This review deals with the utilization of chitin and chitosan derivatives in agriculture. In particular it summarizes recent results concerning the utilization of these compounds to face biotic and abiotic stresses. In addition the review summarizes recent results about the utilization of chitin- and chitosan-based materials for the recovery of waste water and soil contaminaded by different chemicals
- chitin
- chitosan
- defense responses
- plant growth
- recovery
- stress
1. Introduction
Biotic (pathogen attack, herbivores, wounding), abiotic (deficient or excessive water, low or high temperature, high salinity, ultraviolet radiation, heavy metals, various toxic contaminants) stresses and in particular the unpredictable combination of different stresses are very harmful to plant growth and development, and lead to severe crop yield loss worldwide[1]. In consideration of the increasing food demand of the growing world population, it is becoming imperative to enhance crops’ tolerance of multistress. So far, different approaches have been tested to increase plant resistance against stresses. In particular, an increasing resistance has been obtained by the use of different chemicals such as pesticides, fertilizers and phytoregulators. However, the extensive use of these chemicals in agriculture has a great environmental impact, with accumulation in soil, water and in living organisms[2]. This has stimulated the search of more eco-friendly mechanisms to manage plant stresses. Chitin, a naturally occurring long-chain high molecular weight polysaccharide composed of N-acetyl-D-glucosamine and D-glucosamine, a main component of the exoskeleton of arthropods and of the fungal cell wall, and its deacetylated derivative chitosan, seem promising tools to solve this problem (Figure 1).
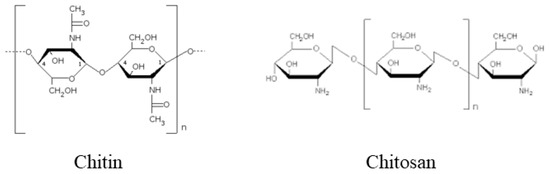
Figure 1. Chemical structure of chitin and chitosan.
In fact, these molecules, easily obtainable from the crustacean shells of crabs, prawns etc., and are non-toxic, biodegradable and biocompatible. This accounts for their potentially broad application in agriculture where they can act as potent stimulators of plant productivity and protectors against pathogens ([3][4]and references therein]). In addition, chitin and chitosan can act as bioadsorbents for remediation of contaminated soil and water. The increasing industrial production and use of synthetic molecules such as dyes, pesticides, fertilizers, molecules containing heavy metal ions or nuclear residues are a major environmental concern. In fact, these compounds accumulate in soil and water and enter into food chains resulting in mutagenesis, carcinogenicity and other serious human health impairments[5]. To date, arrays of methodologies are in use to remediate this situation. Adsorption is the most attractive, in particular when it employs eco-friendly, sustainable, and low-cost materials such as chitin and chitosan[6].
After some pioneering indications in the early 1980′s on the protective effects of chitin and chitosan, an impressive number of papers dealing with the use of these molecules in an agricultural context have been published. These papers have been summarized in many exhaustive reviews ([2][3][4], among others]), thus in this review we summarize only recent results (years 2018–2020) obtained using chitin- and chitosan-based derivatives in plant protection against biotic and abiotic stresses and in recovery of contaminated soil and water.
2. Chitin- and Chitosan-Based Derivatives in Plant Protection against Biotic Stress
The total world population has increased from 2.5 billion in the 1950’s to over 7 billion in the present and is expected to increase to 9 billion by the end of the century. To feed this increasing population, food production must also rise, and in a safe and environmentally sustainable manner. At present, severe crop yield losses occur due to plant diseases and to global climate changes that have increased some phytosanitary emergences[7]. To fight these losses, several chemicals are still in use in modern agriculture. However, these agrochemicals are not without risk for the environment where their residues can easily accumulate. Thus, the implementation of novel strategies to manage plant disease is crucial to respond to the growing demand of safe and healthy food. In this perspective, chitin and chitosan are among the most promising tools. In fact, they can fight several stresses permitting relevant increases in plant productivity. The exact action mechanism of these protective molecules is under investigation and the different possibilities summarized in the following have been proposed (see[4] and references therein). Chitin and chitosan are positively charged molecules that can easily interact with the anionic structures present in the cell wall and in the cellular and nuclear membranes of pathogens like proteins, lipopolysaccharides and negatively charged ions. This can lead to leakage of intracellular components and the death of the microorganism. Interestingly, the negatively charged phosphate groups of nucleic acids can strongly interact with chitin and chitosan. This direct interaction can induce specific modifications in the expression and activity of proteins involved in the stress response. In addition, recent investigations strongly suggest the presence in the cellular plasma membrane of a specific receptor belonging to the glycoprotein family of lectins. The binding with this receptor starts a well-defined signaling cascade that leads to the responses. Finally, it should be noted that chitin and chitosan with their high nitrogen content and very low C/N ratio can directly act as natural fertilizers ([8] and references therein]). Whatever the mode of action, chitin, chitosan and their derivatives (e.g., nanoparticles (≤0.5 µm in size), microparticles (≥1 µm in size) and oligosaccharides, degradation products formed by no more than 12 glucosamine residues) permit a relevant increase in plant productivity by controlling several plant pathogens (Table 1).
