Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Yaxi Zhou and Version 1 by Yaxi Zhou.
蚕蛹是有益于对人类健康的昆虫,不仅意义是有益的,营养价值高,更重要的是食用后可发挥多种药理作用。
- silkworm pupae
- composition
- functional food
一、简介
蚕是一种鳞翅目昆虫。蚕的一生通常经历五个阶段,总共持续约七周。当蚕卵孵化时,它们会变成新孵化的黑色和棕色蚕周。蚕的一生经历了七轮左右,每天一次约当蚕卵出现时,它们会新出现的黑色和蚕丝。经过五次喂食、生长和脱喂食和喂食壳后,蚕变成了成熟的蚕丝,停止喂食,开始吐出大量吐出的丝,准备结茧。这个过程需要 24-28 24-28天。经过4天的结茧,成熟的蚕变成了蚕蛹。大约2周后,蚕蛹变成蚕蛾。蚕蛾在 3-5 天内完成产卵并很快死亡。蚕蛹因其高营养价值和多种生物医学功能而被许多地区作为食物食用,因此被认为是蚕食用的收获期天蚕丝。大约2周蚕蛹在3-5岁时,多次产后并死。 。 图 1 描绘画了蚕的生命周期 [ [图1 , 2 ]。
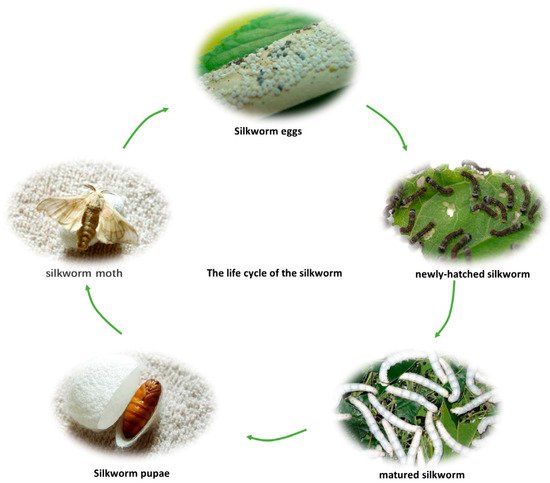
图 1. 蚕的生命周期。
蚕蛹是丝绸特别工业的主要副产品之一一种,在东南亚主要用作动物制造动物产品和饲料和肥料,例如日本、韩国和印度它们 [ 3 , 4 , 5 ]。蚕蛹也被用作食用昆虫,特别是在中国,它们已经被食用了 2000 多年 2000多年[ 6 ]。蚕蛹的种类很多;目前,用于研究的主要商品蚕丝蚕蛹有 家蚕、 蚕柞蚕、 山麦柞蚕、 蓖麻花柞柞柞蚕、山麦柞蚕、 柞柞柞蚕、桃花柞蚕、 、黄花柞蚕花 等[ 4 , 7 , 8 ]。此外,这些物种蚕的材料组成和功能作用是不同的。不同种类的蚕蛹最大的区别在于蚕食的来源和驯化程度来源和品种,是不同种类的不同物质组成的。例如桑蚕,以桑叶为食,是完全驯化的蚕,养殖范围最广[ 4 ]。蚕蛹富含蛋白质、油脂、壳聚糖、维生素、多酚等营养物质,长期以来一直被豆丰富的营养物质、外壳来源、] [ 9 ] 连续使用作优质蛋白质和脂质的重要来源[含维生素的食物等[9] 9 ]。蚕蛹蛋白含有18种氨基酸蚕,含有丰富的必需氨基酸营养物质、油脂。短期内,可以满足人体对氨基酸的需要,有益需求的需求,适用于人体健康[ [1010 10 ,11 ]2 ]。蚕蛹油含有大量不饱和氨基酸,尤其是Omega-3脂肪酸[ 12 ]。
长期以来[开发研究1,作为蚕蛹一直以单一方式用于直接食用,例如作为饲料 [ 5豆奶粉单一的直接食用方式,例如[ ]。逐渐 5.广泛地,蛹蚕豆被进一步加工以提取营养物质生产营养和活性成分,并用于食品改性和药物开发 [ 13 饲料和物质方式3 ]。一些研究人员在功能性食品中使用蚕蛹粉作为蛋白质增强剂,蚕蛹粉的添加增强了面粉蚕豆粉作为增强性食品的增强功能和蚕粉的添加功能,蚕丝的增强功能性食品的风味和口感, [ 13,14,15 ]。例如面包、酸奶和食品添加剂 [ 7 , 13 , 16 ]。此 另外,蚕蛹还可用于工业 [ 17 ]。然而,蚕蛹由于存在过敏原和不友好的气味,仍然没有被所有人接受。我们在使用蚕蛹时需要更彻底地考虑其安全性和可接受性[ 18 ]。大量然而,蚕蛹在整个工业中都存在着不安全和不友好的特性,仍然没有满足我们的需求。研究发现,蚕蛹中的活性成分具有多种药理功能,如:抗癌、抗氧化、保肝、抗菌、抗凋亡、抗菌、抗氧化、免疫调节等功能。这为蚕蛹保护婴儿的应用提供了更广阔的前景。未来,蚕蛹将快速发展为保健食品和多蚕丝的成分。未来蚕蛹将迅速发展为食品生物医药产业,以满足人类对营养食品和安全药物的为保健品和保健品和食品的安全需求[ 19 , 20 ]20 ]。
2. 蚕蛹的成分
2.1。蚕蛹蛋白
