CD19-targeted chimeric antigen receptor (CAR) T (CD19-CAR-T) cell therapy usually causes B cell aplasia because of “on-target off-tumor” toxicity. The aim of the study was to assess the concept that the introduction of an inhibitory CAR (iCAR) into CAR-T cells could alleviate the side effect of CD19-CAR-T cell therapy. The results showed that CD19-CAR-T cells with a novel KIR/PD-1-based inhibitory CAR (iKP-19-CAR-T) exhibited more naïve, less exhausted phenotypes and preserved a higher proportion of central memory T cells (TCM). Furthermore, iKP-19-CAR-T cells exerted the similar level of cytotoxicity on CD19+HLA-C1- Burkitt’s lymphoma cells compared to CD19-CAR-T cells while sparing CD19+HLA-C1+ healthy human B cells both in vitro and in the xenograft model. Our data demonstrates that the KIR/PD-1-based inhibitory CAR can be a promising strategy to avoid B cell aplasia caused by CD19-CAR-T cell therapy.
- CD19
- CAR-T
- KIR
- PD-1
- inhibitory CAR
- on-target off-tumor
- Introduction
1. Introduction
CD19-CAR-T (CD19-targeted chimeric antigen receptor T-) cells are the first cell therapy products for the treatment of relapsed or refractory B cell acute lymphoblastic leukemia (B-ALL) that were approved by US Food and Drug Administration (FDA) in 2017 [1,2]. Since then, CD19-CAR-T has brought a gigantic revolution in the field of immunotherapy because of the high percentage rate of complete remission (CR) in several blood-related malignancies [3,4,5,6]. While some challenges increase the risk of treatment failures, such as an “on-target off-tumor” adverse event instigating B cell aplasia, i.e., CD19-CAR-T cells kill all healthy B cells because of CD19 expressed in all B cells [7,8,9]. B cell aplasia contributes to hypogammaglobulinemia, which is one of the main factors leading to infection in patients [10]. Although these patients were administrated an intravenous immunoglobulin to maintain IgG levels, concomitant bacterial, viral, and fungal infections were still observed [11,12].
CD19-CAR-T (CD19-targeted chimeric antigen receptor T-) cells are the first cell therapy products for the treatment of relapsed or refractory B cell acute lymphoblastic leukemia (B-ALL) that were approved by US Food and Drug Administration (FDA) in 2017[1][2]. Since then, CD19-CAR-T has brought a gigantic revolution in the field of immunotherapy because of the high percentage rate of complete remission (CR) in several blood-related malignancies[3][4][5][6]. While some challenges increase the risk of treatment failures, such as an “on-target off-tumor” adverse event instigating B cell aplasia, i.e., CD19-CAR-T cells kill all healthy B cells because of CD19 expressed in all B cells[7][8][9][10]. B cell aplasia contributes to hypogammaglobulinemia, which is one of the main factors leading to infection in patients[10]. Although these patients were administrated an intravenous immunoglobulin to maintain IgG levels, concomitant bacterial, viral, and fungal infections were still observed [11][12].
At present, some strategies have been developed to overcome the “on-target off-tumor” effect. Since the ideal specific target is almost non-existent in reality, it is a good idea to target structurally differentiated proteins. For example, the engineered CAR-T cells targeting the integrin β7 activated conformation was specifically effective against multiple myeloma (MM) without damaging normal hematopoietic cells [13]. Similarly, recognition of the Tn-glyco form of MUC1 by engineering CAR-T cells exhibited target-specific cytotoxicity to cellular adenocarcinoma [14]. According to another strategy, splitting 4-1BB domain and CD3ζ domain and fusing them together with two different single chain fragment variable regions (scFv) respectively, T cells would be entirely active if only two antigens were recognized at the same time [15]. Likewise, the same result was found using the “And gate” approach, in which logical control of CAR-T cells responses needed two different antigen engagements [16,17,18]. Although these strategies can reduce the incidence of the “on-target off-tumor” effect, they are not universal and not easy to implement.
