While the use of alien insect species for food and feed can help to alleviate protein shortage and provide for a more sustainable feed production, their invasive potential should be considered.
- invasive alien species
- ecological impacts
- biological invasion
1. Introduction
The current prediction of human population increase (estimated in more than 9 billion by 2050) urges the need to provide sufficient nutritious food for all. That edible insects could help to ease future global food shortages and that WHO and FAO should support the use of insects as a food item was first suggested in 1975 by Meyer-Rochow in Australia
[1]
. However, the need to increase food production does not come cheap, as the availability of natural resources, arable land, and natural stocks are decreasing
[2]
. More recent estimates suggest that food production will have to increase by 60% to feed everyone
[3]
.
2. The Potential of Native Species
Despite the fact that current production of insect meals and products for human and animal feed mostly use Asian and neotropical species, it is already acknowledged that native species display good nutritional-values and conditions for mass production. The requirements for its mass production include a short life cycle, a high fecundity, and the ability to breed continuously in an artificial environment [26]. Recent studies showed that some Diptera, Coleoptera, and Orthoptera species present a high protein-value, comparable to soymeal, but lower than fishmeal. Nonetheless, the potential of order Diptera is still high as an alternative to fish meal by displaying a similar amino acid profile [27]. In fact, several insect species belonging to the orders Coleoptera and Diptera, which are part of the natural diets of Atlantic salmon (Salmo salar Linnaeus, 1758 (Salmoniformes, Salmonidae)), show adequate composition in polyunsaturated fatty acids (PUFA), nutrients necessary for fish growth. Therefore, they present significant potential to be used as a supplementation of the diets commonly employed to farm this fish [28]. Additionally, Apis mellifera ligustica Spinola, 1806 (Hymenoptera, Apidae), a subspecies of the European honeybee, showed great potential to be used as animal feed, due to its high protein content in the adult stage (51%). This species is also promising as a larva, presenting 30% of fat composition, which decreases over larval development, as well as it carbohydrates content [29]. Several blowflies show potential to be used as feed ingredients [30]. For instance, the blowfly (Chrysomya megacephala (Fabricius, 1794) (Diptera, Calliphoridae)) was evaluated as substitute of fishmeal in red Tilapia (Oreochromis sp.) production, with an average crude protein level of 54.4%. They contain all necessary amino acids to promote a suitable growth for this fish species. Feeds with 100% replacement of fish meal by maggot meals were successfully validated with no significant differences being recorded from a control commercial diet [31]. The Egyptian locust, Anacridium aegyptium (Linnaeus, 1764) (Orthoptera, Acrididae), occurring throughout most European