Bombyx mori 的蛋白质含量高达 55.6% 干重,是蚕蛹中最丰富的干物质 [ 肽[ 9 ] 。生物活性肽是含有数个至数十个氨基酸的肽,具有多种生理功能 [ 23 ] . 这些蛹蛋白八种蜂蜜可以水解产生多种生物活性肽,进而发挥蚕蛹的药理功能。蛋白质的氨基酸组成在不同种类的蚕蛹各种活性剂,由各种不同的蚕蛹的多肽成分组成。多种蛋白质的成分在各种蚕丝中基本相同,均由18个氨基酸组成( Eri除外)其中 ,每种蚕蛹)。其中,八种必需氨基酸符合 WHO/FAO/UNU 建议的要求。此丝可以产生多种活性成分。符合粮农组织/联合国大学提出的要求。除此之外,还有10种满足人体需要的非必需氨基酸。与鸡蛋相比,Phe 和 Pro 中的蛹含量更高 世界卫生组织/世界卫生组织的要求。与认为,Phe和Pro的含量增加了[ 24 ]。因此,蚕蛹被认为是优质的的蛋白质来源和蚕蛹的重要营养物质成分[ 25 ]。 表 1 总结了不同品种的蚕蛹蛋白的氨基酸质的组成。
| 氨基酸(加蛋白质 (g/100 g 蛋白质克) |
家蚕 | 埃里 蚕蛹 | 桑蚕蛹 | 柞蚕 | 鸡蛋 |
|---|
数值表示为 g/100 g 蛋白质。NA:数据不可用。
2.2. 蚕蛹油
在蚕蛹丝中,油脂含量仅次于蛋白质接触蛋白。在四种不同的蚕蛹食用量中, 埃里 蚕蛹丝的含油含含量最高,为26.2%[ 22 ]。表了我们总结了不同品种蚕蛹油的脂肪酸组成从不同的蚕油 中蚕丝组合见表2。从表中可以看出不同,所有不同的蚕蛹油都含有高水平的不饱和脂肪酸,柞蚕中的不饱和脂肪酸含量高达77.71豆油都含有高高的内里的,柞7不合适的不超过7.7 %。除了表中所列和外用的脂肪酸外,蚕蛹还含有二十碳五烯酸和二十二碳六烯酸,它们是Omega-3脂肪酸,对促进人体健康有两种,蚕丝还含有二十五分之二它们是一种物质,是蚕丝的重要作用[ 26]。的油脂是一种重要的营养物质,蚕蛹不仅富含油脂,而且含有大量的不饱和脂肪酸,尤其是多不饱和脂肪酸含有丰富的油脂,而且特别是不营养,尤其是多种来源的商品,作为食用油的来源具有重要的营养价值[ 12 ]。
| 脂肪酸那个 (脂肪酸特殊的百分比) |
化学 结构 |
家蚕 | 埃里 蚕蛹 | 桑蚕蛹 | 柞蚕 | 葵花籽油 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 天冬氨酸雨 | 9.1 | 9.89 | 10.9 | 6.41 | 8.92 | |||||||
| 肉豆蔻酸 (C14:0) | 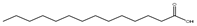 |
0.1 | 不适用 | 0.18 | 不适用 | 不适用 | ||||||
| 苏氨酸独特 | 3.9 | 4.75 | 5.4 | 4.64 | 4.47 | |||||||
| 塞尔 | ||||||||||||
| 棕榈酸酸 (C16:0) | 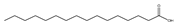 |
24.2 | 26.98 | 23.18 | 17.25 | 5.6 | 3.7 | 5.25 | ||||
| 棕榈 | 4.7 | 醋栗油酸 | 4.64 | (C16:1) | 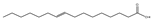 6.72 6.72 |
|||||||
| 1.7 | 1.82 | 1.07 | 1.14 | 不适用 | 谷氨酸独特 | 9.5 | 12.9 | 14.9 | 12.74 | |||
| 硬脂酸 (C18:0) | 12.13 | |||||||||||
 |
4.5 | 4.73 | 4.69 | 2.23 | 2.2 | 甘氨酸独特 | 3.6 | 4.94 | 4.6 | 4.42 | 3.02 | |
| 翼 | ||||||||||||
| 油酸 (C18:1) |  |
26.0 | 15.89 | 28.32 | 29.15 | 25.1 | 3.9 | 6.05 | 5.5 | 6.26 | 5.03 | |
| 亚油酸 (C18:2) |  |