Table 1. Chitin- and chitosan-based derivatives in plant protection against biotic stress.
| Plant Species | Characteristics of the Protective Molecules and Method of Administration | Protective Effect | Reference |
|---|---|---|---|
| Capsicum annuum L. | 1% chitosan, foliar application | Resistance against Phytophthora capsici | [9] |
| Melissa officinalis | 0.005, 0.01, 0.015% chitosan, shoot spraying | Accumulation of defense-related enzymes and phenolic compounds | [10] |
| Phoenix dactylifera L. | 0.1% chitosan nanoparticles, seedling irrigation | Enhancement of the innate immunity | [11] |
| Solanum lycopersicum | 0.001, 0.01, 0.1% chitosan microparticles, foliar application | Accumulation of defense-related enzymes | [12] |
| Stone fruit trees | 0.001% chitosan-Ag nanoparticles, foliar application | Resistance against Pseudomonas syringae | [13] |
| Beta vulgaris | 0.2% chitosan; 0.05% nano chitosan, foliar sprayjng | Resistance against Pegomya hyoscyami | [14] |
| Solanum tuberosum L. | 0.4% chitosan, tuber immersion | Resistance against Fusarium spp. | [15] |
| Oryza sativa L. | 0.3% chitosan oligosaccharide, seedlings sprayjng | Resistance against Fusarium oxysporum | [16] |
| Citrus reticulata Blanco | 0.05% chitin oligosaccharide, leaf infiltration | Resistance against Candidatus Liberibacter asiaticus | [17] |
| “in vitro” test | 0.5% chitin oligosaccharide diluted in culture medium | Inhibition of Botrytis cinerea spores germination | [18] |
Foliar application of chitosan enhances growth and modulates expression of defense genes in chilli pepper (Capsicum annuum L.) thus reducing the severe losses in chilli production induced by Phytophthora capsici infection[9]. Shoot cultures of Melissa officinalis treated with chitosan accumulate several defense-related enzymes and phenolic compounds with antimicrobial activity[10]. Similar results have been obtained in date palm (Phoenix dactylifera L.) seedlings treated with chitosan nanoparticles[11] and in tomato (Solanum lycopersicum) seedlings treated with chitosan microparticles[12]. A silver-chitosan nanocomposite reduces canker disease induced by Pseudomonas syringae pv. syringae in stone fruit trees[13] and chitosan and nano chitosan treatments efficiently control beet fly (Pegomya hyoscyami) infection in sugarbeet (Beta vulgaris) plants[14]. Similarly, chitosan alleviates diseases induced by Fusarium spp. in potato (Solanum tuberosum L.)[15] and by Fusarium oxysporum in rice (Oryza sativa L.)[16]. Interestingly, in Sun Chu Sha mandarin (Citrus reticulata Blanco) plants, hexaacetyl-chitohexaose, a chitin-derived oligosaccharide, affects the vitality of Asian citrus psyllid (Diaphorina citri), the hemipteran vector of Candidatus Liberibacter asiaticus, the pathogen associated with citrus greening disease[17]. In addition, efficacy of undiluted chitin-based cultures of Paenibacillus elgii HOA73 bacteria, a biocontrol agent that limits the damage caused to plants by microbial pathogens, insects, and nematodes, is comparable to that of standard chemical pesticides, suggesting a possible alternative to these chemicals in eco-friendly agriculture[18]. The suppressive effect of chitin added to the soil against pathogens often involves a change in the composition of the soil microbiota with an increase in the presence and activity of chitinolytic microorganisms that hydrolyze the chitinous hyphae of pathogenic fungi, and with an increase in secondary responders to added chitin that may affect pathogens[19]. However, it has been reported that deacetylation by specific enzymes of chitin oligomers converting them to ligand-inactive chitosan, is a strategy largely used by soil-borne fungal pathogens to prevent the protective effect of chitin[20]. These results indicate that more investigations are needed to exactly clarify the virulence strategy of soil-borne fungal pathogens.
