Antioxidants are compounds that normally prevent lipid and protein oxidation. They play a major role in preventing many adverse conditions in the human body, including inflammation and cancer. Synthetic antioxidants are widely used in the food industry to prevent the production of adverse compounds that harm humans. However, plant and animal-based antioxidants are more appealing to consumers than synthetic antioxidants. Plant-based antioxidants are mainly phenolic compounds, carotenoids, and vitamins, while animal-based antioxidants are mainly whole protein or the peptides of meat, fish, egg, milk, and plant proteins. Plant-based antioxidants mainly consist of aromatic rings, while animal-based antioxidants mainly consist of amino acids. The phenolic compounds and peptides act differently in preventing oxidation and can use in the food and pharmaceutical industries. Therefore, compared with the animal-based antioxidants, plant-based compounds are more practical in the food industry. Even though plant base antioxidant compounds are good sources of antioxidants, animal-based peptides (individual peptides) cannot be considered antioxidants compounds to add to food. However, they can consider an ingredient that will enhance the antioxidant capacity. This review mainly compares plant and animal-based antioxidants' structure, efficacy, mechanisms, and applications.
- plant-based antioxidants
- phenolic compounds
- natural antioxidants
- flavonoids
1. Introduction
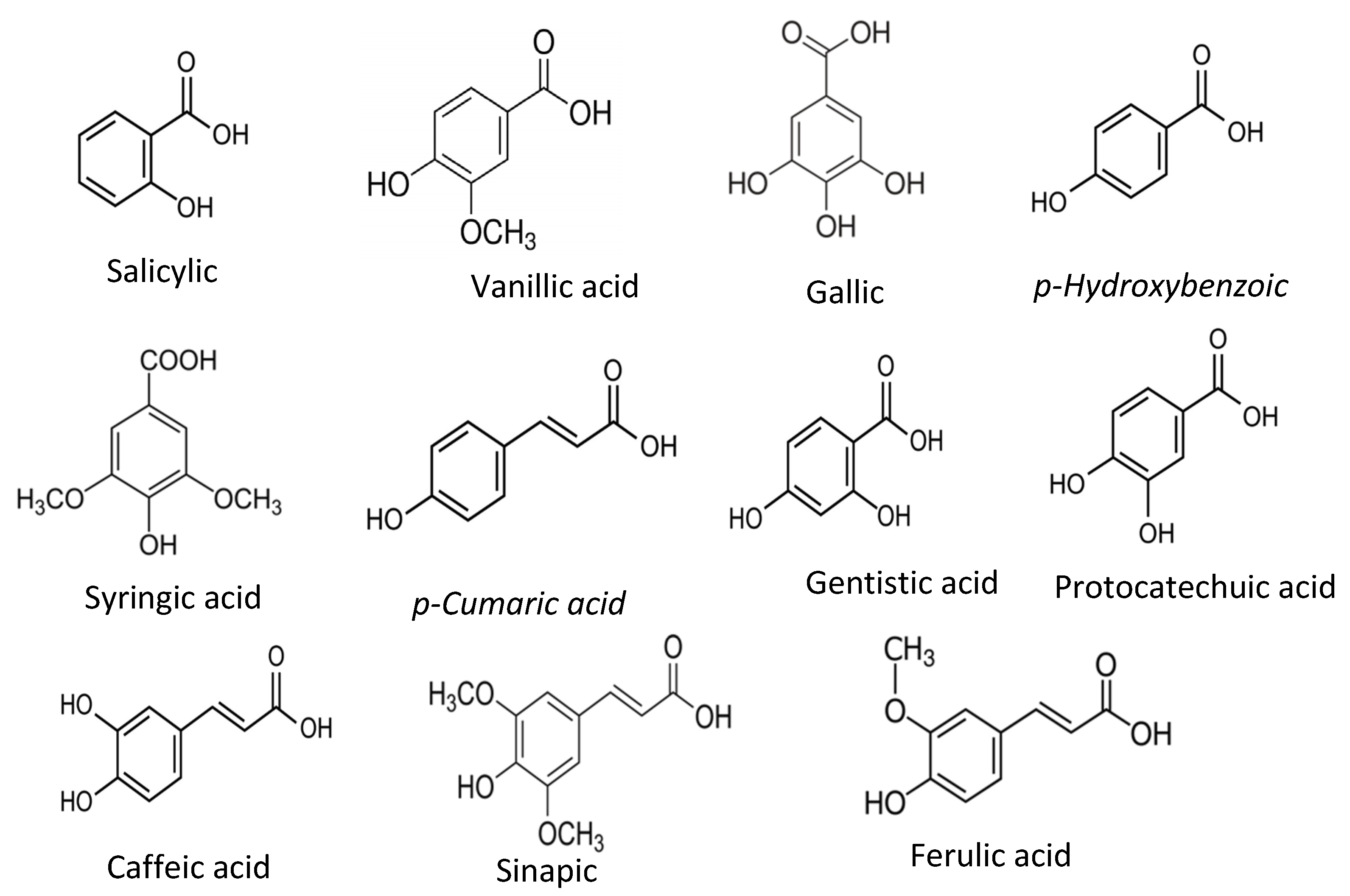
2. Phenolic Acids
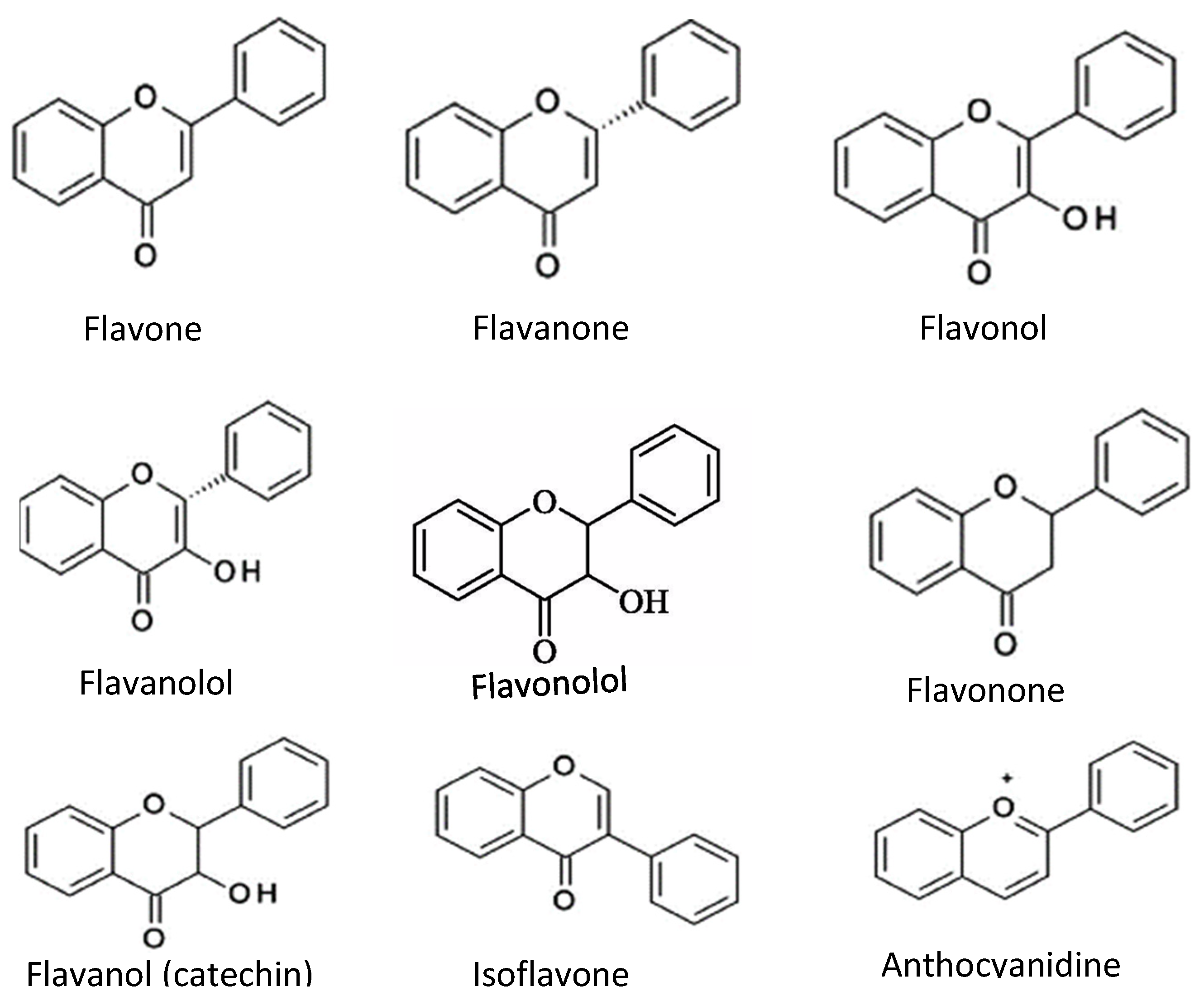
3. Flavonoids
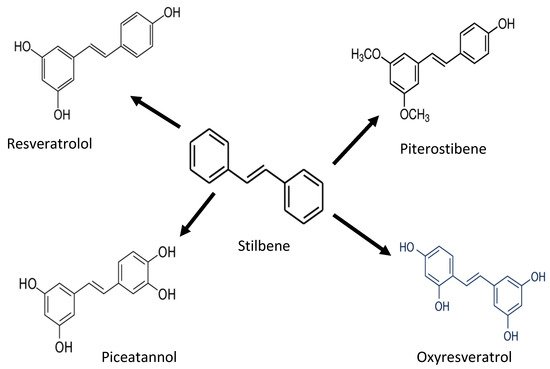
4. Stilbenes
5. Lignans