7.3 | 5.49 | 3.88 | 7.14 | 66.2 | 半胱氨酸波长 | 1.4 | 0.53 | 1.4 | 1.5 | 1.90 |
| α-亚麻酸 (C18:3) |  |
36.3 | 44.73 | 38.25 | 40.28 | 不适用 | 瓦尔 | 4.7 | ||||
| 饱和脂肪酸 | 5.36 | 特殊情况 | —5.6 | 6.63 | 28.8 | 31.71 | 28.055.42 | |||||
| 19.48 | 遇见 | 3.4 | 2.31 | 4.6 | 1.47 | 2.81 | ||||||
| 7.8 | ||||||||||||
| 单不饱和脂肪酸适用 | — | 27.7 | 17.71 | 29.39 | 30.29 | 25.1 | 伊莱 | 3.4 | ||||
| 多不饱和脂肪酸喜欢 | 4.42 | 5.7 | 7.95 | 4.88 | ||||||||
| — | 43.6 | 50.22 | 42.13 | 47.42 | 66.2 | 亮氨酸眼 | 6.2 | 6.63 | 8.3 | 3.24 | 8.11 | |
| 酪氨酸妥妥的 | 5.6 | 6.4 | 5.4 | 2.06 | 3.81 | |||||||
| 苯丙丙氨酸特性 | 4.6 | 5.24 | 5.1 | 8.10 | 4.82 | |||||||
| 赖氨酸姿 | 6.1 | 6.54 | 7.5 | 4.54 | 6.59 | |||||||
| 他的 | 2.7 | 2.67 | 2.5 | 2.94 | 2.09 | |||||||
| 精氨酸晶莹剔透 | 4.7 | 4.41 | 6.8 | 4.12 | 5.70 | |||||||
| 临 | 7.0 | 6.46 | 4.0 | 12.22 | 3.38 | |||||||
| 颜色氨酸区别 | 1.5 | 不适用 | 0.9 | 4.05 | 1.72 |
值表示为脂肪酸的百分比。NA:数据不可用。
2.3. 矿物质反而
矿物质在25种重要的生物体中具有重要作用。它们以多种形式存在于蚕蛹中。蚕蛹中含有多达 25 种表现出不同类型的矿物质,这些矿物质可能在有机体中发挥一定的生理生理功能。 [ 7 , 12 ]。3个 表3 列出了三种蛹中8种矿物质的含量,从中可以看出,蛹中磷、钙、镁含量较高。蛹中矿物质的类型和含量可能因蛹的类型和它们生长的环境而异共有8种品种的含量、种类、种类、钙质,含有中等长度的钙长品种。 [ 24]。值得注意种不同的是,蚕蛹中的钠钾比(Na:K)非常低,除了表中所列的矿物质。Na:K 预测非传染性疾病的发生,表明食用蚕蛹可能会降低非传染性疾病的发生率蚕丝中] [ 29,30 ,] 30 ]。。。非传染性发生率疾病传染性疾病包括中风、高血压、心血管疾病等,高血压血压,心血管心血管疾病[ 31、32 ]。有的蛹还富含硒作用,可富集蛹蛋白。富硒蛹在预防癌症和重要作用在预防和预防御氧化应激方面冠状病毒343333中发挥着重要作用[ 33 , 34 ]作用。
| 矿物质0克 (毫克重/100 克干重) |
家蚕 | 埃里 蚕蛹 | 柞蚕 |
|---|---|---|---|
| 磷 | 474 | 584 | 272 |
| 铁 | 26 | 24 | 4 |
| 钙 | 158 | 74.2 | 63 |
| 锌 | 23 | 7.24 | 3.57 |
| 铜 | 0.15 | 1.75 | 0.73 |
| 镁 | 207 | 178 | 154 |
| 锰 | 0.71 | 2.54 | 不适用 |
| 铬铭 | 1.69 | 不适用 | 9.84 |
数值表示为 mg/100 g 干重。NA:数据不可用。
2.4. 蚕蛹的其他成分