3. Chitin- and Chitosan-Based Derivatives in Plant Protection against Abiotic Stress
To secure healthy and safe food of high nutritional quality to the growing world population and in particular, to consent adequate food access even in the present condition of global climate changes, it is necessary to increase plant resistance against abiotic stress too. In fact, abiotic stresses (temperature, water, salt, heavy metals, and UV radiation, among others) account for relevant losses in agricultural production worldwide[1]. To this end, in addition to selection of more performing crop genotypes, evaluation of new agronomic techniques and/or new agrochemicals without adverse ecological impact is needed. Water scarcity is regarded as the key restriction point for food production worldwide[21]. Thus, many investigations have been recently performed to ameliorate water utilization. Among the investigated substances, chitin and chitosan are able to confer tolerance to several abiotic stresses (Table 2).
Table 2. Chitin- and chitosan-based derivatives in plant protection against abiotic stress.
| Plant Species | Characteristics of the Protective Molecules and Methods of Administration | Protective Effect | Reference |
|---|---|---|---|
| Zea mays L. cv. White Pearl | 0, 2 and 4 g chitin added to 1 kg of soil | Drought stress tolerance | [22] |
| Triticum aestivum L. | 0.0125% chitosan, foliar application | Drought stress tolerance | [23] |
| Triticum aestivum cv. pishtaz | 0.0009% chitosan nanoparticles, soil and foliar application | Drought stress tolerance | [24] |
| Zea mays L. | 0.01% chitosan, foliar application | Drought stress tolerance | [25] |
| Sesamum indicum L. | 0.00048, 0.00064% chitosan, foliar application | Drought stress tolerance | [26] |
| Origanum majorana | 0.005, 0.02, 0.05% chitosan, plant irrigation | Drought stress tolerance | [27] |
| Brassica napus L. | 0.2% chitosan, seedling soaking | Drought stress tolerance | [28] |
| Arabidopsis thaliana | 0.01% chitin, plant spraying | Drought stress tolerance | [29] |
| Triticum aestivum L., Zea mays L. | 25, 50, 75% chitosan, seed coating | Salt stress tolerance | [30] |
| Zea mays cv. Arifiye | 0.1% chitosan, foliar application | Salt stress tolerance | [31] |
| Solanum lycopersicum Mill. | Chitosan–aggregated growth-promoting bacteria | Salt stress tolerance | [32] |
| Zea mays L. | 0.01% chitosan, seedling soaking | Cadmium stress tolerance | [33] |
| Solanum melongena L. | 0.0125, 0.0150, 0.02% chitosan, foliar application | Heat stress tolerance | [34] |
| Capsicum annuum L. | 0.00125, 0.00250, 0.00375% chitosan, plant spraying | Heat stress tolerance | [35] |
| Solanum lycopersicum Mill. | 0.003, 0.006, 0.009, 0.012% chitosan, foliar application | Heat stress tolerance | [36] |
| Solanum tuberosum L. | 0.25, 0.5% chitosan, foliar application | Poor soil tolerance | [37] |
| Phaseolus vulgaris cv. Contender | 10% chitosan nanoparticles loaded with NPK fertilizers, seed priming and foliar application | Poor soil tolerance | [38] |
| Mokara Orchids Hybrids | 0.002, 0.004, 0.008% chitosan, foliar application | Poor fertilization tolerance | [39] |
| Solanum lycopersicum | 1 mg/plant of chitosan, applied to the soil in the transplant cavity | Poor fertilization tolerance | [40] |
| Fragaria x ananassa Duch. cv. Elsanta | 0.001% chitosan, foliar application | Poor fertilization tolerance | [41] |
For example, chitin added to the soil at the beginning of the experiment induces water-stress tolerance in maize (Zea mays L.) plants grown under regulated deficit irrigation[22]. Chitosan application has the potential to mitigate the water deficit effects on yield and quality of wheat (Triticum aestivum L.)[23], and in the same plant species chitosan nanoparticles decrease the adverse effects of drought stress[24]. Similarly, leaf application of chitosan renders maize (Zea mays L.) hybrids more tolerant to water stress[25] and decreases the plant damage under drought stress in sesame (Sesamum indicum L.)[26]. Chitosan furnished as water solution alleviates water stress in marjoram (Origanum majorana L.)