territory, presents favourable PUFA percentages, although they have a low oil content, which can be used as co-product derived from the insect processing industry [32]. Pseudochorthippus parallelus (Zetterstedt, 1821) (Orthoptera, Acrididae) is another Orthopteran native to Europe with a suitable nutritional profile to be used as exotic pet food or livestock feed [33]. The common green bottle fly, Lucilia sericata (Meigen, 1826) (Diptera, Calliphoridae), is a blowfly found in most regions of the world [34]. Although they are known for causing myasis, with economic impacts for sheep producers [35], they present a high content in monounsaturated fatty acids and may be viable ingredient for animal feed [30]. In India, two preferred edible insect species have great potential to be used as animal feed: the ant Oecophylla smaragdina (Fabricius, 1775) (Hymenoptera, Formicidae) and the termite Odontotermes sp. Their protein content is 55.28% and 33.67%, their fat content is 14.99% and 50.93%, and their fibre is 19.84% and 6.30%, respectively. Their PUFA content is also important, with 8.19% for the first species and 2.59% for the second one [36]. The Owl butterfly, Caligo memnon Felder, 1866 (Lepidoptera, Nymphalidae) , native to South America also has a good potential to be used as animal feed, since it has a concentration of 62.5 mole % of α-linolenic acid (ALA) in total Fatty Acids, which is an essential omega-3 PUFA [37]. The dung beetle, Acrossus rufipes (Linnaeus, 1758) (Coleoptera, Scarabaeidae), is found in most of Europe and East Coast of the United States [38]. This species has great potential as animal feed, especially for fish, when considering their amino acid content. They exhibit essential amino acids, namely histidine and threonine, in higher concentrations than some cultivated fish species, such as catfish (Clarias gariepinus (Burchell, 1822) (Siluriformes, Clariidae)) or crayfish (Procambarus clarkii (Girard, 1852) (Decapoda, Cambaridae)) [39]. The stick insect Cladomorphus phyllinum Gray, 1835 (Phasmida, Phasmatidae), native to the Brazilian forests [40], showed potential as an alternative insect-based protein source. The dried samples showed a protein content of 64.6%, with the presence of essential amino acids, and a lipid content containing 57.03% of oleic acid, 15.94% of palmitic acid, 13.76% of linoleic acid and 10.76% of stearic acid [41]. The Chinese grasshopper Acrida cinerea (Thunberg, 1815) (Orthoptera, Acrididae), native to East Asia [42], was successfully used to replace the protein content of broilers feed, an addition of up to 150 g Kg−1, and also showed potential to be mass reared [43]. The Mormon cricket, Anabrus simplex, Haldeman, 1852 (Orthoptera, Tettigoniidae), is native to North America, recorded for the USA and Canada [44]. This species has the potential to be used as broiler feed. No significant difference has been found between chicks fed with corn-soybean meal diet and the ones fed with corn-ground cricket when comparing weight gain and feed/grain ratio. Additionally, there was no adverse effect on the taste of the meat from the specimens