA mutase (MMCoAMut), which converts methylmalonyl-CoA to succinyl-CoA into the mitochondria (Figure 3) [30]. Succinyl-CoA is involved in the succinylation processes, and is a major component of the tricarboxylic acid (TCA) Krebs cycle, which produces ATP, NADH and FADH2, the latter two to feed the respiratory chain to produce ATP [31]. Therefore, the MMCoAMut activity on one hand feeds the TCA cycle, and on the other hand contributes to lysine succinylation and downstream-associated posttranslational modifications [32].

Figure 3. Metabolic pathways directly or indirectly related to the adenosylcobalamin. Notes: Adenosylcobalamin is a cofactor for MMCoAMut enzyme that synthesizes succinyl-CoA, which is a component of the TCA cycle and the substrate for lysine succinylation. aB12: adenosylcobalamin; MMA: methylmalonic acid; MMCoAMut: methylmalonyl-CoA mutase; TCA cycle: tricarboxylic acid cycle (Krebs cycle). Figure created with BioRender.com.
2. Cobalamin and solid cancers
The identification of metabolic pathways promoting cancer cell growth is of major interest in oncology [33][34]. The cobalamin is essential for cell proliferation [2]; consequently, cobalamin-dependent pathways are of high interest to target cancer cells. Cobalamin as a cofactor for methionine synthase and MMCoAMut is implicated in methylation, purine bases synthesis, succinylation and ATP production [2]. These functions are crucial within tumor cells for their proliferation, explaining why the avidity of tumor cells for cobalamin is crucial [12], while the inhibition of B12 uptake in vitro has anti-proliferating effects [35].
In addition to the high B12 needs and uptake by cancer cells, the link between cobalamin and neoplasms also involves B12-binding proteins, the transcobalamins. Before the identification of transcobalamins in the 1960s, it was demonstrated that the plasma cobalamin-binding capacity was raised in myeloid blood malignancies (not detailed here) and in solid cancers [36][37], paralleling the accumulation of a yet unknown cobalamin-binding protein [37]. A few years later, the identification and dosage of transcobalamins [38][39] led to the observation of high transcobalamin levels in solid cancers [40][41][42]. The elevation of total plasma B12 (tB12) and plasma transcobalamins was further associated with solid cancer diagnosis [43][44], and also with metastases [45] and a worse clinical prognosis [46].
Today, cobalamin and transcobalamins can be considered in solid cancers either as potential biomarkers of the diagnosis [45][47][48][49] or the prognosis of cancers [46][50], or as therapeutic targets to affect the avidity of cancer cells for cobalamin [35][50][51][52] or the cobalamin-dependent metabolic pathways [53][54][55][56][57].
References
- Fang, H.; Kang, J.; Zhang, D. Microbial Production of Vitamin B12: A Review and Future Perspectives. Microb. Cell Fact. 2017, 16, 15.
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 Deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040.
- Watanabe, F.; Bito, T. Vitamin B12 Sources and Microbial Interaction. Exp. Biol. Med. 2018, 243, 148–158.
- Doscherholmen, A.; Hagen, P.S. A Dual Mechanism of Vitamin B12 Plasma Absorption1. J. Clin. Investig. 1957, 36, 1551–1557.
- Lacombe, V.; Roquin, G.; Vinatier, E.; Lavigne, C.; Urbanski, G. Parietal Cell Antibodies: Evolution of Plasma Vitamin B12 during Oral Supplementation to Differentiate True and False Positives for Pernicious Anemia. Pol. Arch. Intern. Med. 2020, 130, 813–815.
- Stabler, S.P. Vitamin B12 Deficiency. N. Engl. J. Med. 2013, 368, 149–160.
- Johnston, J.; Bollekens, J.; Allen, R.H.; Berliner, N. Structure of the CDNA Encoding Transcobalamin I, a Neutrophil Granule Protein. J. Biol. Chem. 1989, 264, 15754–15757.
- Quadros, E.V. Advances in the Understanding of Cobalamin Assimilation and Metabolism. Br. J. Haematol. 2010, 148, 195–204.
- MacDonald, C.M.L.A.; Farquharson, J.; Bessent, R.G.; Adams, J.F. The Forms of Vitamin B12 on the Transcobalamins. Clin. Sci. 1977, 52, 215–218.