[27], and application of chitosan solution during sowing increases resistance against drought stress and the amount of oil in rape (Brassica napus L.)[28]. Basic studies conducted in the model plant Arabidopsis thaliana suggest that chitin modulates water balance through its action on vascular bundle sheath and mesophyll cells[29]. About other stresses, chitosan application alleviates salt stress thus improving growth performance in Triticum aestivum L. and Zea mays L.[30], and confers tolerance to salt stress in maize seedlings by enhancing the expression and activation of alternative oxidase[31]. Interestingly, inoculation of chitosan-immobilized plant growth-promoting bacteria improves growth of tomato plant (Solanum lycopersicum Mill.) under salt stress conditions suggesting the use of this eco-friendly and sustainable approach to fight salt stress[32]. Finally, chitin and chitosan use permits the cultivation of crops even in non-optimal conditions and the conservation of energy and other resources. For example, chitosan treatment alleviates cadmium stress in maize (Zea mays L.) seedlings, permitting their growth in areas subject to heavy metal stress[33] and application of chitosan protects eggplants in field (Solanum melongena L.) against heat and high irradiance stresses[34]. Chitosan spraying on sweet pepper plants (Capsicum annuum L.) permits their growth in unheated greenhouse conditions [35]. Similarly, foliar application of chitosan or chitosan nanoparticles has positive effects on different plant species. In fact, it ameliorates growth and the quality of tomato (Solanum lycopersicum Mill.) plants under plastic tunnel conditions[36]. It also stimulates tuber yields of potato (Solanum tuberosum L.) plants grown under newly reclaimed sandy soil conditions[37]. Finally, when supplemented as nanoparticles loaded with an NPK fertilizer, it ameliorates growth and productivity of French bean (Phaseolus vulgaris cv. Contender) plants grown in clay–sandy soil[38]. Last but not least, chitin and chitosan treatments permit relevant savings in the use of very expensive and high ecological impacting chemical fertilizers and cultivation in soil with limited nutrients. Chitosan ameliorates inflorescence quality and commercial half-life of Mokara orchid hybrids grown at half the regular application fertilizer dosage[39]. Chitosan stimulates growth of tomato (Solanum lycopersicum) plants[40] in poor soil conditions, and it promotes growth, fruit yield and quality in strawberry plants (Fragaria x ananassa Duch.) cv. Elsanta grown under nutrient limitations[41].
Chitin and chitosan can protect plants against abiotic stress with different mechanism with respect to different stresses. For example, their foliar application induces drought tolerance by direct antitranspirant coating, induction of stomatal closure, accumulation of stress protective enzymes and metabolites ([42]and references therein]). Similarly, chitin and chitosan can affect heat stress by abscissic acid accumulation, which is linked with the previous reported induction of stomatal closure ([42] and references therein]). Salinity stress is relieved by accumulation of antioxidant enzymes and reduction of lipid peroxidation ([42] and references therein]). Finally, due to the presence of functional amino and hydroxyl groups, chitin and chitosan are also able to form complexes with several heavy metals, thus reducing their bioavailability and alleviating their phytotoxicity ([42]and references therein]).
References
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771. He, M.; He, C.-Q.; Ding, N.-Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996–1010. Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996–1010. [Google Scholar]
- Malerba, M.; Cerana, R. Recent advances of chitosan application in plants. Polymers 2018, 10, 118–127. Malerba, M.; Cerana, R. Recent advances of chitosan application in plants. Polymers 2018, 10, 118–127. [Google Scholar]
