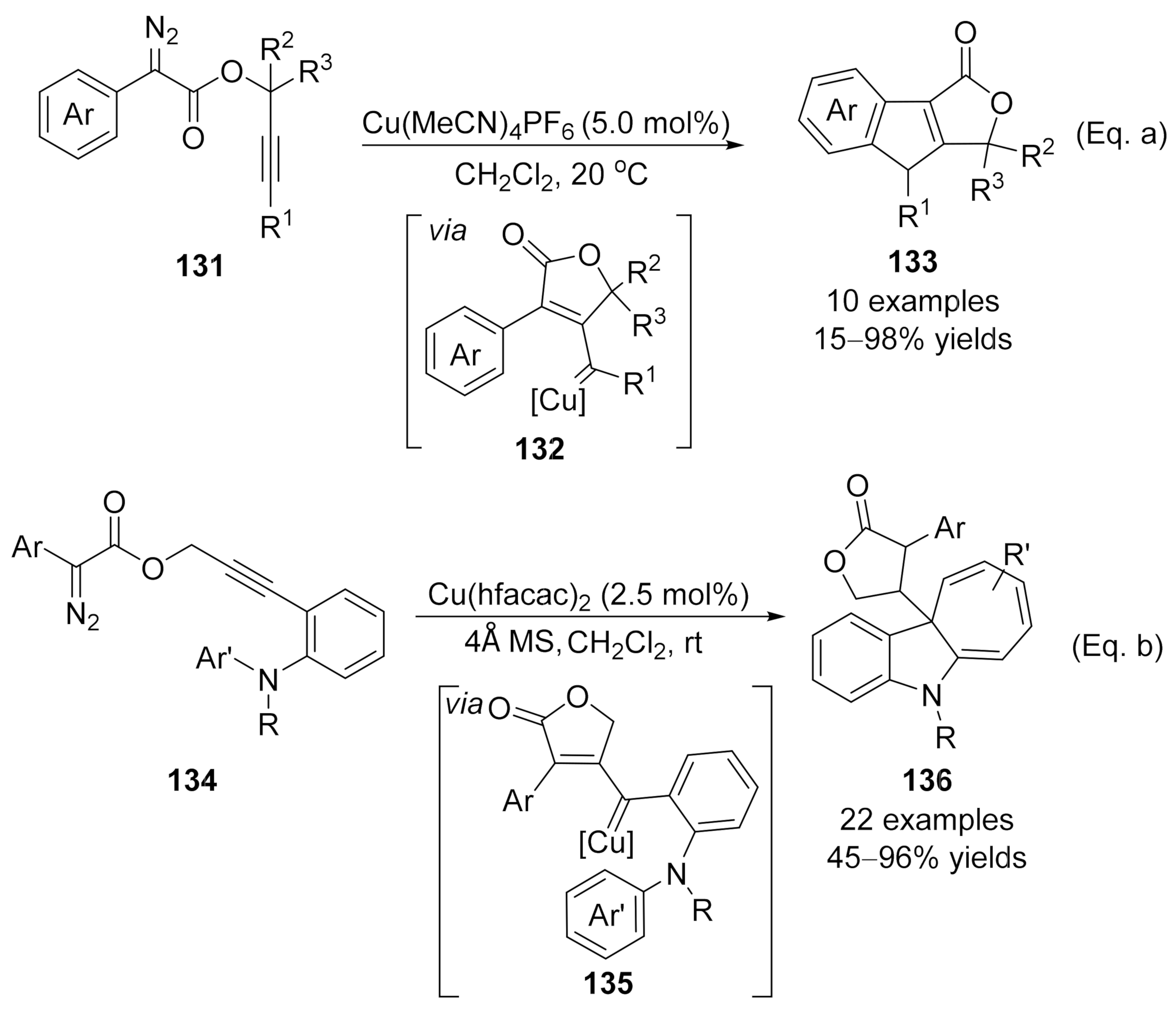
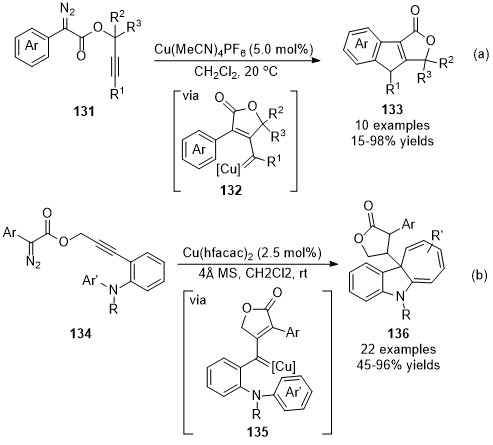
- copper catalysis
- carbene intermediate
- alkyne functionalization
1. Alkynylation
1.1. Alkynylation Terminated by Protonation
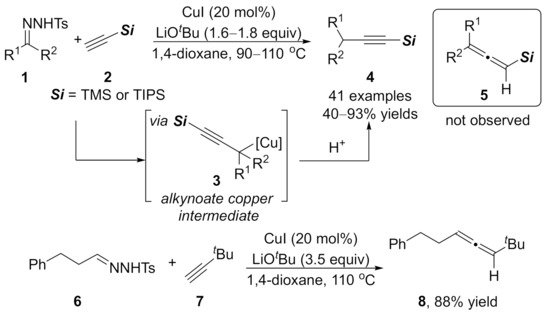
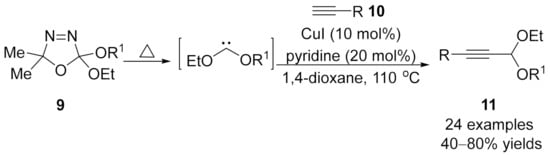
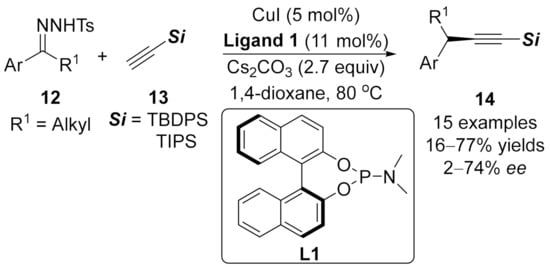
1.2. Alkynylation Terminated by Electrophilic Addition
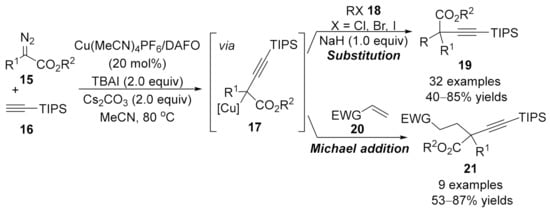
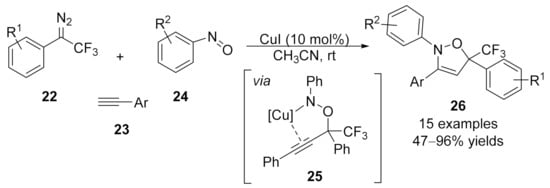
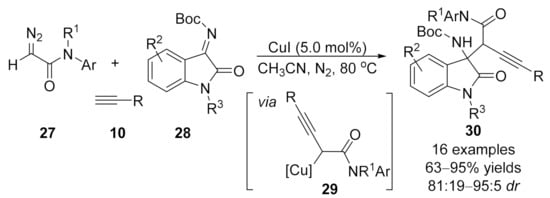
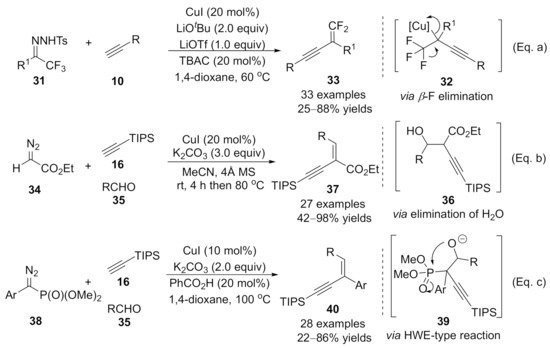
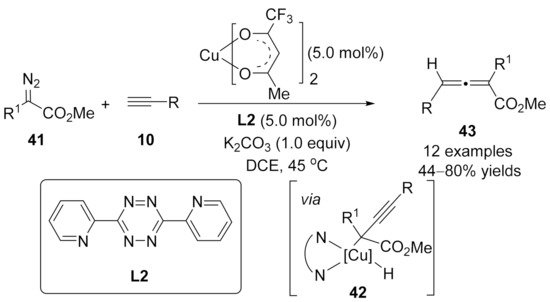
1.3. Alkynylation Terminated by β-Elimination
2. Allenylation
2.1. Allenylation Terminated by Protonation
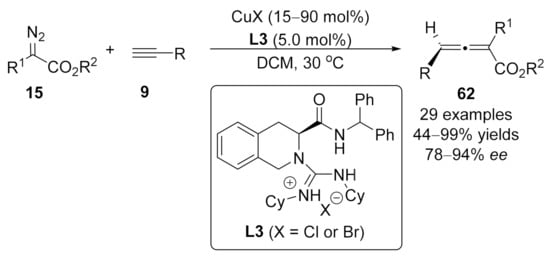