At present, some strategies have been developed to overcome the “on-target off-tumor” effect. Since the ideal specific target is almost non-existent in reality, it is a good idea to target structurally differentiated proteins. For example, the engineered CAR-T cells targeting the integrin β7 activated conformation was specifically effective against multiple myeloma (MM) without damaging normal hematopoietic cells[13]. Similarly, recognition of the Tn-glyco form of MUC1 by engineering CAR-T cells exhibited target-specific cytotoxicity to cellular adenocarcinoma [14]. According to another strategy, splitting 4-1BB domain and CD3ζ domain and fusing them together with two different single chain fragment variable regions (scFv) respectively, T cells would be entirely active if only two antigens were recognized at the same time[15]. Likewise, the same result was found using the “And gate” approach, in which logical control of CAR-T cells responses needed two different antigen engagements[16][17][18]. Although these strategies can reduce the incidence of the “on-target off-tumor” effect, they are not universal and not easy to implement.
In contrast to the strategies outlined earlier, inhibitory CAR (iCAR) is a versatile and implementable solution to subdue the “on-target off-tumor” effect by providing a negative signal to regulate T cell activation. In 2013, Fedorov et al. developed a PD-1-based iCAR strategy and they provided a proof of concept that CAR-T cells expressing an iCAR could discriminate between off target cells and target cells and functioned in a temporary and reversible manner [19].
In contrast to the strategies outlined earlier, inhibitory CAR (iCAR) is a versatile and implementable solution to subdue the “on-target off-tumor” effect by providing a negative signal to regulate T cell activation. In 2013, Fedorov et al. developed a PD-1-based iCAR strategy and they provided a proof of concept that CAR-T cells expressing an iCAR could discriminate between off target cells and target cells and functioned in a temporary and reversible manner[19].
KIRs are the most important inhibitory receptors expressed predominantly in NK cells and a small subset of T cells [20]. They can dampen the activation of NK cells after interacting with the human leukocyte antigen (HLA) ligands expressed on the surface of normal cells [21,22]. Tumor cells downregulate HLA to escape from the T cells immune surveillance [23,24,25]. Data from the Human Protein Atlas Database demonstrate that HLA-C is low or non-expressed on most tumor cell lines, but highly or moderately expressed in normal tissues. PD-1 is an inhibitory protein expressed in activated T cells to limit the excessive activation of T cells [26,27,28]. In this study, we engineered a novel iCAR consisting of the extracellular domain of KIR2DL2, CD8a hinge and transmembrane, and the intracellular domain of PD-1. This KIR/PD-1-based iCAR was termed as iKP CAR. We speculated that when KIR2DL2 recognized HLA-C1 on normal cells, iKP CAR would deliver a negative signal to inhibit T cells response via the PD-1 domain, meanwhile, iKP CAR did not work in the absence of HLA-C1 on tumor cells, so that iKP CAR could discriminate between normal cells (HLA-C1
KIRs are the most important inhibitory receptors expressed predominantly in NK cells and a small subset of T cells [20]. They can dampen the activation of NK cells after interacting with the human leukocyte antigen (HLA) ligands expressed on the surface of normal cells[21][22]. Tumor cells downregulate HLA to escape from the T cells immune surveillance[23][24][25]. Data from the Human Protein Atlas Database demonstrate that HLA-C is low or non-expressed on most tumor cell lines, but highly or moderately expressed in normal tissues. PD-1 is an inhibitory protein expressed in activated T cells to limit the excessive activation of T cells[26][27][28]. In this study, we engineered a novel iCAR consisting of the extracellular domain of KIR2DL2, CD8a hinge and transmembrane, and the intracellular domain of PD-1. This KIR/PD-1-based iCAR was termed as iKP CAR. We speculated that when KIR2DL2 recognized HLA-C1 on normal cells, iKP CAR would deliver a negative signal to inhibit T cells response via the PD-1 domain, meanwhile, iKP CAR did not work in the absence of HLA-C1 on tumor cells, so that iKP CAR could discriminate between normal cells (HLA-C1
+
) and tumor cells (HLA-C1
−
). Simultaneously, we hoped that CD19-CAR-T cells with an iKP CAR could eliminate CD19
+
HLA-C1
−
malignant B cells, while reducing the damage to CD19
+
HLA-C1
+
healthy B cells.