- Nexø, E.; Andersen, J. Unsaturated and Cobalamin Saturated Transcobalamin I and II in Normal Human Plasma. Scand. J. Clin. Lab. Investig. 1977, 37, 723–728.
- Russell-Jones, G.J.; Alpers, D.H. Vitamin B12 Transporters. In Membrane Transporters as Drug Targets; Amidon, G.L., Sadée, W., Eds.; Pharmaceutical Biotechnology; Kluwer Academic Publishers: Boston, MA, USA, 2002; Volume 12, pp. 493–520. ISBN 978-0-306-46094-4.
- Collins, D.A.; Hogenkamp, H.P.C.; O’Connor, M.K.; Naylor, S.; Benson, L.M.; Hardyman, T.J.; Thorson, L.M. Biodistribution of Radiolabeled Adenosylcobalamin in Patients Diagnosed With Various Malignancies. Mayo Clin. Proc. 2000, 75, 568–580.
- Pacifico, F.; Laviola, L.; Ulianich, L.; Porcellini, A.; Ventra, C.; Consiglio, E.; Avvedimento, V.E. Differential Expression of the Asialoglycoprotein Receptor in Discrete Brain Areas, in Kidney and Thyroid. Biochem. Biophys. Res. Commun. 1995, 210, 138–144.
- Sun, P.; Zheng, J.; She, G.; Wei, X.; Zhang, X.; Shi, H.; Zhou, X. Expression Pattern of Asialoglycoprotein Receptor in Human Testis. Cell Tissue Res. 2013, 352, 761–768.
- Mu, J.-Z.; Fallon, R.J.; Swanson, P.E.; Carroll, S.B.; Danaher, M.; Alpers, D.H. Expression of an Endogenous Asialoglycoprotein Receptor in a Human Intestinal Epithelial Cell Line, Caco-2. Biochim. Biophys. Acta—Mol. Cell Res. 1994, 1222, 483–491.
- Witzigmann, D.; Quagliata, L.; Schenk, S.H.; Quintavalle, C.; Terracciano, L.M.; Huwyler, J. Variable Asialoglycoprotein Receptor 1 Expression in Liver Disease: Implications for Therapeutic Intervention: ASGR1 Expression in Liver Disease. Hepatol. Res. 2016, 46, 686–696.
- Trerè, D.; Fiume, L.; De Giorgi, L.B.; Di Stefano, G.; Migaldi, M.; Derenzini, M. The Asialoglycoprotein Receptor in Human Hepatocellular Carcinomas: Its Expression on Proliferating Cells. Br. J. Cancer 1999, 81, 404–408.
- Rothenberg, S.P.; Quadros, E.V. Transcobalamin II and the Membrane Receptor for the Transcobalamin II-Cobalamin Complex. Bailliere’s Clin. Haematol. 1995, 8, 499–514.
- Li, N.; Seetharam, S.; Rosenblatt, D.S.; Seetharam, B. Expression of Transcobalamin II MRNA in Human Tissues and Cultured Fibroblasts from Normal and Transcobalamin II-Deficient Patients. Biochem. J. 1994, 301, 585–590.
- Fràter-Schröder, M.; Porck, H.J.; Erten, J.; Müller, M.R.; Steinmann, B.; Kierat, L.; Arwert, F. Synthesis and Secretion of the Human Vitamin B12-Binding Protein, Transcobalamin II, by Cultured Skin Fibroblasts and by Bone Marrow Cells. Biochim. Biophys. Acta 1985, 845, 421–427.
- Begley, J.A.; Colligan, P.D.; Chu, R.C. Synthesis and Secretion of Transcobalamin II by Cultured Astrocytes Derived from Human Brain Tissue. J. Neurol. Sci. 1994, 122, 57–60.
- Rabinowitz, R.; Rachmilewitz, B.; Rachmilewitz, M.; Schlesinger, M. Production of Transcobalamin II by Various Murine and Human Cells in Culture. Isr. J. Med. Sci. 1982, 18, 740–745.
- Lindemans, J.; Kroes, A.C.; van Geel, J.; van Kapel, J.; Schoester, M.; Abels, J. Uptake of Transcobalamin II-Bound Cobalamin by HL-60 Cells: Effects of Differentiation Induction. Exp. Cell Res. 1989, 184, 449–460.