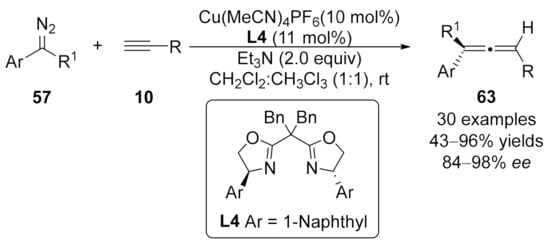
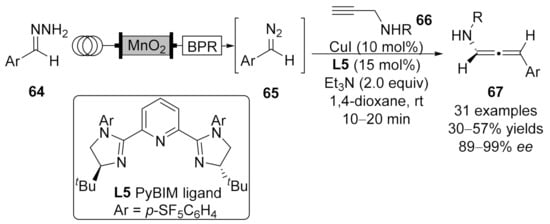
2.2. Allenylation Terminated by Electrophilic Addition
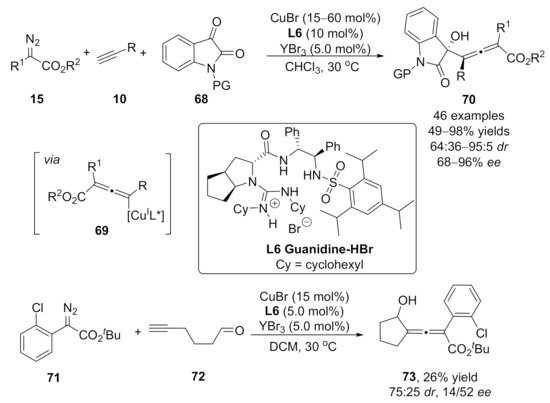
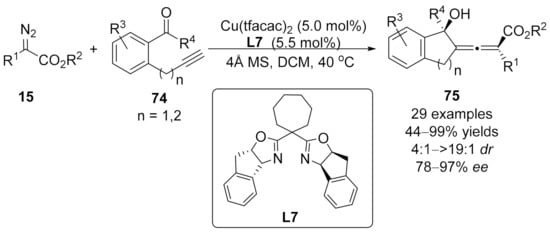
2.3. Allenylation Terminated by Allylation
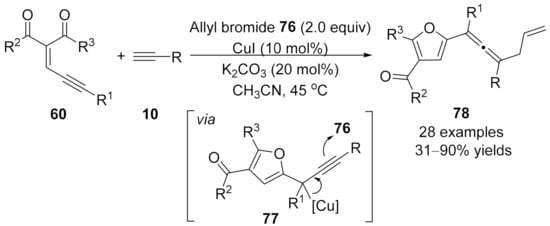
2.4 Cascade Transformations Involving Allenylation Process
Nucleophilic addition with allenoic ester or its isomeric compound is generally used synthetic strategy for the expeditious construction of highly functionalized carbocycle or heterocycle structures [24][25][26][27][28][29][30][31][32]. Thus, a variety of inter- or intramolecular cascade reactions have been developed through different nucleophilic addition processes of the allene derivatives generated from cross-coupling between alkynes and copper carbenes.
In 2011, a one-pot synthesis of phenanthrenes 81 via ligand-free CuBr2-catalyzed coupling reaction/intramolecular cyclization of terminal alkynes 23 with N-tosylhydrazones 79 derived from o-formyl biphenyls was developed by Wang and co-workers [33] Allene intermediates 85 were initially generated in this cascade reaction through a cross-coupling reaction of N-tosylhydrazones 79 with terminal alkynes 23, followed by a 6π-cyclization and isomerization deliver the phenanthrene products 81 with broad functional group compatibility (Scheme 19).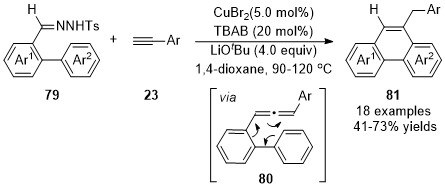
Scheme 19. Copper-catalyzed allenylation followed by 6π-cyclization.