- CD19-CAR-T Cells and CD19+HLA-C1− Malignant B Cells
2. CD19-CAR-T Cells and CD19+HLA-C1− Malignant B Cells
CARs use scFv structure to recognize target antigens on cancer cells. The safety of CAR-T cells depends on the specificity of target antigens. However, most antigens are also expressed in normal cells, hence the “on-target off-tumor” effect is inevitable. This behavior of CAR-T cells causes severe side effects in the body systems expressing the target antigens [29]. Current clinical solutions are to use high-dose corticosteroid for treatment when “on-target off-tumor” events occur [30]. This immunosuppressive drug controls off-target toxicity at the cost of abolishing the T cells antitumor effect.
CARs use scFv structure to recognize target antigens on cancer cells. The safety of CAR-T cells depends on the specificity of target antigens. However, most antigens are also expressed in normal cells, hence the “on-target off-tumor” effect is inevitable. This behavior of CAR-T cells causes severe side effects in the body systems expressing the target antigens[29]. Current clinical solutions are to use high-dose corticosteroid for treatment when “on-target off-tumor” events occur[30]. This immunosuppressive drug controls off-target toxicity at the cost of abolishing the T cells antitumor effect.
Fedorov et al. provided a model to elucidate the possibility to apply iCAR to regulate the function of CAR-T cells [19]. As we know, NK cells can discriminate between normal cells and abnormal cells that do not express adequate amounts of HLA such as cancer cells, virus-infected cells, etc [31]. Whether they are activated depends on the integrated signal of positive and negative signals. KIR is one of the important inhibitory receptors and exerts an inhibitory function to constrain NK cell response upon recognizing HLA on normal cells [32]. Based on the activation mechanism of NK cells, we fused the PD-1 intracellular signal domain and the extracellular recognition domain of KIR2DL2 to develop an iKP/PD-1-based iCAR (iKP CAR), and the data demonstrated that T cells co-expressing CD19 CAR and iKP CAR could discern between malignant B cells and normal B cells in vitro and in vivo.
Fedorov et al. provided a model to elucidate the possibility to apply iCAR to regulate the function of CAR-T cells [19]. As we know, NK cells can discriminate between normal cells and abnormal cells that do not express adequate amounts of HLA such as cancer cells, virus-infected cells, etc[31]. Whether they are activated depends on the integrated signal of positive and negative signals. KIR is one of the important inhibitory receptors and exerts an inhibitory function to constrain NK cell response upon recognizing HLA on normal cells[32]. Based on the activation mechanism of NK cells, we fused the PD-1 intracellular signal domain and the extracellular recognition domain of KIR2DL2 to develop an iKP/PD-1-based iCAR (iKP CAR), and the data demonstrated that T cells co-expressing CD19 CAR and iKP CAR could discern between malignant B cells and normal B cells in vitro and in vivo.
Two factors are important for iCAR design. Firstly, the target of iCAR should be widely expressed on normal tissue cells, but rarely expressed on cancer cells. Obviously, HLA is an ideal target for iCAR. As HLA-C1 subtype has a high expression frequency in humans [33], we choose the extracellular domain of KIR2DL2 (whose ligand is HLA-C1) as the recognition domain of iCAR. Secondly, the intracellular signal domain should respond quickly and strongly in a transiently reversible manner once the extracellular recognition domain binds to the target. PD-1 is a powerful inhibitory molecule in T cells that dephosphorylates the TCR signal in a few hours after interacting with PD-L1 [34]. The dephosphorylation event is a dynamic and reversible process, which can ensure that T cell activity can restore during engagement of target cells. Therefore, we used the intracellular domain of PD-1 as the signaling domain of iCAR in the current study.
Two factors are important for iCAR design. Firstly, the target of iCAR should be widely expressed on normal tissue cells, but rarely expressed on cancer cells. Obviously, HLA is an ideal target for iCAR. As HLA-C1 subtype has a high expression frequency in humans[33], we choose the extracellular domain of KIR2DL2 (whose ligand is HLA-C1) as the recognition domain of iCAR. Secondly, the intracellular signal domain should respond quickly and strongly in a transiently reversible manner once the extracellular recognition domain binds to the target. PD-1 is a powerful inhibitory molecule in T cells that dephosphorylates the TCR signal in a few hours after interacting with PD-L1[34]. The dephosphorylation event is a dynamic and reversible process, which can ensure that T cell activity can restore during engagement of target cells. Therefore, we used the intracellular domain of PD-1 as the signaling domain of iCAR in the current study.