| Antioxidant Type | Subgroups | Examples | Applications | References |
|---|---|---|---|---|
| Phenolic compounds | Phenolic acids | Salicylic acid, Gentisic acid, p-Hydoxybenzoic acid, Protocatechuic acid, Vanillic acid, Syringic acid, Gallic acid, p-coumaric acid, Ferulic acid, Caffeic acid, Sinapic acid | As naturally present, primary antioxidant. | [21,[25,1035]][15][25] |
| Stilbenes | Piceid, Resveratrol, Piceatannol, Pterostilbene | Antioxidant activity against proteins and lipids | [27,28][17][18] | |
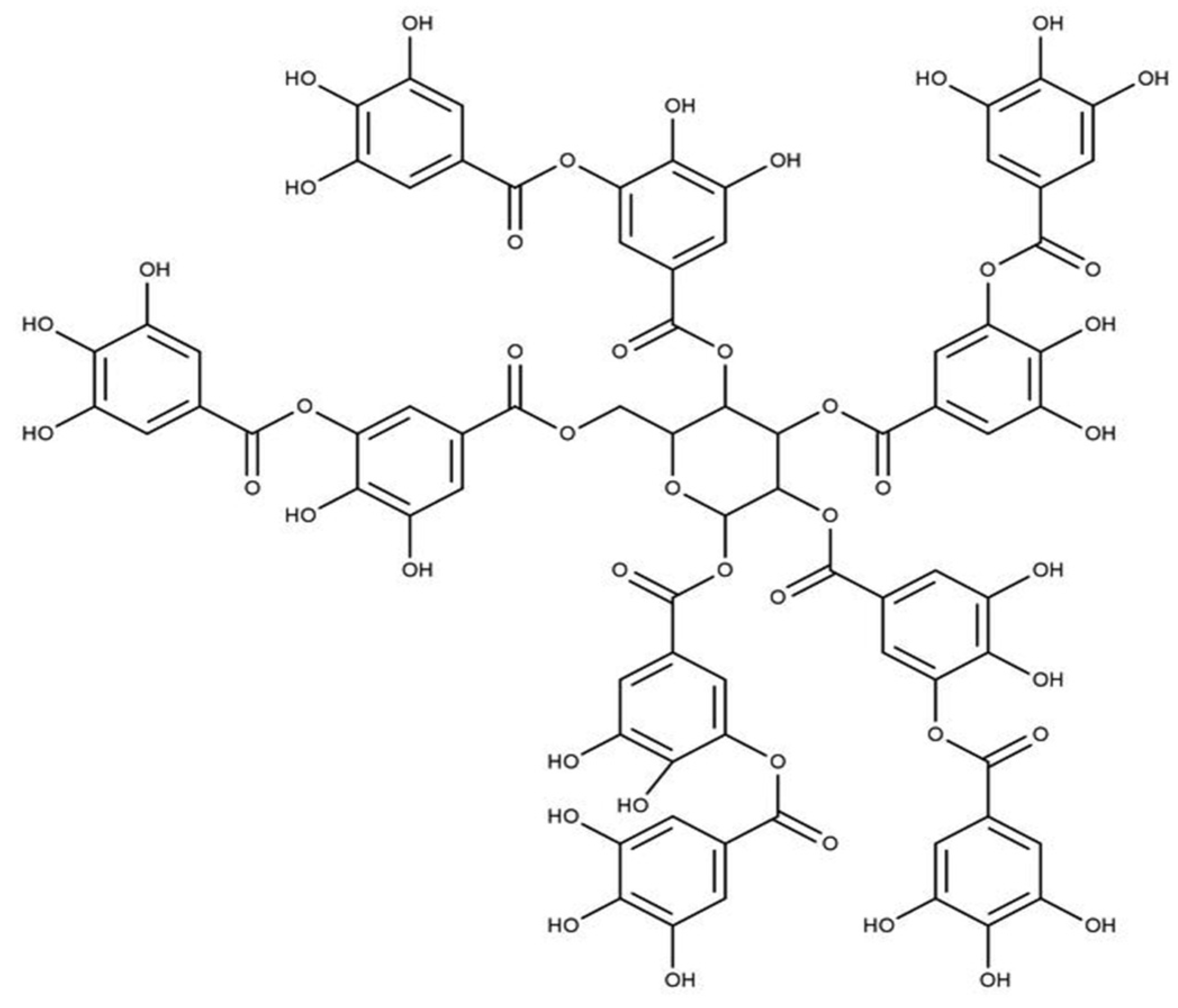
| Tannins | Biopolymers based on flavan-3-ols and Gallic and Ellagic acid | Strong antioxidant activities than flavonoids and phenolic acids | [36,37,38,39,40,41][26][27][28][29][30][31] | |
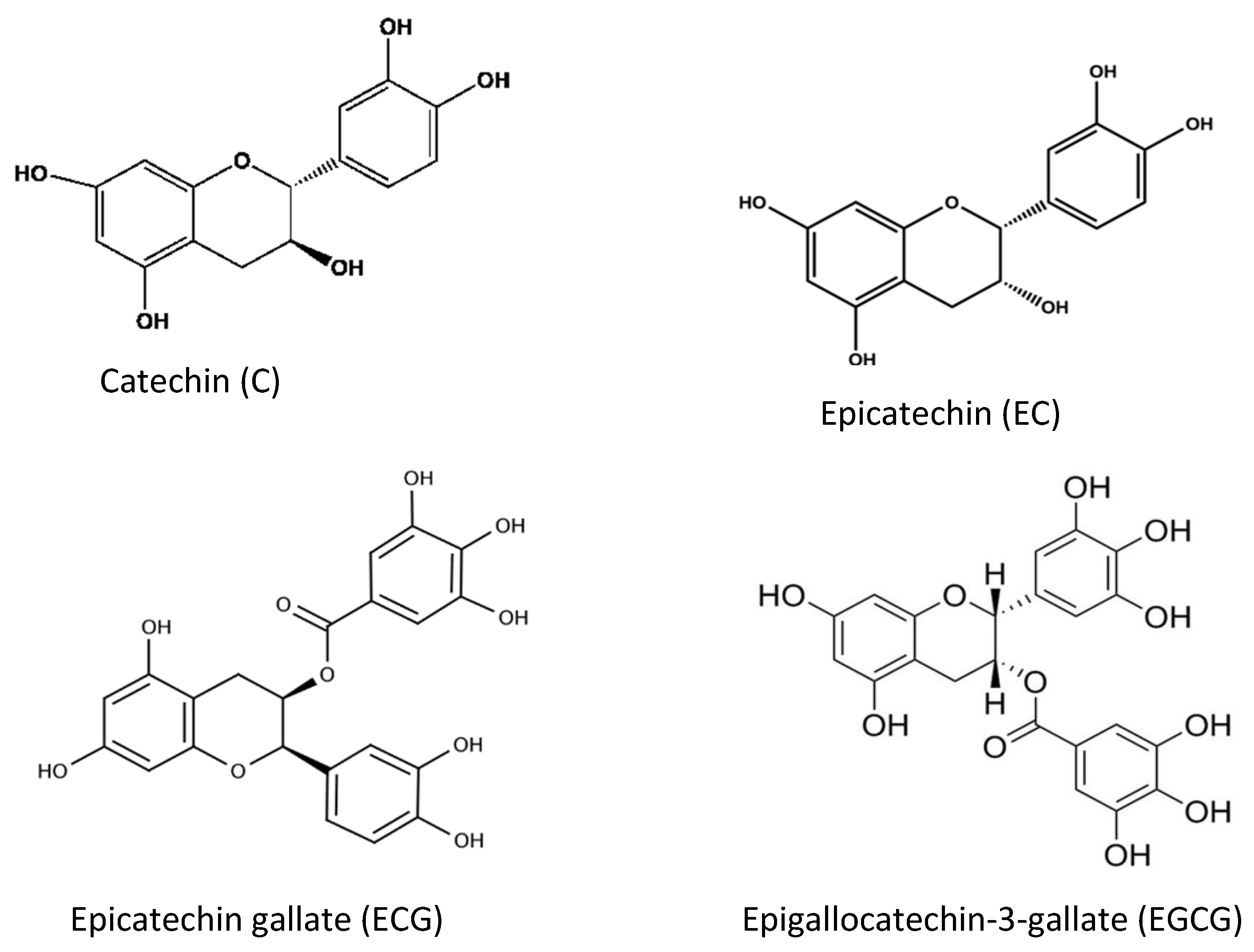