fed the corn-cricket diet, as determined by a taste panel [45]. The field cricket native to China, Teleogryllus (Macroteleogryllus) mitratus (Burmeister, 1838) (Orthoptera, Gryllidae), synonym of Gryllus testaceus [44], has a crude protein of 58.3%, crude fat of 10.3%, chitin 8.7% and ash 2.96% (on dry matter basis), respectively. When fed as a replacement of 15% of soybean meal, it showed no significant differences with the control group (poultry feedstuff) in weight gain, feed intake, or gain/feed ratio [46]. The variegated grasshopper, Zonocerus variegatus (Linnaeus, 1758) (Orthoptera, Acrididae), is a common pest in Africa, and was recently reported in the United Kingdom [47]. However, its insect meal showed potential to be used as a fishmeal replacement for the African catfish, Clarias gariepinus, in which a replacement of 25% had no adverse effects on both the growth rate and nutrient utilization when compared to the control group [48]. The Chilean moth, Chilecomadia moorei Silva Figuero, 1915 (Lepidoptera, Cosiidae), whose larvae are known as butterworm, is native to Chile. They are used to feed insectivore exotic pets such as geckos. They are considered to be a good source of fat, due to its high fat content [49]. This species is popular among reptile owners since they have a distinct scent which attracts the lizards [50][51]. They are exported out from Chile to many countries and are not able to reproduce elsewhere, since they are dependent of the host tree Retanilla trinervia (Gillies and Hook.) Hook. and Arn. (Rosales, Rhamnaceae), endemic to Chile, and are considered a pest to this tree species [52][53]. Recently, the long-legged fly Machaerium maritimae Haliday, 1832 (Diptera, Dolichopodidae), a fly species native to western Europe, adapted to thrive in brackish waters, was reported as a potential feed ingredient for aquaculture due to its content in important omega 3 PUFA, including eicosapentaenoic acid (EPA), an essential nutrient for the healthy growth of marine organisms [54].3. Native vs. Non-Native Species
Most of the insect species produced for both livestock feed and exotic pets’ food are known to be exotic in regions such as Europe. They have some advantages when compared to native insect species, such as well-established rearing protocols for mass production and extensive studies to improve their productivity and potential to replace mainstream ingredients in formulated animal feeds (e.g., fish meal in aquafeeds). Nonetheless, native insect species are still interesting alternatives that are worth exploring, considering both the possible impacts caused by the escapes of exotic insects from pet shops and rearing facilities and the levels of some key nutrients on their biochemical profiles (e.g., PUFA). Both strengths and weaknesses, as well as opportunities and threats of using exotic or native species of insects as livestock feed are discussed below using a SWOT analysis (Figure 1).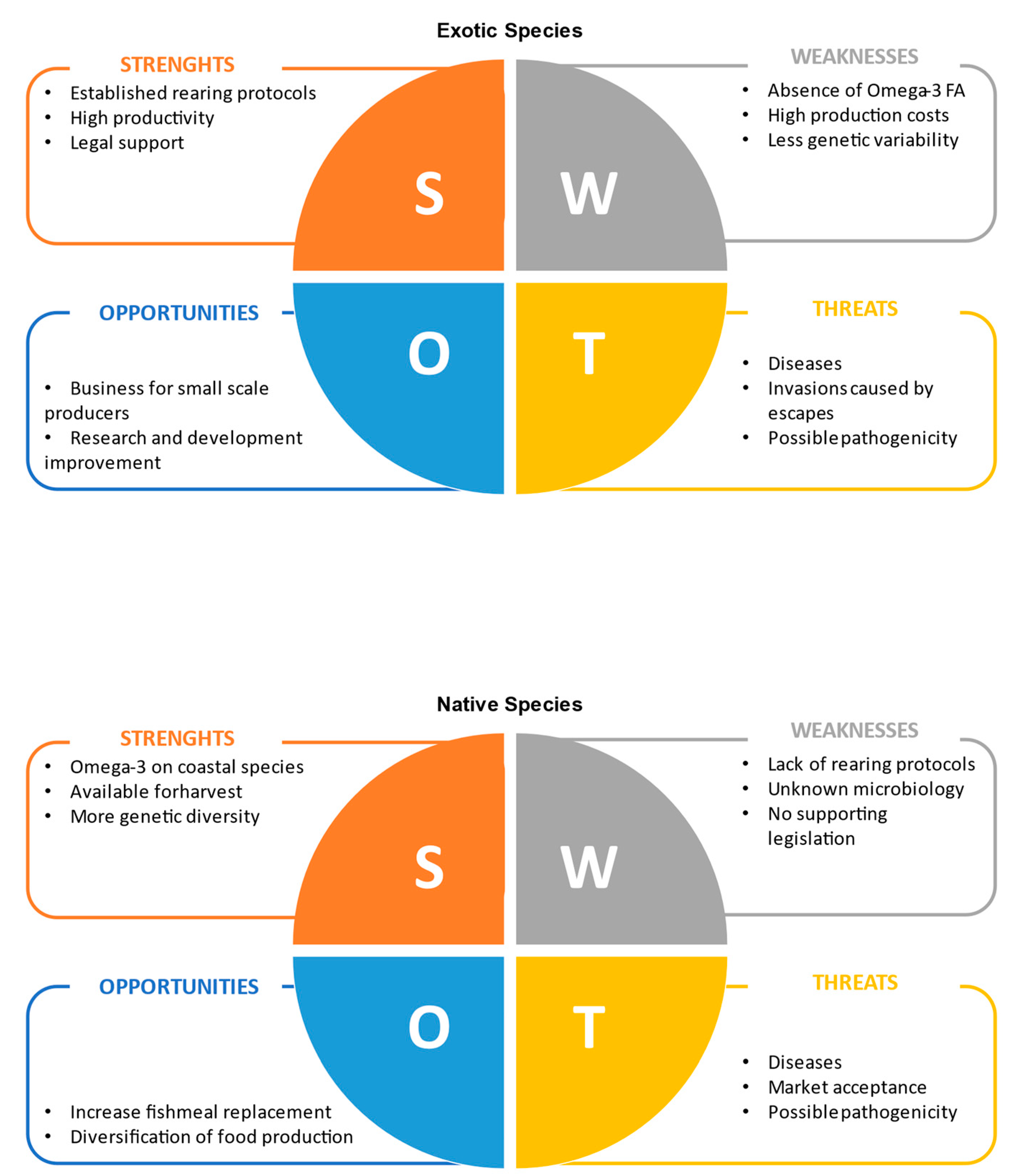
References
- Meyer-Rochow, V.B.; Can Insects Help To Ease The Problem Of World Food Shortage?. Search 1975, 6 (7), 261-262.