- Banerjee, R.V.; Matthews, R.G. Cobalamin-Dependent Methionine Synthase. FASEB J. 1990, 4, 1450–1459.
- Newman, A.C.; Maddocks, O.D.K. One-Carbon Metabolism in Cancer. Br. J. Cancer 2017, 116, 1499–1504.
- Zeisel, S. Choline, Other Methyl-Donors and Epigenetics. Nutrients 2017, 9, 445.
- Hoffman, R.M.; Machover, D. Recombinant Methioninase as a DNA Demethylation Agent. Methods Mol. Biol. 2019, 1866, 279–284.
- Hasan, T.; Arora, R.; Bansal, A.K.; Bhattacharya, R.; Sharma, G.S.; Singh, L.R. Disturbed Homocysteine Metabolism Is Associated with Cancer. Exp. Mol. Med. 2019, 51, 1–13.
- Tao, B.-B.; Cai, W.-J.; Zhu, Y.-C. H2S Is a Promoter of Angiogenesis: Identification of H2S “Receptors” and Its Molecular Switches in Vascular Endothelial Cells. Handb. Exp. Pharmacol. 2015, 230, 137–152.
- Smith, A.D.; Warren, M.J.; Refsum, H. Vitamin B12. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 83, pp. 215–279. ISBN 978-0-12-811803-0.
- Anderson, N.M.; Mucka, P.; Kern, J.G.; Feng, H. The Emerging Role and Targetability of the TCA Cycle in Cancer Metabolism. Protein Cell 2018, 9, 216–237.
- Alleyn, M.; Breitzig, M.; Lockey, R.; Kolliputi, N. The Dawn of Succinylation: A Posttranslational Modification. Am. J. Physiol.-Cell Physiol. 2018, 314, C228–C232.
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic Pathways Promoting Cancer Cell Survival and Growth. Nat. Cell Biol. 2015, 17, 351–359.
- Heiden, M.G.V.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669.
- McLean, G.R.; Quadros, E.V.; Rothenberg, S.P.; Morgan, A.C.; Schrader, J.W.; Ziltener, H.J. Antibodies to Transcobalamin II Block In Vitro Proliferation of Leukemic Cells. Blood 1997, 89, 235–242.
- Cooper, B.A.; Paranchych, W. Selective Uptake of Specifically Bound Cobalt-58 Vitamin B12 by Human and Mouse Tumour Cells. Nature 1961, 191, 393–395.
- Meyer, L.M.; Bertcher, R.W.; Cronkite, E.P.; Suarez, R.M.; Miller, I.F.; Mulzac, C.W.; Olivarreta, S.T. Co60 Vitamin B12 Binding Capacity of Serum in Persons with Hematologic Disorders, Various Medical Diseases and Neoplasms. Acta Med. Scand. 1961, 169, 557–575.
- Hall, C.A.; Finkler, A.E. The dynamics of transcobalamin ii. a vitamin B12 binding substance in plasma. J. Lab. Clin. Med. 1965, 65, 459–468.
- Hall, C.A.; Finkler, A.E. A Second Vitamin B12-Binding Substance in Human Plasma. Biochim. Biophys. Acta 1963, 78, 234–236.
- Carmel, R. Extreme Elevation of Serum Transcobalamin I in Patients with Metastatic Cancer. N. Engl. J. Med. 1975, 292, 282–284.
- Kane, S.P.; Murray-Lyon, I.M.; Paradinas, F.J.; Johnson, P.J.; Williams, R.; Orr, A.H.; Kohn, J. Vitamin B12 Binding Protein as a Tumour Marker for Hepatocellular Carcinoma. Gut 1978, 19, 1105–1109.
- Gimsing, P.; Hippe, E. Increased Concentration of Transcobalamin I in a Patient with Metastatic Carcinoma of the Breast. Scand. J. Haematol. 1978, 21, 243–249.
- Arendt, J.F.B.; Pedersen, L.; Nexo, E.; Sørensen, H.T. Elevated Plasma Vitamin B12 Levels as a Marker for Cancer: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2013, 105, 1799–1805.