| Flavonoids | Flavone, Flavanol, Flavanone, Flavanonol, Flavonone, Flanononol, Flavanol (catechin), Isoflavone, Anthocyanidin | Act as antioxidant compounds if fruits, berries | [24,26,42,43,44][14][16][32][33][34] | |
| Lignans | Secoisolariciresinol, Matairesinol, Pinoresinol, Lariciresinol, sesamin, sesamolin | Strong antioxidant activities | [30,32][20][22] |
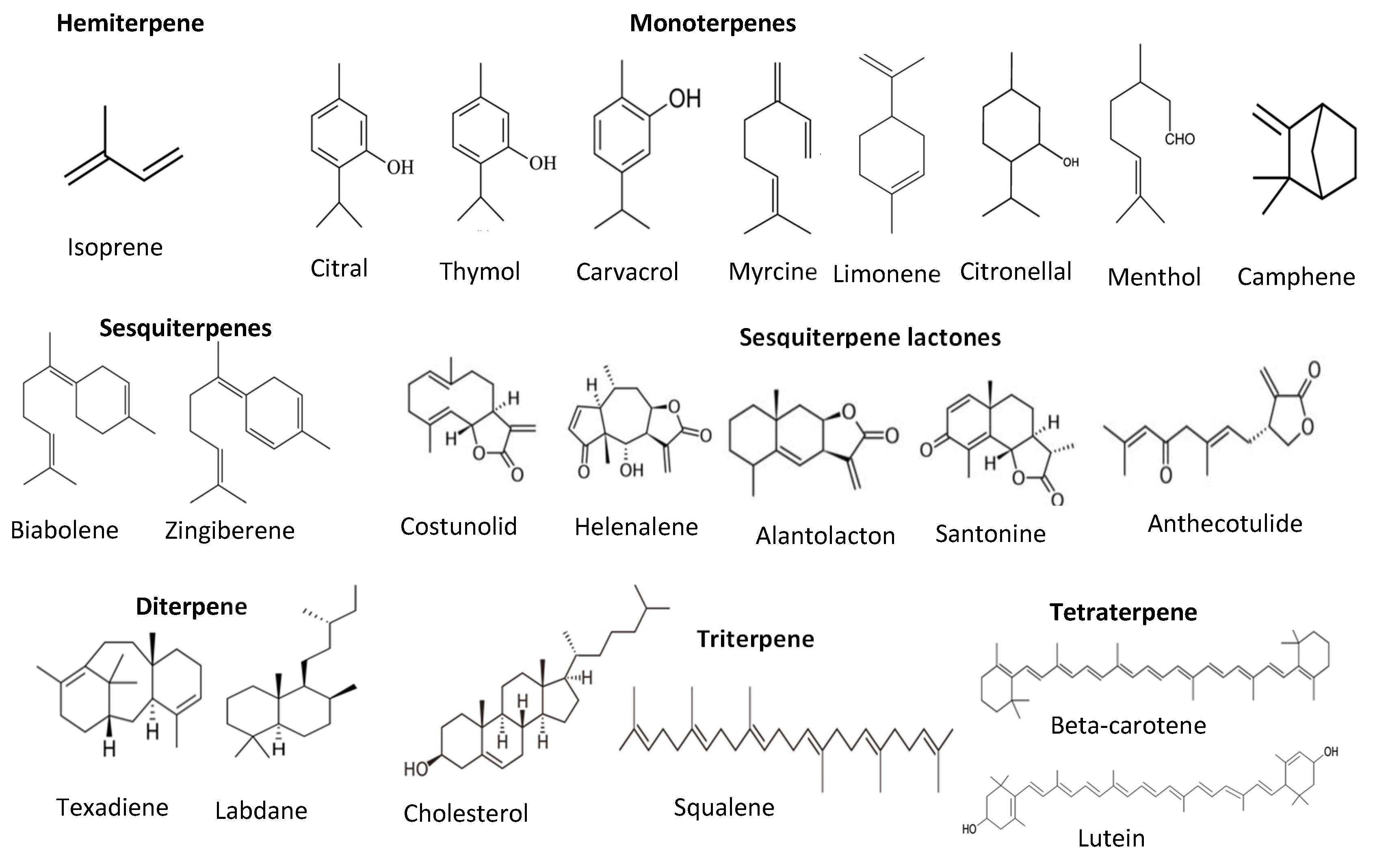
6. Terpenes and Terpenoids
7. Tannins
8. Sources of Plant Antioxidants
References
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyashi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67.
- Chen, X.; Touyz, R.M.; Park, J.B.; Schiffrin, E.L. Antioxidant effects of vitamin C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 2001, 38, 606–611.
- Traber, M.G.; Stevens, J.F. Vitamin C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013.
- Alshkh, N.; de Camargo, A.D.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038.
- Leopoldina, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922.
- Klein, E.; Lukeš, V. DFT/B3LYP study of the substituent effect on the reaction enthalpies of the individual steps of single-electron transfer-proton transfer and sequential proton loss electron transfer mechanisms of phenols antioxidant action. J. Phys. Chem. A 2006, 110, 12312–12320.
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394.
- Abdel-Shafy, H.; Mansour, M.S.M. Polyphenols: Properties, occurrence, Content in food, and potential effects. In Environmental Science and Engineering; Volume 6: Toxicology; Chandra, R., Gurjar, B.R., Govil, J.N., Eds.; Studium Press LLC: Houston, TX, USA, 2017; pp. 232–261.
- Abourashed, E.A. Bioavailability of plant-derived antioxidants. Antioxidants 2013, 2, 309–325.