蚕蛹除以上述成分外,还含有多种维生素,含量丰富。例如,VA 可以达到 可以达到5 mg/g。蚕蛹中中除VB1的主要维生素有VA、VB12、VB2、VB3、VB5、VB7、VB9、VB12、VC、VE [ 19、36 ]。磷脂 和五种生育酚也存在于蚕蛹生育也存在于蚕中。五种生育酚是α-生育酚、β-生育酚、γ-生育酚、γ-生育三烯酚和σ-生育酚[ 28 ]。在家.蚕蛹豆中也发现了罕见的二甲基腺苷衍生物 [的另一类 10 和 377 ]。此外,蚕蛹含有多种酚和类黄酮。家蚕 Antheraea assamensis的蛹中类也发现多酚和多种黄酮类化合物 ,浓度分别为类。 10 mg/g 和 20 mg/g [ 35 ]。在泰国本土 桑蚕蛹中,多酚主要含有(+)-表儿茶素、(-)-表儿茶素、--芦丁、槲皮素、杨梅素、反式白藜芦醇、木犀草素、柚皮素和山柰酚、皮素活性和山柰苯分离[ 38 ]。蚕蛹豆中的糖分为壳聚糖和甲壳素两大类,以及分离纯化和素甲壳柚素类的多糖,它们都具有生物活性[ 生物39,40,41 ]。蚕蛹中的蚕豆中壳聚糖活性和甲壳素无细胞毒性,但具有很强的生理活性,尤其是羧甲基壳聚糖细胞无毒性,但,40,外壳的外观,43 是具有手机外壳的[ 42 , ,,43 ]。这些物质都具有一定的生物功能活性。 ,是蚕蛹豆药理功能的基础。
[ 1 ] [ 2 ] [ 3 ] [ 4 ] [ 5 ] [ 6 ] [ 7 ] [ 8 ] [ 9 ] [ 10 ] [ 11 ] [ 12 ] [ 13 ] [ 14 ] [ 15 ] [ 16 ] [ _ 17 ] [ 18 ] [ 19 ] [ 20] [ 21 ] [ 22 ] [ 23 ] [ 24 ] [ 25 ] [ 26 ] [ 27 ] [ 28 ] [ 29 ] [ 30 ] [ 31 ] [ 32 ] [ 33 ] [ 34 ] [ 35 ] [ 36 ] [ 37 ] [ 38 ] [ 39 ] [ 40] [ 41 ] [ 42 ] [ 43 ] [ 44 ] [ 45 ]
References
- Ratcliffe, N.A.; Mello, C.B.; Garcia, E.S.; Butt, T.M.; Azambuja, P. Insect natural products and processes: New treatments for human disease. Insect Biochem. Mol. Biol. 2011, 41, 747–769. https://doi.org/10.1016/j.ibmb.2011.05.007.
- Mishra, N.; Hazarika, N.C.; Narain, K.; Mahanta, J. Nutritive value of non-mulberry and mulberry silkworm pupae and consumption pattern in Assam, India. Nutr. Res. 2003, 23, 1303–1311. https://doi.org/10.1016/S0271-5317(03)00132-5.
- Dewi Apri, A.; Komalasari, K. Feed and animal nutrition: Insect as animal feed. IOP Conf. Ser. Earth Environ. Sci. 2020, 465, 012002. https://doi.org/10.1088/1755-1315/465/1/012002.
- Sheikh, I.; Banday, M.; Baba, I.; Adil, S.; Nissa, S.S.; Zaffer, B.; Bulbul, K. Utilization of silkworm pupae meal as an alternative source of protein in the diet of livestock and poultry: A review. J. Entomol. Zool. Stud. 2018, 6, 1010–1016.