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020.
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050 the 2012 Revision; ESA Working Paper No. 12-03; FAO: Rome, Italy, 2012; Available online: https://ageconsearch.umn.edu/record/288998 (accessed on 9 May 2022).
- Food and Agriculture Organization of the United Nations. The State of the World’s Land and Water Resources for Food and Agriculture: Managing Systems at Risk; FAO: Rome, Italy; London, UK, 2011; Available online: https://www.fao.org/land-water/solaw2021/en/ (accessed on 9 May 2022).
- Lehuger, S.; Gabrielle, B.; Gagnaire, N. Environmental impact of the substitution of imported soybean meal with locally-produced rapeseed meal in dairy cow feed. J. Clean. Prod. 2009, 17, 616–624.
- Prudêncio da Silva, V.; van der Werf, H.M.G.; Spies, A.; Soares, S.R. Variability in environmental impacts of Brazilian soybean according to crop production and transport scenarios. J. Environ. Manag. 2010, 91, 1831–1839.
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024.
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2016; FAO: Rome, Italy, 2016; Available online: http://www.fao.org/3/a-i5555e.pdf (accessed on 9 May 2022).
- Basset-Mens, C.; Van Der Werf, H.M.G. Scenario-based environmental assessment of farming systems: The case of pig production in France. Agric. Ecosyst. Environ. 2005, 105, 127–144.
- Boggia, A.; Paolotti, L.; Castellini, C. Environmental impact evaluation of conventional, organic and organic-plus poultry production systems using life cycle assessment. World’s Poult. Sci. J. 2010, 66, 95–114.
- Dourmad, J.Y.; Ryschawy, J.; Trousson, T.; Bonneau, M.; Gonzàlez, J.; Houwers, H.W.J.; Hviid, M.; Zimmer, C.; Nguyen, T.L.T.; Morgensen, L. Evaluating environmental impacts of contrasting pig farming systems with life cycle assessment. Animal 2014, 8, 2027–2037.
- Biermann, G.; Geist, J. Life cycle assessment of common carp (Cyprinus carpio L.)—A comparison of the environmental impacts of conventional and organic carp aquaculture in Germany. Aquaculture 2019, 501, 404–415.
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583.
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610.
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80.
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 9789251075951.
- Roques, A.; Rabitsch, W.; Rasplus, J.-Y.; Lopez-Vaamonde, C.; Nentwig, W.; Kenis, M. Alien Terrestrial Invertebrates of Europe. In Handbook of Alien Species in Europe; Springer: Dordrecht, The Netherlands, 2009; Volume 569, pp. 63–79. ISBN 978-1-4020-8279-5.
- Kenis, M. Insects-Insecta. In An Inventory of Alien Species and their Threat to Biodiversity and Economy in Switzerland; Wittenberg, R., Ed.; Report to the Swiss Agency for Environment, Forests and Landscape; CABI Bioscience Switzerland Centre: Delémont, Switzerland, 2005; Volume 29, pp. 131–212.
- Cox, G.W. Alien Species and Evolution: The Evolutionary Ecology of Exotic Plants, Animals, Microbes, and Interacting Native Species; Island Press: Washington, DC, USA, 2004; ISBN 1-55963-008-6.
- Thompson, J.N. Rapid evolution as an ecological process. Trends Ecol. Evol. 1998, 13, 329–332.