Later in the same year, instead of using
o
-aryl substituted
N
-tosylhydrazones,
o
-hydroxy- or
o
-amino-substituted
N
-tosylhydrazones were introduced by the same group as carbene precursors in an analogous cascade transformation, a ligand-free CuBr-catalyzed coupling reaction/intramolecular cyclization sequence, achieving the synthesis of benzofuran or indole derivatives
84
in moderate to excellent yields
[34]
. The initially formed allene intermediates
83
were trapped through a nucleophilic addition by the embedded
o
-hydroxy- or
o
-amino group to afford the cyclized products
84
(Scheme 20).
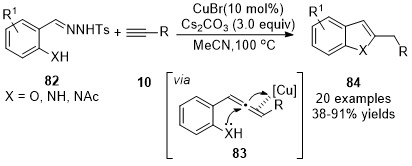
Scheme 20. Copper-catalyzed allenylation followed nucleophilic addition.
In 2011, a similar catalytic strategy was developed by Balakishan’s group. They reported a simple procedure for synthesizing aza- and oxacycles
via
a copper-catalyzed coupling reaction of functionalized terminal alkynes
85
with diazoesters
86
[35]
. Initially, the allene intermediates were formed in the presence of CuI, followed by an intramolecular aza- or oxa-Michael cycloaddition and isomerization to generate the cyclized five- or six-membered products
87
in generally good yields (Scheme 21).
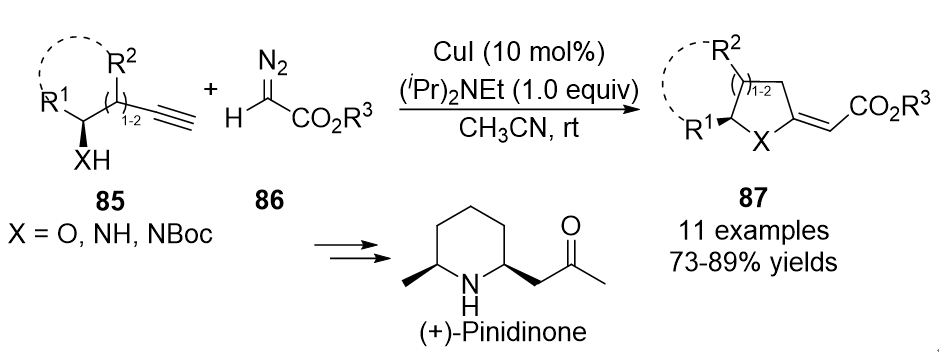
Scheme 21. Copper-catalyzed allenylation followed by cyclization.
In 2015, a stereo-divergent synthesis of five-membered heterocycles was developed by Sun’s group
[36]. The proposed reaction mechanism involves trapping
. This work described a copper-catalyzed cross-coupling reaction and annulation cascade reaction of amino alkynes 88 with diazo compounds 15. The proposed reaction mechanism involves trapping
in situ
formed allene intermediates, yielding 2-methylenes
89
(when PG = Bn) and 2,3-dihydropyrroles
90
(when PG = Ts) in good yields with broad functional group tolerance under mild conditions. Control experimental results show that
N
-benzyl amino alkynes were more likely to form 2-methylenespyrroles derivatives
89
through
5-exo-dig
cycloaddition, while 2,3-dihydropyrroles
90
generated from
N
-tosylamino alkynes through
5-endo-dig
cycloaddition would be more favorable (Scheme 22).
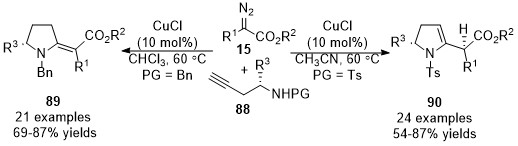
Scheme 22. Copper-catalyzed allenylation followed by divergent annulation.
In 2018, Sun and co-workers expanded the above chemistry to synthesize the four- to six-membered heterocycles with
N
-substituted prop-2-yn-1-amines
91
and diazoacetates
15
[37]
. Generated allenoic species
92
have been proven as the key intermediates for the subsequent diverse annulations under optimized conditions toward functionalized heterocycle in moderate to good yields. Treatment of allenoates
92
with sodium phenolates led to six-membered products
93
; silver nitrate and triethylamine yielded five-membered products
94
; what’s more, four-membered products
95
were generated under lithium
tert
-butoxide conditions (Scheme 23).
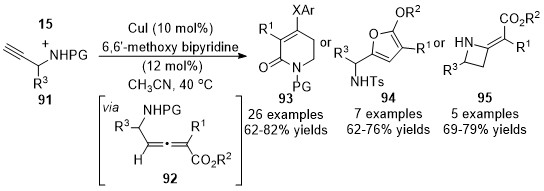
Scheme 23. Divergent synthesis of four- to six-membered heterocycles involving an allenylation process.