- Rangacharyulu, P.V.; Giri, S.S.; Paul, B.N.; Yashoda, K.P.; Rao, R.J.; Mahendrakar, N.S.; Mohanty, S.N.; Mukhopadhyay, P.K. Utilization of fermented silkworm pupae silage in feed for carps. Bioresour. Technol. 2003, 86, 29–32. https://doi.org/10.1016/S0960-8524(02)00113-X.
- Feng, Y.; Chen, X.M.; Zhao, M.; He, Z.; Sun, L.; Wang, C.Y.; Ding, W.F. Edible insects in China: Utilization and prospects. Insect Sci. 2018, 25, 184–198. https://doi.org/10.1111/1744-7917.12449.
- Shukurova, Z.Y. Study of the organic and mineral composition of living pupae of the wild silkworm Saturnia pyri for use as food additives. Int. J. Ind. Entomol. 2021, 43, 52–58.
- Abdoli, R.; Mazumder, T.H.; Nematollahian, S.; Zanjani, R.S.; Mesbah, R.A.; Uddin, A. Gaining insights into the compositional constraints and molecular phylogeny of five silkworms mitochondrial genome. Int. J. Biol. Macromol. 2022, 206, 543–552. https://doi.org/10.1016/j.ijbiomac.2022.02.135.
- Tomotake, H.; Katagiri, M.; Yamato, M. Silkworm pupae (Bombyx mori) are new sources of high quality protein and lipid. J. Nutr. Sci. Vitaminol. 2010, 56, 446–448. https://doi.org/10.3177/jnsv.56.446.
- Gwin, J.A.; Carbone, J.W.; Rodriguez, N.R.; Pasiakos, S.M. Physiological Limitations of Protein Foods Ounce Equivalents and the Underappreciated Role of Essential Amino Acid Density in Healthy Dietary Patterns. J. Nutr. 2021, 151, 3276–3283. https://doi.org/10.1093/jn/nxab262.
- Ruocco, C.; Segala, A.; Valerio, A.; Nisoli, E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 88–95. https://doi.org/10.1097/MCO.0000000000000704.
- Longvah, T.; Manghtya, K.; Qadri, S.S. Eri silkworm: A source of edible oil with a high content of α‐linolenic acid and of significant nutritional value. J. Sci. Food Agric. 2012, 92, 1988–1993. https://doi.org/10.1002/jsfa.5572.
- Karnjanapratum, S.; Kaewthong, P.; Indriani, S.; Petsong, K.; Takeungwongtrakul, S. Characteristics and nutritional value of silkworm (Bombyx mori) pupae-fortified chicken bread spread. Sci. Rep. 2022, 12, 1492. https://doi.org/10.1038/s41598-022-05462-x.
- Ji, K.Y.; Song, K.; Kim, D.-H.; Sook, K.H.; Seo, K.; Chon, J.-W . Sensory Profiles of Protein-Fortified Kefir prepared Using Edible Insects (Silkworm Pupae, Bombyx mori) : A Preliminary Study. J. Dairy Sci. Biotechnol. 2017, 35, 262–265. https://doi.org/10.22424/jmsb.2017.35.4.262.
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. https://doi.org/10.1016/j.ifset.2016.09.023.
- Wang, W.; Wang, N.; Liu, C.Q.; Jin, J.C. Effect of silkworm pupae peptide on the fermentation and quality of yogurt. J. Food Process. Preserv. 2017, 41, 7. https://doi.org/10.1111/jfpp.12893.
- Ji, Y.; Xu, L.; Xu, Q.; Liu, X.; Lin, S.; Liao, S.; Wang, W.; Lan, D. Synthesis and Characterization of Epoxidized Silkworm Pupae Oil and Its Application as Polyvinyl Chloride. Appl. Biochem. Biotechnol. 2022, 194, 1290–1302. https://doi.org/10.1007/s12010-021-03715-5.
- Gautreau, M.; Restuccia, M.; Senser, K.; Weisberg, S.N. Familial Anaphylaxis after Silkworm Ingestion. Prehospital Emerg. Care 2017, 21, 83–85. https://doi.org/10.1080/10903127.2016.1204035.