- Single, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M. Natural polyphenols: Chemical Classification, definition of classes, subcategories, and structure. J. AOAC Int. 2019, 102, 1397–1400.
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512.
- Dirimanov, S.; Högger, P. Screening of inhibitory effects of polyphenols on Akt-phosphorylation in endothelial cells and determination of structure-activity features. Biomolecules 2019, 9, 219.
- Abeyrathne, E.D.N.S.; Nam, K.C.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems—A review. Antioxidants 2021, 10, 1587.
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: Cambridge, UK, 2019; pp. 1–81.
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370.
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Antibacterial and antioxidant effects of five spice and herb extracts as natural preservatives of raw pork. J. Sci. Food Agric. 2009, 89, 1879–1885.
- Markus, M.A.; Morris, B.J. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin. Interv. Aging 2008, 3, 331–339.
- Pandey, K.B.; Rizvi, S.I. Resveratrol may protect plasma proteins from oxidation under conditions of oxidative stress in vitro. J. Braz. Chem. Soc. 2010, 21, 909–913.
- Nagapan, T.S.; Ghazali, A.R.; Basri, D.F.; Lim, W.N. Photoprotective effect of stilbenes and its derivatives against ultraviolet radiation-induced skin disorders. Biomed. Pharmacol. J. 2018, 11, 1199–1208.
- Liu, Z.; Sarrinen, N.M.; Thompson, L.U. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum), as observed in vitro and in rats. J. Nutr. 2006, 136, 906–912.
- Wanasundara, P.K.J.P.D.; Shahidi, F. Process-induced change in edible oils. In Process-Induced Chemical Changes in Food; Shahidi, F., Ho, C.T., Chuyen, N.V., Eds.; Plenum Publishers: New York, NY, USA, 1998; pp. 135–160.
- Kumar, M.C.; Singh, S.A. Bioactive lignans from sesame (Sesamum indicum L.): Evaluation of their antioxidant and antibacterial effects for food applications. J. Food Sci. Technol. 2015, 52, 2934–2941.
- Rosalina, R.; Weerapreeyakul, N. An Insight into sesamolin: Physicochemical properties, pharmacological activities, and future research prospects. Molecules 2021, 26, 5849.
- Takahashi, M.; Nishizaki, Y.; Sugimoto, N.; Takeuchi, H.; Nakagawa, K.; Akiyama, H.; Sato, K.; Inoue, K. Determination and purification of sesamin and sesamolin in sesame seed oil unsaponified matter using reversed-phase liquid chromatography coupled with photodiode array and tandem mass spectrometry and high-speed counter-current chromatography. J. Sep. Sci. 2016, 39, 3898–3905.
- Abdallah, I.I.; Quax, W.J. A Glimpse into the biosynthesis of terpenoids. In NRLS Conference Proceedings, International Conference on Natural Resources and Life Science (2016). KnE Life Sciences; Knoledge E Publisher: Dubai, United Arab Emirates, 2017; pp. 81–98.
- Hatano, T.; Kusuda, M.; Inada, K.; Ogawa, T.O.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 2005, 66, 2047–2055.
- Amarowicz, R.; Estrella, I.; Hernández, T.; Troszynska, A. Antioxidant activity of extract of adzuki bean and its fractions. J. Food Lipids 2008, 15, 119–136.
- Azman, N.A.M.; Gallego, M.G.; Segovia, F.; Abdullah, S.; Md Shaarani, S.; Pablos, M.P.A. Study of the properties of bearberry leaf extract as a natural antioxidant in model foods. Antioxidants 2016, 5, 11.
- Jabri, M.-A.; Rtibi, K.; Ben-Said, A.; Aouadhi, C.; Hosni, K.; Sally, M.; Sebai, H. Antidiarrhoeal, antimicrobial and antioxidant effects of myrtle berries (Myrtus communis L.) seeds extract. J. Pharm. Pharmacol. 2016, 68, 264–274.
- Amarowicz, R.; Karamać, M.; Dueňas, M.; Pegg, R.B. Antioxidant activity and phenolic composition of a red bean (Phaseolus Vulgaris) extract and its fractions. Nat. Prod. Commun. 2017, 12, 541–544.
- Amarowicz, R.; Shahidi, F. Antioxidant activity of Faba bean extract and fractions thereof. J. Food Bioact. 2018, 1, 112–118.
- Patel, M.; Naik, S.N. Gamma-oryzanol from rice bran oil—A review. J. Sci. Ind. Res. 2004, 63, 569–578.
- Lee, S.H.; Kim, H.W.; Lee, M.K.; Lee, M.K.; Kim, Y.J.; Asamenew, G.; Cha, Y.S.; Kim, J.B. Phenolic profiling and quantitative determination of common sage (Salvia plebeian R. Br.) by UPLC-DAD-QTOF/MS. Eur. Food Res. Technol. 2018, 244, 1637–1646.