- Malerba, M.; Cerana, R. Recent applications of chitin- and chitosan-based polymers in plants. Polymers 2019, 11, 839–847. Malerba, M.; Cerana, R. Recent applications of chitin- and chitosan-based polymers in plants. Polymers 2019, 11, 839–847. [Google Scholar]
- Batool, S.; Rashid, S.A.; Moah, M.J.; Sarfraz, M.; Ashraf, M.A. Geographical distribution of persistent organic pollutants in the environment: A review. J. Environ. Biol. 2016, 37, 1125–1134.Batool, S.; Rashid, S.A.; Moah, M.J.; Sarfraz, M.; Ashraf, M.A. Geographical distribution of persistent organic pollutants in the environment: A review. J. Environ. Biol. 2016, 37, 1125–1134. [Google Scholar]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; Bezerra, C.O.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; Bezerra, C.O.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar]
- Iriti, M.; Vitalini, S. Sustainable crop protection, global climate change, food security and safety—Plant immunity at the crossroads. Vaccines 2020, 8, 42. Iriti, M.; Vitalini, S. Sustainable crop protection, global climate change, food security and safety—Plant immunity at the crossroads. Vaccines 2020, 8, 42. [Google Scholar] [CrossRef]
- Shamshina, J.; Oldham, T.; Rogers, R.D. Applications of chitin in agriculture. Sustain. Agric. Rev. 2019, 36, 125–146.Shamshina, J.; Oldham, T.; Rogers, R.D. Applications of chitin in agriculture. Sustain. Agric. Rev. 2019, 36, 125–146. [Google Scholar] [CrossRef]
- Esyanti, R.R.; Dwivany, F.M.; Mahani, S.; Nugrahapraja, H.; Meitha, K. Foliar application of chitosan enhances growth and modulates expression of defense genes in chilli pepper (Capsicum annuum L.). Aust. J. Crop. Sci. 2019, 13, 55–60. Esyanti, R.R.; Dwivany, F.M.; Mahani, S.; Nugrahapraja, H.; Meitha, K. Foliar application of chitosan enhances growth and modulates expression of defense genes in chilli pepper (Capsicum annuum L.). Aust. J. Crop. Sci. 2019, 13, 55–60. [Google Scholar] [CrossRef]
- Fooladi vanda, G.; Shabani, L.; Razavizadeh, R. Chitosan enhances rosmarinic acid production in shoot cultures of Melissa officinalis L. through the induction of methyl jasmonate. Bot. Stud. 2019, 60, 26. Fooladi vanda, G.; Shabani, L.; Razavizadeh, R. Chitosan enhances rosmarinic acid production in shoot cultures of Melissa officinalis L. through the induction of methyl jasmonate. Bot. Stud. 2019, 60, 26. [Google Scholar] [CrossRef]
- Mohamed, E.A. Copper and chitosan nanoparticles as potential elicitors of innate immune response in date palm: A comparative study. Arch. Phytopathol. Plant Prot. 2019, 52, 1276–1288. Mohamed, E.A. Copper and chitosan nanoparticles as potential elicitors of innate immune response in date palm: A comparative study. Arch. Phytopathol. Plant Prot. 2019, 52, 1276–1288. [Google Scholar] [CrossRef]
- Colman, S.L.; Salcedo, M.F.; Mansilla, A.Y.; Iglesias, M.J.; Fiol, D.F.; Martín-Saldaña, S.; Alvarez, V.A.; Chevalier, A.A.; Casalongué, C.A. Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiol. Biochem. 2019, 143, 203–211.Colman, S.L.; Salcedo, M.F.; Mansilla, A.Y.; Iglesias, M.J.; Fiol, D.F.; Martín-Saldaña, S.; Alvarez, V.A.; Chevalier, A.A.; Casalongué, C.A. Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiol. Biochem. 2019, 143, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Shahryari, F.; Rabiei, Z.; Sadighian, S. Antibacterial activity of synthesized silver nanoparticles by sumac aqueous extract and silver-chitosan nanocomposite against Pseudomonas syringae pv. syringae. J. Plant Pathol. 2020. Shahryari, F.; Rabiei, Z.; Sadighian, S. Antibacterial activity of synthesized silver nanoparticles by sumac aqueous extract and silver-chitosan nanocomposite against Pseudomonas syringae pv. syringae. J. Plant Pathol. 2020. [Google Scholar] [CrossRef]
- Sabbour, M.M.; Soleiman, N.Y. Control of beet fly (Pegomya hyoscyami) (Diptera: Anthomyidae) using chitosan and nanochitosan. Plant Arch. 2019, 19 (Suppl. 2), 462–465. Sabbour, M.M.; Soleiman, N.Y. Control of beet fly (Pegomya hyoscyami) (Diptera: Anthomyidae) using chitosan and nanochitosan. Plant Arch. 2019, 19 (Suppl. 2), 462–465. [Google Scholar]