In our study, some characteristics of CD19-CAR-T cells were not affected by iKP CAR. Data showed that iKP-19-CAR-T cells had similar CAR transduction efficacy, cell viability, proliferation and CD8/CD4 ratio to CD19-CAR-T cells in X-VIVO media supplemented with IL-2. However, some characteristics were different. Prior to antigen engagement, iKP-19-CAR-T cells displayed lower differentiated (increased CCR7, decreased CD45RO and GzmB expression), less exhausted (lower PD-1 expression) phenotypes, and had an elevated proportion of T
CM
or less-differentiated cells. The reason might be that the negative signaling of iKP CAR suppressed the IL-2 signal and regulated the related gene expression (up-regulated Eomes expression and down-regulated T-bet expression), thereby inhibiting T cell differentiation and exhaustion simultaneously. A higher percentage of T
CM
or less differentiated cells in the peripheral blood from the mice treated with iKP-19-CAR-T cells had also been observed in vivo. Retrospective analysis from published CAR-T cell clinical studies had revealed that an elevated proportion of T
CM or less differentiated CAR-T cells provided superior antitumor efficacy [35]. Both in vitro and in vivo, we found that HLA-C1 on T cells did not reduce the toxicity of iKP-19-CAR-T cells on Daudi cells. Therefore, we speculated that iKP-19-CAR-T cells obtained an activation pattern similar to NK cells due to iCAR functioning in a temporary and reversible manner [19].
or less differentiated CAR-T cells provided superior antitumor efficacy[35]. Both in vitro and in vivo, we found that HLA-C1 on T cells did not reduce the toxicity of iKP-19-CAR-T cells on Daudi cells. Therefore, we speculated that iKP-19-CAR-T cells obtained an activation pattern similar to NK cells due to iCAR functioning in a temporary and reversible manner[19].
Subsequently, we demonstrated that the novel iKP CAR here had an ability to discern malignant B cells and normal B cells both in vitro and in vivo. Compared to CD19-CAR-T cells, iKP-19-CAR-T cells had an equivalent level of T cell activation, degranulation, and cytotoxic potential against Daudi cells, while all these were reduced significantly against normal B cells in vitro. Furthermore, we found that B cell malignancy could be controlled effectively by both CAR-T cells, but normal B cells were still detectable in the xenograft mice model treated with iKP-19-CAR-T cells while they could not be found in the mice treated with CD19-CAR-T cells. In contrast to CD19-CAR-T cells, the amount of CRS-associated cytokines IL-6, TNF-α and IFN-γ of iKP-19-CAR-T cells was decreased notably during lysing Daudi cells in vitro and this phenomenon was also observed in vivo, which indicated that iKP-19-CAR-T cells were safer than CD19-CAR-T cells. The result was similar to a CD19-CAR-T cells variant reported by Ying et al. [36]. This was possibly because iKP-19-CAR-T cells were not always activated, their activation was suppressed by the negative signaling provided by HLA-C1 expressed in normal B cells or T cells themselves. Therefore, the “missing self” activation mechanism like NK cells confers to iKP-19-CAR-T cells to control malignant B cells effectively and spare normal B cells.
“On-target off-tumor” toxicity has seriously limited the clinical application of CAR-T cells in the treatment of solid tumors. It has been reported that HER2-targeted CAR-T cell treatment for colon cancer caused a patient death because of CAR-T cells off target to pulmonary tissues [37]. In theory, iKP CAR can also reduce the “on-target off-tumor” toxicity of HER2-CAR-T cells to normal tissue cells. So far, our lab has detected the efficiency of iKP CAR in HER2-CAR-T cells therapy in vitro and in vivo, and similar results have been acquired (data not published).