- Oniga, I.; Puşcaş, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077.
- Jaeger, R.; Cuny, E. Terpenoids with special pharmacological significance: A review. Nat. Prod. Commun. 2016, 11, 1373–1390.
- Prishtina, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as potential geroprotectors. Antioxidants 2020, 9, 529.
- Terpins, P.; Čeh, B.; Ulrich, N.P.; Abramovič, H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crops Prod. 2012, 39, 210–217.
- Szydłowska-Czerniak, A. Rapeseed and its products-sources of bioactive compounds: A review of their characteristics and analysis. Crit. Rev. Food Sci. Nutr. 2013, 53, 307–330.
- Lee, L.S.; Kim, Y.C.; Park, J.D.; Kim, Y.B.; Kim, S.H. Changes in major polyphenolic compounds of tea (Camellia sinensis) leaves during the production of black tea. Food Sci. Biotechnol. 2016, 25, 1523–1527.
- Koch, W.; Kukula-Koch, W.; Komsta, Ł.; Maezec, Z.; Szwarc, W.; Głowniak, K. Green tea quality evaluation based on its catechins and metals composition in combination with chemometric analysis. Molecules 2018, 23, 1689.
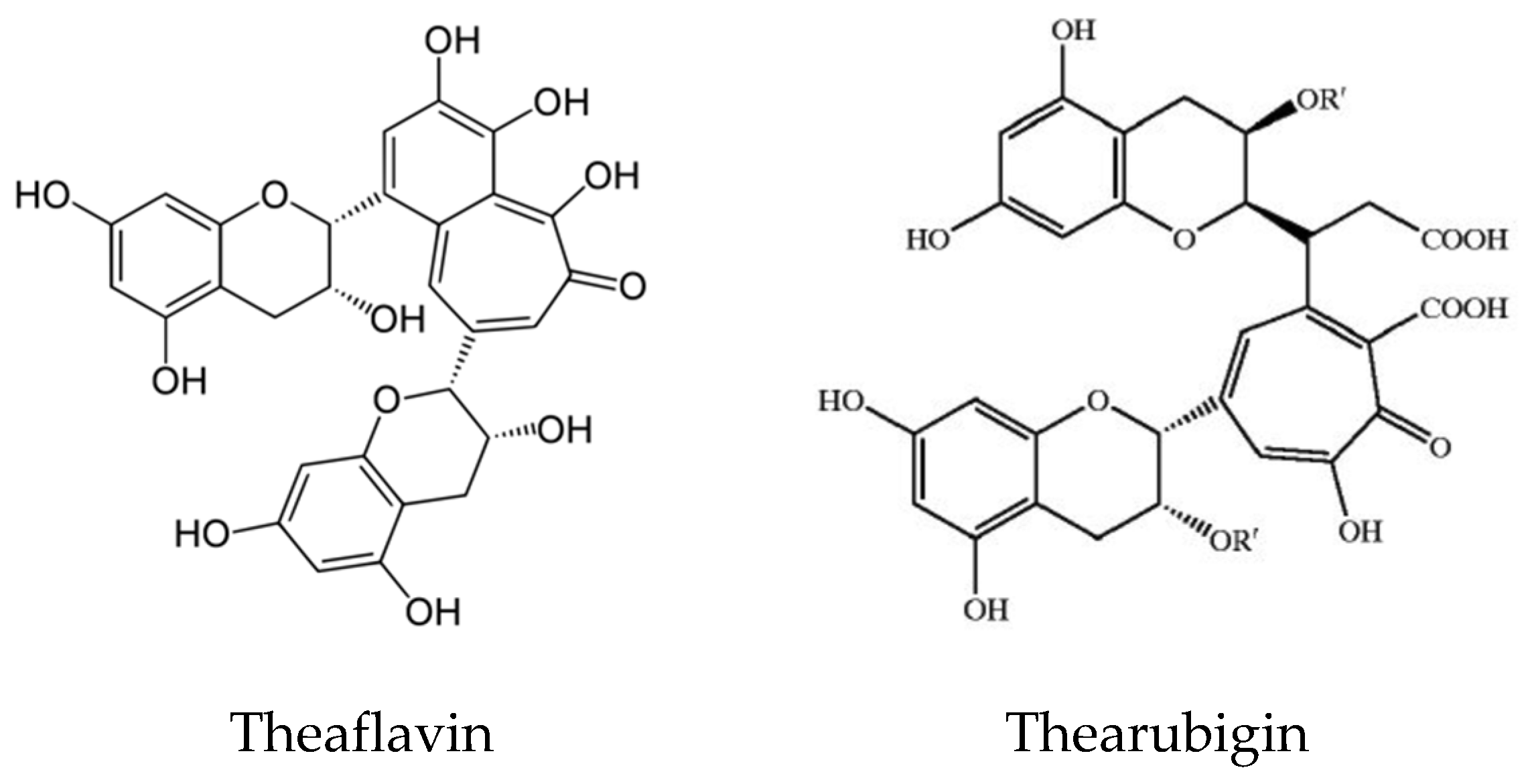
- Takemoto, M.; Takemoto, H. Synthesis of theaflavins and their functions. Molecules 2018, 23, 918.