- Mejdoub-Trabelsi, B.; Touihri, S.; Ammar, N.; Riahi, A.; Daami-Remadi, M. Effect of chitosan for the control of potato diseases caused by Fusarium species. J. Phytopathol. 2020, 168, 18–27.Mejdoub-Trabelsi, B.; Touihri, S.; Ammar, N.; Riahi, A.; Daami-Remadi, M. Effect of chitosan for the control of potato diseases caused by Fusarium species. J. Phytopathol. 2020, 168, 18–27. [Google Scholar] [CrossRef]
- Ma, B.; Wang, J.; Liu, C.; Hu, J.; Tan, K.; Zhao, F.; Yuan, M.; Zhang, J.; Gai, Z. Preventive effects of fluoro-substituted benzothiadiazole derivatives and chitosan oligosaccharide against the rice seedling blight induced by Fusarium oxysporum. Plants 2019, 8, 538. Ma, B.; Wang, J.; Liu, C.; Hu, J.; Tan, K.; Zhao, F.; Yuan, M.; Zhang, J.; Gai, Z. Preventive effects of fluoro-substituted benzothiadiazole derivatives and chitosan oligosaccharide against the rice seedling blight induced by Fusarium oxysporum. Plants 2019, 8, 538. [Google Scholar] [CrossRef]
- Shi, Q.; George, J.; Krystel, J.; Zhang, S.; Lapointe, S.L.; Stelinski, L.L.; Stover, E. Hexaacetyl-chitohexaose, a chitin-derived oligosaccharide, transiently activates citrus defenses and alters the feeding behavior of Asian citrus psyllid. Hortic. Res. 2019, 6, 76.Shi, Q.; George, J.; Krystel, J.; Zhang, S.; Lapointe, S.L.; Stelinski, L.L.; Stover, E. Hexaacetyl-chitohexaose, a chitin-derived oligosaccharide, transiently activates citrus defenses and alters the feeding behavior of Asian citrus psyllid. Hortic. Res. 2019, 6, 76. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hur, J.Y.; Park, S.K. Biocontrol of Botrytis cinerea by chitin-based cultures of Paenibacillus elgii HOA73. Eur. J. Plant Pathol. 2019, 155, 253–263.Kim, Y.C.; Hur, J.Y.; Park, S.K. Biocontrol of Botrytis cinerea by chitin-based cultures of Paenibacillus elgii HOA73. Eur. J. Plant Pathol. 2019, 155, 253–263. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ichino, T.; Saito, A. Transition of the bacterial community and culturable chitinolytic bacteria in chitin-treated upland soil: From Streptomyces to methionine-auxotrophic Lysobacter and other genera. Microbes Environ. 2020, 35.Iwasaki, Y.; Ichino, T.; Saito, A. Transition of the bacterial community and culturable chitinolytic bacteria in chitin-treated upland soil: From Streptomyces to methionine-auxotrophic Lysobacter and other genera. Microbes Environ. 2020, 35. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, B.-S.; Zhao, J.-H.; Huang, J.-F.; Jia, P.S.; Wang, S.; Zhang, J.; Zhou, J.-M.; Guo, H.-S. Deacetylation of chitin oligomers increases virulence in soil-borne fungal pathogens. Nat. Plants 2019, 5, 1167–1176. Gao, F.; Zhang, B.-S.; Zhao, J.-H.; Huang, J.-F.; Jia, P.S.; Wang, S.; Zhang, J.; Zhou, J.-M.; Guo, H.-S. Deacetylation of chitin oligomers increases virulence in soil-borne fungal pathogens. Nat. Plants 2019, 5, 1167–1176. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations—FAO. Deficit Irrigation Practices; Water Reports, No. 22; FAO: Rome, Italy, 2002.Food and Agriculture Organization of the United Nations—FAO. Deficit Irrigation Practices; Water Reports, No. 22; FAO: Rome, Italy, 2002. [Google Scholar]
- Lin, K.-H.; Lin, F.-W.; Wu, C.-W.; Chang, Y.-S. Biostimulation of maize (Zea mays) and irrigation management improved crop growth and water use under controlled environment. Agronomy 2019, 9, 559. Lin, K.-H.; Lin, F.-W.; Wu, C.-W.; Chang, Y.-S. Biostimulation of maize (Zea mays) and irrigation management improved crop growth and water use under controlled environment. Agronomy 2019, 9, 559. [Google Scholar] [CrossRef]
- Farouk, S.; EL-Metwally, I.M. Synergistic responses of drip-irrigated wheat crop to chitosan and/or silicon under different irrigation regimes. Agric. Water Manag. 2019, 226, 105807. Farouk, S.; EL-Metwally, I.M. Synergistic responses of drip-irrigated wheat crop to chitosan and/or silicon under different irrigation regimes. Agric. Water Manag. 2019, 226, 105807. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi-Sarvestani, Z.; Kassaee, M.Z.; Modarres-Sanavy, S.A.M.; Sorooshzadeh, A.; Mokhtassi-Bidgoli, A. Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 2019, 42, 1439–1451.Behboudi, F.; Tahmasebi-Sarvestani, Z.; Kassaee, M.Z.; Modarres-Sanavy, S.A.M.; Sorooshzadeh, A.; Mokhtassi-Bidgoli, A. Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 2019, 42, 1439–1451. [Google Scholar] [CrossRef]