“On-target off-tumor” toxicity has seriously limited the clinical application of CAR-T cells in the treatment of solid tumors. It has been reported that HER2-targeted CAR-T cell treatment for colon cancer caused a patient death because of CAR-T cells off target to pulmonary tissues[37]. In theory, iKP CAR can also reduce the “on-target off-tumor” toxicity of HER2-CAR-T cells to normal tissue cells. So far, our lab has detected the efficiency of iKP CAR in HER2-CAR-T cells therapy in vitro and in vivo, and similar results have been acquired (data not published).
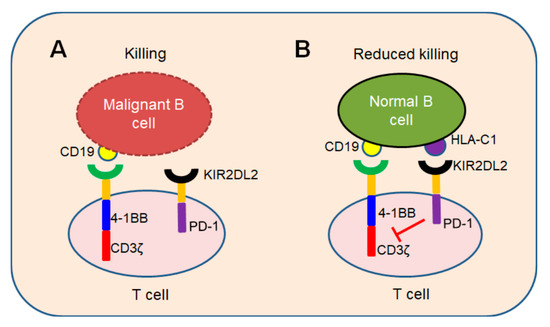
In conclusion, we demonstrated a novel iKP CAR can recognize HLA-C1 and deliver an inhibitory signaling to T cells. T cells can be activated by CD19 CAR and kill malignant B cells because iKP CAR does not work (
A). Once it recognizes “self-HLA-C1” on normal B cells, iKP CAR will deliver an inhibitory signaling via the intracellular PD-1 domain to halt T cell activation mediated by CD19 CAR (
B). This “missing self” activation mechanism like NK cells confers to iKP-19-CAR-T to control malignant B cells effectively and spare normal B cells in vitro and in vivo. The effectiveness of iKP CAR in the human body needs to be verified in future clinical trials.
Figure 1.
“Missing self” mechanism of iKP CAR. T cells co-expressing CD19 CAR and iKP CAR exploit “missing self” activation mechanism similar to NK cells. (
A
) T cells are activated by CD19 CAR and kill malignant B cells upon recognizing CD19 on malignant B cell tumors. (
B) CD19 CAR activation signal is inhibited by PD-1 signal when iKP CAR is engaged to “self-HLA-C1” on normal B cells and iKP-19-CAR-T cells do not kill normal B cells.
Reference
- Thomas, X.; Paubelle, E. Tisagenlecleucel-T for the treatment of acute lymphocyticleukemia. Expert. Opin. Biol. Ther. 2018, 18, 1095–1106. [Google Scholar] [CrossRef]
- Nair, R.; Neelapu, S.S. The promise of CAR T-cell therapy in aggressive B-cell lymphoma. Best Pract. Res. Clin. Haematol. 2018, 31, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Hwang, W.-T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalea, V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef]
- Shah, N.N.; Fry, T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Garfall, A.L.; Maus, M.V.; Hwang, W.-T.; Lacey, S.F.; Mahnke, Y.D.; Melenhorst, J.J.; Zheng, Z.; Vogl, D.T.; Cohen, A.D.; Weiss, B.M. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N. Engl. J. Med. 2015, 373, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Wilson, W.H.; Janik, J.E.; Dudley, M.E.; Stetler-Stevenson, M.; Feldman, S.A.; Maric, I.; Raffeld, M.; Nathan, D.-A.N.; Lanier, B.J. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Feldman, S.A.; Wilson, W.H.; Spaner, D.E.; Maric, I.; Stevenson, M.S.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012, 119, 2709–2720. [Google Scholar] [CrossRef]
- Oluwole, O.O.; Davila, M.L. At the bedside: Clinical review of chimeric antigen receptor (CAR) T cell therapy for B cell malignancies. J. Leukoc. Biol. 2016, 100, 1265–1272. [Google Scholar] [CrossRef]