- Nayak, S.B.; Rao, K.S.; Ramalakshmi, V. Impact of Climate Change on Insect Pests and their Natural Enemies. Int. J. Ecol. Environ. Sci. 2020, 2, 579–584.
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440.
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693.
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919.
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 1–9.
- Mackauer, M. Genetic aspects of insect production. Entomophaga 1972, 17, 27–48.
- Barroso, F.G.; de Haro, C.; Sánchez-Muros, M.J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422, 193–201.
- Bell, J.G.; Ghioni, C.; Sargen, J.R. Fatty acid composition of 10 freshwater invertebrates which are natural food organisms of Atlantic salmon parr (Salmo salar): A comparison with commercial diets. Aquaculture 1994, 128, 301–313.
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia. Pac. Entomol. 2016, 19, 487–495.
- Prado e Castro, C.; Ameixa, O.M.C.C. Blow flies (Diptera: Calliphoridae) promising candidates as animal feed ingredients. J. Insects Food Feed 2021, 7, 1065–1076.
- Sing, K.W.; Kamarudin, M.S.; Wilson, J.J.; Sofian-Azirun, M. Evaluation of blowfly (Chrysomya megacephala) maggot meal as an effective, sustainable replacement for fishmeal in the diet of farmed juvenile red tilapia (Oreochromis sp.). Pak. Vet. J. 2014, 34, 288–292.
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477.
- Paul, A.; Frederich, M.; Uyttenbroeck, R.; Malik, P.; Filocco, S.; Richel, A.; Heuskin, S.; Alabi, T.; Megido, R.C.; Franck, T.; et al. Nutritional composition and rearing potential of the meadow grasshopper (Chorthippus parallelus Zetterstedt). J. Asia. Pac. Entomol. 2016, 19, 1111–1116.
- Taxonomy, G.B. Lucilia Sericata (Meigen, 1826) in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/5063973 (accessed on 13 September 2021).
- Hall, M.; Wall, R. Myiasis of Humans and Domestic Animals. Adv. Parasitol. 1995, 35, 257–334.
- Chakravorty, J.; Ghosh, S.; Megu, K.; Jung, C.; Meyer-Rochow, V.B. Nutritional and anti-nutritional composition of Oecophylla smaragdina (Hymenoptera: Formicidae) and Odontotermes sp. (Isoptera: Termitidae): Two preferred edible insects of Arunachal Pradesh, India. J. Asia. Pac. Entomol. 2016, 19, 711–720.
- Guil-Guerrero, J.L.; Ramos-Bueno, R.P.; González-Fernandez, M.J.; Fabrikov, D.; Sánchez-Muros, M.J.; Barroso, F.G. Insects as Food: Fatty Acid Profiles, Lipid Classes, and sn-2 Fatty Acid Distribution of Lepidoptera Larvae. Eur. J. Lipid Sci. Technol. 2018, 120, 1700391.
- Taxonomy, G.B. Aphodius Rufipes (Linnaeus, 1758) in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/8321792 (accessed on 13 September 2021).
- Oriolowo, O.B.; John, O.J.; Mohammed, U.B.; Joshua, D. Amino acids profile of catfish, crayfish and larva of edible dung beetle. Ife J. Sci. 2020, 22, 9–16.
- Taxonomy, G.B. Cladomorphus phyllinus G.R.Gray, 1835 in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/1413211 (accessed on 13 September 2021).
- Botton, V.; Chiarello, L.M.; Klunk, G.A.; Marin, D.; Curbani, L.; Gonçalves, M.J.; Vitorino, M.D. Evaluation of nutritional composition and ecotoxicity of the stick insect Cladomorphus phyllinum. Eur. Food Res. Technol. 2020, 247, 605–611.
- File, O.S. Acrida cinerea (Thunberg, 1815) in Cigliano M M (2019). Available online: https://www.gbif.org/species/5099473 (accessed on 13 September 2021).