- Veroneze-Júnior, V.; Martins, M.; Mc Leod, L.; Souza, K.R.D.; Santos-Filho, P.R.; Magalhães, P.C.; Carvalho, D.T.; Santos, M.H.; Souza, T.C. Leaf application of chitosan and physiological evaluation of maize hybrids contrasting for drought tolerance under water restriction. Braz. J. Biol. 2019. Veroneze-Júnior, V.; Martins, M.; Mc Leod, L.; Souza, K.R.D.; Santos-Filho, P.R.; Magalhães, P.C.; Carvalho, D.T.; Santos, M.H.; Souza, T.C. Leaf application of chitosan and physiological evaluation of maize hybrids contrasting for drought tolerance under water restriction. Braz. J. Biol. 2019. [Google Scholar] [CrossRef]
- Khordadi Varamin, J.; Fanoodi, F.; Sinaki, J.M.; Rezvan, S.; Damavandi, A. Physiological response of sesame (Sesamum indicum L.) to application of chitosan and magnesium-nano fertilizers under irrigation cut-off in a sustainable agriculture system. Iran. J. Plant Physiol. 2018, 9, 2629–2639.Khordadi Varamin, J.; Fanoodi, F.; Sinaki, J.M.; Rezvan, S.; Damavandi, A. Physiological response of sesame (Sesamum indicum L.) to application of chitosan and magnesium-nano fertilizers under irrigation cut-off in a sustainable agriculture system. Iran. J. Plant Physiol. 2018, 9, 2629–2639. [Google Scholar]
- Al-Ghamdi, A.A. Marjoram physiological and molecular performance under water stress and chitosan treatment. Acta Physiol. Plant. 2019, 41.Al-Ghamdi, A.A. Marjoram physiological and molecular performance under water stress and chitosan treatment. Acta Physiol. Plant. 2019, 41. [Google Scholar] [CrossRef]
- Monfared, B.B.; Noormohamadi, G.; Rad, A.H.S.; Hervan, E.M. Effects of sowing date, cultivar and chitosan on quality and quantity of rapeseed (Brassica napus L.) oil. J. Agric. Sci. 2019, 25, 508–517.Monfared, B.B.; Noormohamadi, G.; Rad, A.H.S.; Hervan, E.M. Effects of sowing date, cultivar and chitosan on quality and quantity of rapeseed (Brassica napus L.) oil. J. Agric. Sci. 2019, 25, 508–517. [Google Scholar] [CrossRef]
- Attia, Z.; Dalal, A.; Moshelion, M. Vascular bundle sheath and mesophyll cells modulate leaf water balance in response to chitin. Plant J. 2019. Attia, Z.; Dalal, A.; Moshelion, M. Vascular bundle sheath and mesophyll cells modulate leaf water balance in response to chitin. Plant J. 2019. [Google Scholar] [CrossRef]
- Peykani, L.S.; Sepehr, M.F. Effect of chitosan on antioxidant enzyme activity, proline, and malondialdehyde content in Triticum aestivum L. and Zea maize L. under salt stress condition. Iran. J. Plant Physiol. 2018, 9, 2661–2670. Peykani, L.S.; Sepehr, M.F. Effect of chitosan on antioxidant enzyme activity, proline, and malondialdehyde content in Triticum aestivum L. and Zea maize L. under salt stress condition. Iran. J. Plant Physiol. 2018, 9, 2661–2670. [Google Scholar]
- Turk, H. Chitosan-induced enhanced expression and activation of alternative oxidase confer tolerance to salt stress in maize seedlings. Plant Physiol. Biochem. 2019, 141, 415–422.Turk, H. Chitosan-induced enhanced expression and activation of alternative oxidase confer tolerance to salt stress in maize seedlings. Plant Physiol. Biochem. 2019, 141, 415–422. [Google Scholar] [CrossRef]
- Chanratana, M.; Joe, M.M.; Roy Choudhury, A.; Anandham, R.; Krishnamoorthy, R.; Kim, K.; Jeon, S.; Choi, J.; Choi, J.; Sa, T. Physiological response of tomato plant to chitosan-immobilized aggregated Methylobacterium oryzae CBMB20 inoculation under salinity stress. 3 Biotech 2019, 9, 397.Chanratana, M.; Joe, M.M.; Roy Choudhury, A.; Anandham, R.; Krishnamoorthy, R.; Kim, K.; Jeon, S.; Choi, J.; Choi, J.; Sa, T. Physiological response of tomato plant to chitosan-immobilized aggregated Methylobacterium oryzae CBMB20 inoculation under salinity stress. 3 Biotech 2019, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.Y.; Gu, W.R.; Zhang, L.G.; Li, C.F.; Chen, X.C.; Li, J.; Li, L.J.; Xie, T.L.; Wei, S. Role of chitosan in the regulation of the growth, antioxidant system and photosynthetic characteristics of maize seedlings under cadmium stress. Russ. J. Plant Physiol. 2019, 66, 140–151.Qu, D.Y.; Gu, W.R.; Zhang, L.G.; Li, C.F.; Chen, X.C.; Li, J.; Li, L.J.; Xie, T.L.; Wei, S. Role of chitosan in the regulation of the growth, antioxidant system and photosynthetic characteristics of maize seedlings under cadmium stress. Russ. J. Plant Physiol. 2019, 66, 140–151. [Google Scholar] [CrossRef]