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915.
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Biol. 2007, 18, 23–36.
- Fanali, C.; Tripodo, G.; Russo, M.; Della Posta, S.; Pasqualetti, V.; de Gara, L. Effect of solvent on the extraction on the phenolic compounds and antioxidant capacity of the hazelnut kernel. Electrophoresis 2018, 39, 1683–1691.
- Rusu, M.E.; Gheldiu, A.M.; Mocan, A.; Moldovan, C.; Popa, D.S.; Tomita, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814.
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538.
- Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.J.; del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of antioxidant capacity, protective effect on human erythrocytes and phenolic compounds identification in two varieties of plum fruit (Spondias spp.) by UPLC-MS. Molecules 2018, 23, 3200.
- Skrovankova, S.; Sumczynski, D.; Macek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706.
- Khoshnoudi-Na, S.; Niakosari, M.; Tahiri, Z. Subcritical water technology for extraction phytochemical compound. J. Med. Plants 2017, 9, 94–107.
- Marchante, L.; Izquierdo-Caǹas, P.M.; Gómez-Alonso, S.; Alaǹón, S.; García-Romero, M.E.; Pérez-Coello, M.S.; Díaz-Maroto, M. Oenological potential of extracts from winery and cooperage by-products in combination with colloidal silver as natural substitutes to sulphur dioxide. Food Chem. 2019, 276, 485–493.
- Nick, M.; Shahidi, F. Phenolics in cereals, fruits, and vegetables: Occurrence, extraction, and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542.
- Liang, J.; Zago, E.; Nandasiri, R.; Khattab, R.; Eskin, N.A.M.M.; Eck, P.; Holländer, U.T. Effect of solvent preheating temperature and time on the ultrasonic extraction of phenolic compounds from cold-pressed hempseed cake. J. Am. Oil Chem. Soc. 2018, 95, 1319–1327.
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods. A review. Food Chem. 2014, 152, 46–55.
- Mullen, W.; Nemzer, B.; Ou, B.; Stalmach, A.; Hunter, J.; Clifford, M.; Combet, E. The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by extraction procedures. J. Agric. Food Chem. 2011, 59, 3754–3762.
- Lee, S.G.; Terrence, M.; Vance, T.M.; Nam, T.G.; Kim, D.O.; Koo, S.I.; Chun, O.K. Contribution of anthocyanin composition of total antioxidant capacity of berries. Plant Food Hum. Nutr. 2005, 25, 523–1527.
- Kozłowska, M.; Gruczynska, E. Comparison of the oxidative stability of soybean and sunflower oils enriched with herbal plant extracts. Chem. Pap. 2018, 72, 2607–2615.
- Dos Reis, L.C.R.; Facco, E.M.P.; Flôres, S.H.; Rios, A.D.O. Stability of functional compounds and antioxidant activity of fresh and pasteurized orange passion fruit (Passiflora caerulea) during cold storage. Food Res. Int. 2018, 106, 481–486.
- İnan, Ö.; Özczn, M.M.; Aljuhaimi, F. Effect of location and Citrus species on total phenolic, antioxidant and radical scavenging activities of some Citrus seed and oil. J. Food Process. Preserv. 2018, 42, 1215–1219.
- Do Couto, C.A.; de Souza, E.R.B.; Morgado, C.M.A.; Ogata, T.; Cunha Júnior, L.C. Citrus Sinensis cultivars: Alternatives for diversification of Brazilian orchards. Rev. Bras. Food Sci. 2018, 50, 905–991.
- Konieczyski, P.; Viapiana, A.; Mark Wesolowski, M. Comparison of infusions from black and green teas (Camellia sinensis L. Kuntze) and erva-mate (Ilex paraguariensis A. St.-Hil) based on the content of essential elements, secondary metabolites, and antioxidant activity. Food Anal. Methods 2017, 10, 3063–3070.
- Liu, Y.-F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530.
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46.
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132.
- Wijeratne, S.S.K.; Amarowicz, R.; Shahidi, F. Antioxidant activity of almonds and their by-products in food model. J. Am. Oil Chem. Soc. 2006, 83, 223–230.
- Rojas, M.C.; Brewer, M.S. Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. J. Food Sci. 2007, 72, S282–S288.
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the US. A Review. Meat Sci. 2017, 145, 469–479.
- Gramza-Michałowska, A.; Korczak, J.; Reguła, J. Use of plant extracts in summer and winter season butter oxidative stability improvement. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 85–88.
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: A detailed review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140.