- Pennell, C.A.; Barnum, J.L.; McDonald-Hyman, C.S.; Panoskaltsis-Mortari, A.; Riddle, M.J.; Xiong, Z.; Loschi, M.; Thangavelu, G.; Campbell, H.M.; Storlie, M.D. Human CD19-targeted mouse T cells induce B cell aplasia and toxicity in human CD19 transgenic mice. Mol. Ther. 2018, 26, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Li, D.; Hay, K.A.; Green, M.L.; Cherian, S.; Chen, X.; Riddell, S.R.; Maloney, D.G.; Boeckh, M.; Turtle, C.J. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018, 131, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hosen, N.; Matsunaga, Y.; Hasegawa, K.; Matsuno, H.; Nakamura, Y.; Makita, M.; Watanabe, K.; Yoshida, M.; Satoh, K.; Morimoto, S. The activated conformation of integrin β7 is a novel multiple myeloma-specific target for CAR T cell therapy. Nat. Med. 2017, 23, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Posey, A.D., Jr.; Schwab, R.D.; Boesteanu, A.C.; Steentoft, C.; Mandel, U.; Engels, B.; Stone, J.D.; Madsen, T.D.; Schreiber, K.; Haines, K.M. Engineered CAR T cells targeting the cancer-associated tn-glycoform of the membrane mucin muc1 control adenocarcinoma. Immunity 2016, 44, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, K.; Jiang, H.; Song, F.; Gao, H.; Pan, X.; Shi, B.; Bi, Y.; Wang, H.; Wang, H. Development of T cells carrying two complementary chimeric antigen receptors against glypican-3 and asialoglycoprotein receptor 1 for the treatment of hepatocellular carcinoma. Cancer Immunol. Immunother. 2017, 66, 475–489. [Google Scholar] [CrossRef]
- Roybal, K.T.; Rupp, L.J.; Morsut, L.; Walker, W.J.; Mcnally, K.A.; Park, J.S.; Lim, W.A. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 2016, 164, 770–779. [Google Scholar] [CrossRef]
- Morsut, L.; Roybal, K.T.; Xiong, X.; Gordley, R.M.; Goyle, S.M.; Thomson, M.; Lim, W.A. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell 2016, 164, 780–791. [Google Scholar] [CrossRef]
- Roybal, K.T.; Williams, J.Z.; Morsut, L.; Rupp, L.J.; Kolinko, I.; Choe, J.H.; Walker, W.J.; McNally, K.A.; Lim, W.A. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 2016, 167, 419–432. [Google Scholar] [CrossRef]
- Fedorov, V.D.; Themeli, M.; Sadelain, M. PD-1– and CTLA-4–based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 2013, 5, 215ra172. [Google Scholar] [CrossRef]
- Martinet, L.; Smyth, M.J. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 2015, 15, 243–254. [Google Scholar] [CrossRef]
- Handgretinger, R.; Lang, P.; Andre, M.C. Exploitation of natural killer cells for the treatment of acute leukemia. Blood 2016, 127, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Khong, H.T.; Restifo, N.P. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat. Immunol. 2002, 3, 999–1005. [Google Scholar] [CrossRef]
- Tsukahara, T.; Kawaguchi, S.; Torigoe, T.; Asanuma, H.; Nakazawa, E.; Shimozawa, K.; Nabeta, Y.; Kimura, S.; Kaya, M.; Nagoya, S. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006, 97, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Kono, K.; Maruyama, T.; Watanabe, M.; Kawaguchi, Y.; Kamimura, K.; Fujii, H. Downregulation of HLA class I molecules in the tumor is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br. J. Cancer 2008, 99, 1462–1467. [Google Scholar] [CrossRef]
- Pedoeem, A.; Azoulay-Alfaguter, I.; Strazza, M.; Silverman, G.J.; Mor, A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014, 153, 145–152. [Google Scholar] [CrossRef]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 pathway in the immune response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef]
- Chikuma, S. Basics of PD-1 in self-tolerance, infection, and cancer immunity. Int. J. Clin. Oncol. 2016, 21, 448–455.