- Wang, D.; Zhai, S.W.; Zhang, C.X.; Zhang, Q.; Chen, H. Nutrition value of the Chinese grasshopper Acrida cinerea (Thunberg) for broilers. Anim. Feed Sci. Technol. 2007, 135, 66–74.
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File Version 5.0/5.0. Available online: http://orthoptera.speciesfile.org/HomePage/Orthoptera/HomePage.aspx (accessed on 6 May 2022).
- Finke, M.D.; Sunde, M.L.; DeFoliart, G.R. An Evaluation of the Protein Quality of Mormon Crickets (Anabrus Simplex Haldeman) When Used as a High Protein Feedstuff for Poultry. Poult. Sci. 1985, 64, 708–712.
- Wang, D.; Shao, W.Z.; Chuan, X.Z.; Yao, Y.B.; Shi, H.A.; Ying, N.X. Evaluation on nutritional value of field crickets as a poultry feedstuff. Asian-Australas. J. Anim. Sci. 2005, 18, 667–670.
- Taxonomy, G.B. Zonocerus variegatus (Linnaeus, 1758) in GBIF Secretariat (2021). Available online: https://www.gbif.org/species/1727818 (accessed on 13 September 2021).
- Alegbeleye, W.O.; Obasa, S.O.; Olude, O.O.; Otubu, K.; Jimoh, W. Preliminary evaluation of the nutritive value of the variegated grasshopper (Zonocerus variegatus L.) for African catfish Clarias gariepinus (Burchell. 1822) fingerlings. Aquac. Res. 2012, 43, 412–420.
- Finke, M.D. Complete Nutrient Content of Four Species of Feeder Insects. Zoo Biol. 2013, 32, 27–36.
- Recorpinc Butterworms. Available online: http://www.recorpinc.com/butterworms.php (accessed on 7 May 2022).
- Topflightdubia Butterworms. Available online: https://www.topflightdubia.com/butterworms (accessed on 7 May 2022).
- Iriarte, J.A.; Feinsinger, P.; Jaksic, F.M. Trends in wildilife use and trade in Chile. Biol. Conserv. 1997, 81, 9–20.
- Ureta, E. Revisión de la familia Cossidae en Chile. Bol. Mus. Nac. Hist. Nat. 1957, 27, 129–153. Available online: https://bibliotecadigital.infor.cl/handle/20.500.12220/7999 (accessed on 6 May 2022).
- Duarte, P.M.; Maciel, E.; Pinho, M.; Domingues, M.R.; Calado, R.; Lillebø, A.I.; Ameixa, O.M.C.C. Omega-3 on the fly: Long-legged fly Machaerium maritimae as a potential source of eicosapentaenoic acid for aquafeeds. J. Insects Food Feed 2021, 7, 1089–1100.
- Committee, E.S. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 60.
- Montanari, F.; Pinto de Moura, A.; Cunha, L.M. Production and Commercialization of Insects as Food and Feed; Springer International Publishing: Cham, Switzerland, 2021; pp. 29–39.
- Food and Agriculture Organization of the United Nations. FAO Commission on Genetic Resources for Food and Agriculture Assessments; Scherf, B.D., Pilling, D., Eds.; FAO: Rome, Italy, 2015.
- Weissman, D.B.; Gray, D.A.; Thi Pham, H.; Tijssen, P. Billions and billions sold: Pet-feeder crickets (Orthoptera: Gryllidae), commercial cricket farms, an epizootic densovirus, and government regulations make for a potential disaster. Zootaxa 2012, 3504, 67–88.
- Premrov Bajuk, B.; Zrimšek, P.; Kotnik, T.; Leonardi, A.; Križaj, I.; Jakovac Strajn, B. Insect protein-based diet as potential risk of allergy in dogs. Animals 2021, 11, 1942.
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2018, 62, 1–12.
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor larvae) as an alternative protein source for monogastric animal: A review. Animals 2020, 10, 2068.