- Liaqat, A.; Ihsan, M.Z.; Rizwan, M.S.; Mehmood, A.; Ijaz, M.; Alam, M.; Abdullah, M.; Wajid, M.; Hussain, R.; Naeem, M.; et al. Inducing effect of chitosan on the physiological and biochemical indices of eggplant (Solanum melongena L.) genotypes under heat and high irradiance. Appl. Ecol. Environ. Res. 2019, 17, 11273–11287.Liaqat, A.; Ihsan, M.Z.; Rizwan, M.S.; Mehmood, A.; Ijaz, M.; Alam, M.; Abdullah, M.; Wajid, M.; Hussain, R.; Naeem, M.; et al. Inducing effect of chitosan on the physiological and biochemical indices of eggplant (Solanum melongena L.) genotypes under heat and high irradiance. Appl. Ecol. Environ. Res. 2019, 17, 11273–11287. [Google Scholar] [CrossRef]
- Al-Hassani, F.A.M.; Majid, B.H. Effect of arginine, chitosan and agryl mulching on the growth and yield of pepper plant under the conditions of unheated greenhouses. Plant Arch. 2019, 19, 256–262.Al-Hassani, F.A.M.; Majid, B.H. Effect of arginine, chitosan and agryl mulching on the growth and yield of pepper plant under the conditions of unheated greenhouses. Plant Arch. 2019, 19, 256–262. [Google Scholar]
- Hussain, I.; Ahmad, S.; Ullah, I.; Basit, A.; Ahmad, I.; Sajid, M.; Alam, M.; Khan, S.; Ayaz, S. Foliar application of chitosan modulates the morphological and biochemical characteristics of tomato. Asian J. Agric. Biol. 2019, 7, 365–372. Hussain, I.; Ahmad, S.; Ullah, I.; Basit, A.; Ahmad, I.; Sajid, M.; Alam, M.; Khan, S.; Ayaz, S. Foliar application of chitosan modulates the morphological and biochemical characteristics of tomato. Asian J. Agric. Biol. 2019, 7, 365–372. [Google Scholar]
- Shaheen, A.M.; Ragab, M.E.; Rizk, F.A.; Mahmoud, S.H.; Soliman, M.M.; Omar, N.M. Effect of some active stimulants on plant growth, tubers yield and nutritional values of potato plants grown in newly reclaimed soil. Anim. Plant Sci. 2019, 29, 215–225.Shaheen, A.M.; Ragab, M.E.; Rizk, F.A.; Mahmoud, S.H.; Soliman, M.M.; Omar, N.M. Effect of some active stimulants on plant growth, tubers yield and nutritional values of potato plants grown in newly reclaimed soil. Anim. Plant Sci. 2019, 29, 215–225. [Google Scholar]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Impact of engineered nanomaterials either alone or loaded with NPK on growth and productivity of French bean plants: Seed priming vs foliar application. S. Afr. J. Bot. 2019, 125, 102–108.Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Impact of engineered nanomaterials either alone or loaded with NPK on growth and productivity of French bean plants: Seed priming vs foliar application. S. Afr. J. Bot. 2019, 125, 102–108. [Google Scholar] [CrossRef]
- Obsuwan, K.; Ngampanya, B.; Uthairatanakij, A.; Thepsithar, C. Effects of fertiliser ratio and chitosan on inflorescence quality and display life of a Mokara hybrid. Acta Hortic. 2019, 1262.Obsuwan, K.; Ngampanya, B.; Uthairatanakij, A.; Thepsithar, C. Effects of fertiliser ratio and chitosan on inflorescence quality and display life of a Mokara hybrid. Acta Hortic. 2019, 1262. [Google Scholar] [CrossRef]
- El Amerany, F.; Rhazi, M.; Wahbi, C.; Taourirte, M.; Meddich, A. The effect of chitosan, arbuscular mycorrhizal fungi, and compost applied individually or in combination on growth, nutrient uptake, and stem anatomy of tomato. Sci. Hortic. 2020, 261, 109015.El Amerany, F.; Rhazi, M.; Wahbi, C.; Taourirte, M.; Meddich, A. The effect of chitosan, arbuscular mycorrhizal fungi, and compost applied individually or in combination on growth, nutrient uptake, and stem anatomy of tomato. Sci. Hortic. 2020, 261, 109015. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326.