- Lamers, C.H.; Sleijfer, S.; Vulto, A.G.; Kruit, W.H.; KLiffen, M.; Debets, R.; Gratama, J.W.; Stoter, G.; Oosterwijk, E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhyfrase IX: First clinical experience. J. Clin. Oncol. 2006, 24, e20–e22. [Google Scholar] [CrossRef] [PubMed]
- Akpek, G.; Lee, S.M.; Anders, V.; Vogelsang, G.B. A high-dose plus steroid regimen for controlling active chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2001, 7, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Karre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986, 319, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for HLA class I molecules in human natural killer cells. Annu. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Silva, D.; Srinivasan, M.; Sharma, S.; Lee, C.M.; Wagner, D.L.; Davis, T.H.; Rouce, R.H.; Bao, G.; Brenner, M.K.; Mamonkin, M. CD7-edited T cells espressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 2017, 130, 285–296. [Google Scholar] [CrossRef]
- Mary, E.K.; Manish, J.B.; Gordon, J.F.; Arlene, H.S. PD-1 and Its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M. The who's who of T-cell differentiation: Human memory T-cell subsets. J. Immunol. 2013, 43, 2797–2809.[Google Scholar] [CrossRef]
- Ying, Z.; Huang, X.F.; Xiang, X.; Liu, Y.; Kang, X.; Song, Y.; Guo, X.; Liu, H.; Ding, N.; Zhang, T. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 2019, 25, 947–953. [Google Scholar] [CrossRef]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851.[Google Scholar] [CrossRef]
) CD19 CAR activation signal is inhibited by PD-1 signal when iKP CAR is engaged to “self-HLA-C1” on normal B cells and iKP-19-CAR-T cells do not kill normal B cells.
References
- Thomas, X.; Paubelle, E. Tisagenlecleucel-T for the treatment of acute lymphocyticleukemia. Expert. Opin. Biol. Ther. 2018, 18, 1095–1106. [Google Scholar] [CrossRef]
- Nair, R.; Neelapu, S.S. The promise of CAR T-cell therapy in aggressive B-cell lymphoma. Best Pract. Res. Clin. Haematol. 2018, 31, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Hwang, W.-T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalea, V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef]
- Shah, N.N.; Fry, T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Garfall, A.L.; Maus, M.V.; Hwang, W.-T.; Lacey, S.F.; Mahnke, Y.D.; Melenhorst, J.J.; Zheng, Z.; Vogl, D.T.; Cohen, A.D.; Weiss, B.M. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N. Engl. J. Med. 2015, 373, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Wilson, W.H.; Janik, J.E.; Dudley, M.E.; Stetler-Stevenson, M.; Feldman, S.A.; Maric, I.; Raffeld, M.; Nathan, D.-A.N.; Lanier, B.J. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Feldman, S.A.; Wilson, W.H.; Spaner, D.E.; Maric, I.; Stevenson, M.S.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012, 119, 2709–2720. [Google Scholar] [CrossRef]
- Oluwole, O.O.; Davila, M.L. At the bedside: Clinical review of chimeric antigen receptor (CAR) T cell therapy for B cell malignancies. J. Leukoc. Biol. 2016, 100, 1265–1272. [Google Scholar] [CrossRef]
- Pennell, C.A.; Barnum, J.L.; McDonald-Hyman, C.S.; Panoskaltsis-Mortari, A.; Riddle, M.J.; Xiong, Z.; Loschi, M.; Thangavelu, G.; Campbell, H.M.; Storlie, M.D. Human CD19-targeted mouse T cells induce B cell aplasia and toxicity in human CD19 transgenic mice. Mol. Ther. 2018, 26, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Li, D.; Hay, K.A.; Green, M.L.; Cherian, S.; Chen, X.; Riddell, S.R.; Maloney, D.G.; Boeckh, M.; Turtle, C.J. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018, 131, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hosen, N.; Matsunaga, Y.; Hasegawa, K.; Matsuno, H.; Nakamura, Y.; Makita, M.; Watanabe, K.; Yoshida, M.; Satoh, K.; Morimoto, S. The activated conformation of integrin β7 is a novel multiple myeloma-specific target for CAR T cell therapy. Nat. Med. 2017, 23, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Posey, A.D., Jr.; Schwab, R.D.; Boesteanu, A.C.; Steentoft, C.; Mandel, U.; Engels, B.; Stone, J.D.; Madsen, T.D.; Schreiber, K.; Haines, K.M. Engineered CAR T cells targeting the cancer-associated tn-glycoform of the membrane mucin muc1 control adenocarcinoma. Immunity 2016, 44, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, K.; Jiang, H.; Song, F.; Gao, H.; Pan, X.; Shi, B.; Bi, Y.; Wang, H.; Wang, H. Development of T cells carrying two complementary chimeric antigen receptors against glypican-3 and asialoglycoprotein receptor 1 for the treatment of hepatocellular carcinoma. Cancer Immunol. Immunother. 2017, 66, 475–489. [Google Scholar] [CrossRef]