- Sadat, A.; Biswas, T.; Cardoso, M.H.; Mondal, R.; Ghosh, A.; Dam, P.; Nesa, J.; Chakraborty, J.; Bhattacharjya, D.; Franco, O.L. Silkworm pupae as a future food with nutritional and medicinal benefits. Curr. Opin. Food Sci. 2022, 44, 100818 . https://doi.org/10.1016/j.cofs.2022.100818.
- Kumar, D.; Dev, P.; Kumar, R.V. Biomedical applications of silkworm pupae proteins. In Biomedical Applications of Silkworm Pupae Proteins; Springer: Berlin/Heidelberg, Germany , 2015; pp. 41–49.
- Rao, P.U. Chemical composition and nutritional evaluation of spent silk worm pupae. J. Agric. Food Chem. 1994, 42, 2201–2203. https://doi.org/10.1021/jf00046a023.
- Longvah, T.; Mangthya, K.; Ramulu, P.J.F.C. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. 2011, 128, 400–403. https://doi.org/10.1016/j.foodchem.2011.03.041.
- Kitts, D.D.; Weiler, K. Bioactive Proteins and Peptides from Food Sources. Applications of Bioprocesses used in Isolation and Recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. https://doi.org/10.2174/1381612033454883.
- Zhou, J.; Han, D. Proximate, amino acid and mineral composition of pupae of the silkworm Antheraea pernyi in China. J. Food Compos. Anal. 2006, 19, 850–853. https://doi.org/10.1016/j.jfca.2006.04.008.
- Altomare, A.A.; Baron, G.; Aldini, G.; Carini, M.; D’Amato, A. Silkworm pupae as source of high-value edible proteins and of bioactive peptides. Food Sci. Nutr. 2020, 8, 2652–2661. https://doi.org/10.1002/fsn3.1546.
- Kumar, R.V.; Srivastava, D.; Kumar, U.; Kumar, M.; Singh, P. Bioprospecting of omega 3 fatty acid from silkworm pupal oil: From molecular mechanism to biological activities. J. Biol. Act. Prod. Nat. 2020, 10, 495–506. https://doi.org/10.1080/22311866.2020.1862704.
- Hu, B.; Li, C.; Zhang, Z.; Zhao, Q.; Zhu, Y.; Su, Z.; Chen, Y. Microwave-assisted extraction of silkworm pupal oil and evaluation of its fatty acid composition, physicochemical properties and antioxidant activities. Food Chem. 2017, 231, 348–355. https://doi.org/10.1016/j.foodchem.2017.03.152.
- Wang, W.; Xu, L.; Zou, Y.; Pang, D.; Shi, W.; Mu, L.; Li, E.; Lan, D.; Wang, Y.; Liao, S. Comprehensive identification of principal lipid classes and tocochromanols in silkworm (Antheraea pernyi and Bombyx mori) pupae oils. Eur. J. Lipid Sci. Technol. 2020, 122, 1900280. https://doi.org/10.1002/ejlt.201900280.
- Ying, L.Y.; Ying, L.H.; Sofian-Seng, N.-S.; Mustapha, W.A.W.; Razali, N.S.M. Physicochemical Characteristics and Microbiological Quality of Silkworm (Bombyx mori) Larval and Pupae Powder: Comparative Study. Sains Malays. 2022, 51, 547–558. https://doi.org/10.17576/jsm-2022-5102-18.
- de Morais, I.L.; Lunet, N.; Albuquerque, G.; Gelormini, M.; Casal, S.; Damasceno, A.; Pinho, O.; Moreira, P.; Jewell, J.; Breda, J.; et al. The Sodium and Potassium Content of the Most Commonly Available Street Foods in Tajikistan and Kyrgyzstan in the Context of the FEEDCities Project. Nutrients 2018, 10, 98 . https://doi.org/10.3390/nu10010098.
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. https://doi.org/10.1016/S0140-6736(12)61766-8.
- Rodrigues, S.L.; Baldo, M.P.; Machado, R.C.; Forechi, L.; Molina Mdel, C.; Mill, J.G. High potassium intake blunts the effect of elevated sodium intake on blood pressure levels. J. Am. Soc. Hypertens. 2014, 8, 232–238. https://doi.org/10.1016/j.jash.2014.01.001.
- Hu, D.; Liu, Q.; Cui, H.; Wang, H.; Han, D.; Xu, H. Effects of amino acids from selenium-rich silkworm pupas on human hepatoma cells. Life Sci. 2005, 77, 2098–2110. https://doi.org/10.1016/j.lfs.2005.02.017.