- Arendt, J.F.H.; Sørensen, H.T.; Horsfall, L.J.; Petersen, I. Elevated Vitamin B12 Levels and Cancer Risk in UK Primary Care: A THIN Database Cohort Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 814–821.
- Urbanski, G.; Hamel, J.-F.; Prouveur, B.; Annweiler, C.; Ghali, A.; Cassereau, J.; Lozac’h, P.; Lavigne, C.; Lacombe, V. Strength of the Association of Elevated Vitamin B12 and Solid Cancers: An Adjusted Case-Control Study. J. Clin. Med. 2020, 9, 474.
- Arendt, J.F.H.; Farkas, D.K.; Pedersen, L.; Nexo, E.; Sørensen, H.T. Elevated Plasma Vitamin B12 Levels and Cancer Prognosis: A Population-Based Cohort Study. Cancer Epidemiol. 2016, 40, 158–165.
- Arendt, J.F.B.; Nexo, E. Cobalamin Related Parameters and Disease Patterns in Patients with Increased Serum Cobalamin Levels. PLoS ONE 2012, 7, e45979.
- Arendt, J.F.B.; Nexo, E. Unexpected High Plasma Cobalamin/Proposal for a Diagnostic Strategy. Clin. Chem. Lab. Med. 2013, 51, 489–496.
- Lacombe, V.; Chabrun, F.; Lacout, C.; Ghali, A.; Capitain, O.; Patsouris, A.; Lavigne, C.; Urbanski, G. Persistent Elevation of Plasma Vitamin B12 Is Strongly Associated with Solid Cancer. Sci. Rep. 2021, 11, 13361.
- Valentin Lacombe; Anne Patsouris; Estelle Delattre; Carole Lacout; Geoffrey Urbanski; Evolution of plasma vitamin B12 in patients with solid cancers during curative versus supportive care. Archives of Medical Science 2021, 17, 1811-1815, 10.5114/aoms/140974.
- Huang, H.; Wang, J.; Zhang, J.; Cai, J.; Pi, J.; Xu, J.-F. Inspirations of Cobalt Oxide Nanoparticle Based Anticancer Therapeutics. Pharmaceutics 2021, 13, 1599.
- Bauer, J.A. Effects of Interferon Beta on Transcobalamin II-Receptor Expression and Antitumor Activity of Nitrosylcobalamin. CancerSpectrum Knowl. Environ. 2002, 94, 1010–1019.
- Gupta, Y.; Kohli, D.V.; Jain, S.K. Vitamin B12-Mediated Transport: A Potential Tool for Tumor Targeting of Antineoplastic Drugs and Imaging Agents. Crit. Rev. Ther. Drug Carrier Syst. 2008, 25, 347–379.
- Hoffman, R.M.; Tan, Y.; Li, S.; Han, Q.; Zavala, J.; Zavala, J. Pilot Phase I Clinical Trial of Methioninase on High-Stage Cancer Patients: Rapid Depletion of Circulating Methionine. In Methionine Dependence of Cancer and Aging; Hoffman, R.M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1866, pp. 231–242. ISBN 978-1-4939-8795-5.
- Thivat, E.; Farges, M.-C.; Bacin, F.; D’Incan, M.; Mouret-Reynier, M.-A.; Cellarier, E.; Madelmont, J.-C.; Vasson, M.-P.; Chollet, P.; Durando, X. Phase II Trial of the Association of a Methionine-Free Diet with Cystemustine Therapy in Melanoma and Glioma. Anticancer Res. 2009, 29, 5235–5240.
- Durando, X.; Farges, M.-C.; Buc, E.; Abrial, C.; Petorin-Lesens, C.; Gillet, B.; Vasson, M.-P.; Pezet, D.; Chollet, P.; Thivat, E. Dietary Methionine Restriction with FOLFOX Regimen as First Line Therapy of Metastatic Colorectal Cancer: A Feasibility Study. Oncology 2010, 78, 205–209.
- Valentin Lacombe; Guy Lenaers; Geoffrey Urbanski; Diagnostic and Therapeutic Perspectives Associated to Cobalamin-Dependent Metabolism and Transcobalamins’ Synthesis in Solid Cancers. Nutrients 2022, 14, 2058, 10.3390/nu14102058.
More