- Roybal, K.T.; Rupp, L.J.; Morsut, L.; Walker, W.J.; Mcnally, K.A.; Park, J.S.; Lim, W.A. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 2016, 164, 770–779. [Google Scholar] [CrossRef]
- Morsut, L.; Roybal, K.T.; Xiong, X.; Gordley, R.M.; Goyle, S.M.; Thomson, M.; Lim, W.A. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell 2016, 164, 780–791. [Google Scholar] [CrossRef]
- Roybal, K.T.; Williams, J.Z.; Morsut, L.; Rupp, L.J.; Kolinko, I.; Choe, J.H.; Walker, W.J.; McNally, K.A.; Lim, W.A. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 2016, 167, 419–432. [Google Scholar] [CrossRef]
- Fedorov, V.D.; Themeli, M.; Sadelain, M. PD-1– and CTLA-4–based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 2013, 5, 215ra172. [Google Scholar] [CrossRef]
- Martinet, L.; Smyth, M.J. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 2015, 15, 243–254. [Google Scholar] [CrossRef]
- Handgretinger, R.; Lang, P.; Andre, M.C. Exploitation of natural killer cells for the treatment of acute leukemia. Blood 2016, 127, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Khong, H.T.; Restifo, N.P. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat. Immunol. 2002, 3, 999–1005. [Google Scholar] [CrossRef]
- Tsukahara, T.; Kawaguchi, S.; Torigoe, T.; Asanuma, H.; Nakazawa, E.; Shimozawa, K.; Nabeta, Y.; Kimura, S.; Kaya, M.; Nagoya, S. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006, 97, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Kono, K.; Maruyama, T.; Watanabe, M.; Kawaguchi, Y.; Kamimura, K.; Fujii, H. Downregulation of HLA class I molecules in the tumor is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br. J. Cancer 2008, 99, 1462–1467. [Google Scholar] [CrossRef]
- Pedoeem, A.; Azoulay-Alfaguter, I.; Strazza, M.; Silverman, G.J.; Mor, A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014, 153, 145–152. [Google Scholar] [CrossRef]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 pathway in the immune response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef]
- Chikuma, S. Basics of PD-1 in self-tolerance, infection, and cancer immunity. Int. J. Clin. Oncol. 2016, 21, 448–455.
- Lamers, C.H.; Sleijfer, S.; Vulto, A.G.; Kruit, W.H.; KLiffen, M.; Debets, R.; Gratama, J.W.; Stoter, G.; Oosterwijk, E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhyfrase IX: First clinical experience. J. Clin. Oncol. 2006, 24, e20–e22. [Google Scholar] [CrossRef] [PubMed]
- Akpek, G.; Lee, S.M.; Anders, V.; Vogelsang, G.B. A high-dose plus steroid regimen for controlling active chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2001, 7, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Karre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986, 319, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for HLA class I molecules in human natural killer cells. Annu. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Silva, D.; Srinivasan, M.; Sharma, S.; Lee, C.M.; Wagner, D.L.; Davis, T.H.; Rouce, R.H.; Bao, G.; Brenner, M.K.; Mamonkin, M. CD7-edited T cells espressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 2017, 130, 285–296. [Google Scholar] [CrossRef]
- Mary, E.K.; Manish, J.B.; Gordon, J.F.; Arlene, H.S. PD-1 and Its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M. The who's who of T-cell differentiation: Human memory T-cell subsets. J. Immunol. 2013, 43, 2797–2809.[Google Scholar] [CrossRef]
- Ying, Z.; Huang, X.F.; Xiang, X.; Liu, Y.; Kang, X.; Song, Y.; Guo, X.; Liu, H.; Ding, N.; Zhang, T. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 2019, 25, 947–953. [Google Scholar] [CrossRef]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851.[Google Scholar] [CrossRef]