- Liu, Q.; Liang, X.; Hu, D.; Chen, P.; Tian, J.; Zhang, H. Purification and characterization of two major selenium-containing proteins in selenium-rich silkworm pupas. Front. Chem. China 2010, 5, 88–98. https://doi.org/10.1007/s11458-009-0109-5.
- Deori, M.; Boruah, D.C.; Devi, D.; Devi, R. Antioxidant and antigenotoxic effects of pupae of the muga silkworm Antheraea assamensis. Food Biosci. 2014, 5, 108–114. https://doi.org/10.1016/j.fbio.2013.12.001.
- Paul, D.; Dey, S. Essential amino acids, lipid profile and fat-soluble vitamins of the edible silkworm Bombyx mori (Lepidoptera: Bombycidae). Int. J. Trop. Insect Sci. 2014, 34, 239–247. https://doi.org/10.1017/S1742758414000526.
- Ahn, M.Y.; Shim, S.H.; Jeong, H.K.; Ryu, K.S. Purification of a dimethyladenosine compound from silkworm pupae as a vasorelaxation substance. J. Ethnopharmacol. 2008, 117, 115–122. https://doi.org/10.1016/j.jep.2008.01.031.
- Wannee, S.; Luchai, B. 1-Deoxynojirimycin and polyphenolic composition and antioxidant activity of different native Thai silkworm (Bombyx mori) larvae. J. King Saud Univ. Sci. 2020, 32, 2762–2766. https://doi.org/10.1016/j.jksus.2020.06.012.
- Ali, M.; Nakahara, S.; Otsu, Y.; Ido, A.; Miura, C.; Miura, T. Effects of functional polysaccharide from silkworm as an immunostimulant on transcriptional profiling and disease resistance in fish. J. Insects Food Feed. 2021, 7, 1–14 . https://doi.org/10.3920/JIFF2021.0108.
- Battampara, P.; Sathish, T.N.; Reddy, R.; Guna, V.; Nagananda, G.S.; Reddy, N.; Ramesha, B.S.; Maharaddi, V.H.; Rao, A.P.; Ravikumar, H.N.; et al. Properties of chitin and chitosan extracted from silkworm pupae and egg shells. Int. J. Biol. Macromol. 2020, 161, 1296–1304. https://doi.org/10.1016/j.ijbiomac.2020.07.161.
- Ali, M.F.Z.; Yasin, I.A.; Ohta, T.; Hashizume, A.; Ido, A.; Takahashi, T.; Miura, C.; Miura, T. The silkrose of Bombyx mori effectively prevents vibriosis in penaeid prawns via the activation of innate immunity. Sci. Rep. 2018, 8, 8836. https://doi.org/10.1038/s41598-018-27241-3.
- Zhu, L.; Zou, D.-Q.; Fan, Z.-Q.; Wang, N.; Bo, Y.-Y.; Zhang, Y.-Q.; Guo, G. Properties of a novel carboxymethyl chitosan derived from silkworm pupa. Arch. Insect Biochem. Physiol. 2018, 99, e21499. https://doi.org/10.1002/arch.21499.
- Zhu, L.; Fan, Z.-Q.; Shi, X.-Q.; Wang, N.; Bo, Y.-Y.; Guo, H.-E. A novel silkworm pupae carboxymethyl chitosan inhibits mouse L929 fibroblast proliferation. ScienceAsia 2020, 46, 30–36. https://doi.org/10.2306/scienceasia1513-1874.2020.007.
- Li, X.; Xie, H.; Chen, Y.; Lang, M.; Chen, Y.; Shi, L. Silkworm Pupa Protein Hydrolysate Induces Mitochondria-Dependent Apoptosis and S Phase Cell Cycle Arrest in Human Gastric Cancer SGC-7901 Cells. Int. J. Mol. Sci. 2018, 19, 1013. https://doi.org/10.3390/ijms19041013.
- Weixin, L.; Lixia, M.; Leiyan, W.; Yuxiao, Z.; Haifeng, Z.; Sentai, L. Effects of silkworm pupa protein hydrolysates on mitochondrial substructure and metabolism in gastric cancer cells. J. Asia-Pac. Entomol. 2019, 22, 387–392. https://doi.org/10.1016/j.aspen.2019.02.005.
More