In addition to the cyclization through addition with a heteroatom, carbon-based nucleophilic species could also be served as the nucleophile to addition with these allenes, forming the C-C bond instead of the C-X bond
. In 2015, Kumaraswamy’s group developed a cooper catalyzed cross-coupling reaction/intramolecular Michael addition cascade reaction
[40]
, achieving the formation of indene and dihydronaphthalene derivatives
97
in good yields with broad functional group tolerance (Scheme 24a). Later in 2017, Sun’s group reported an analogous approach toward five- or six-membered carbo-/heterocycles with diazo compounds
15
and alkyne-substituted malonates
98 [41]
. In this reaction, the ligand significantly enhanced the reaction yields and inhibited the Conia-ene side reaction. As a result, the polyfunctionalized cyclohexenes, tetrahydropyridines, and dihydropyrans have been prepared in moderate to high yields under mild reaction conditions (Scheme 24b).
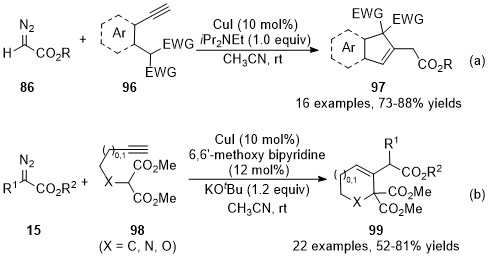
Scheme 24. Copper-catalyzed allenylation followed by Michael addition.
In 2015, a Cu(I)-catalyzed denitrogenative annulation reaction of pyridotriazoles
100
with terminal alkynes
10
was developed by Gevorgyan’s group
[42]
. Initially,
α
-pyridyl copper carbenes were generated from pyridotriazoles
100
in the presence of the copper catalyst, followed by a cross-coupling reaction with terminal alkyne to form either propargylic or allenoic intermediates
101
, which were terminated by copper-catalyzed cycloisomerization to furnish the indolizines
102
in moderate to excellent yields (Scheme 25).
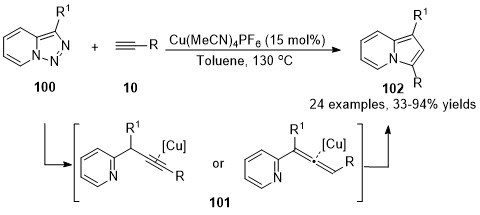
Scheme 25.
Copper-catalyzed allenylation followed by cycloisomerization.
In 2018, Wang and co-workers reported a copper-catalyzed geminal difunctionalization reaction of terminal alkynes
[43]
. The key step in this cascade reaction is trapping the
in situ
generated allenoic species
105
with a sulfonyl anion to form the carbon-sulfur bond, providing a variety of vinyl sulfones
106
in good yields with excellent stereoselectivities under mild reaction conditions. It was noted that the excellent stereoselectivities might be due to the influence of steric hindrance, and no ligand and additive were required in this transformation (Scheme 26).
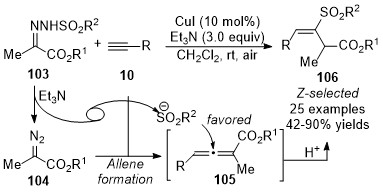
Scheme 26. Copper-catalyzed allenylation followed by sulfonylation.
Recently, Sun and co-workers demonstrated a copper-catalyzed three-component reaction of terminal alkynes with diazo compounds and B
2
pin
2
for the synthesis of trisubstituted alkenylboronates
[44]
. Copper catalysts played dual roles in these alkynes’ difunctionalizations. Initially, copper catalyzed the cross-coupling to form an allenoic intermediate, followed by a copper-catalyzed stereoselective boration reaction with B
2
pin
2
. When diazo compounds
53
were used as carbene precursors, the steric interaction forced the boron group to attack the
β
-carbon from the opposite side of the
γ
-phenyl group on the allenoic species
107
, leading to the favored (
Z
)-isomers
108
as major products. Whereas, in the case with
N
-tosylhydrones
51
as carbene precursors, the addition of Cu-Bpin complex to corresponding allenoic species
109
provided allyl copper intermediate, which was more favored to form a six-membered ring transition state with the association of MeOH, finally furnishing the more thermodynamically stable (
E
)-products
110 (Scheme 27).
(Scheme 27).
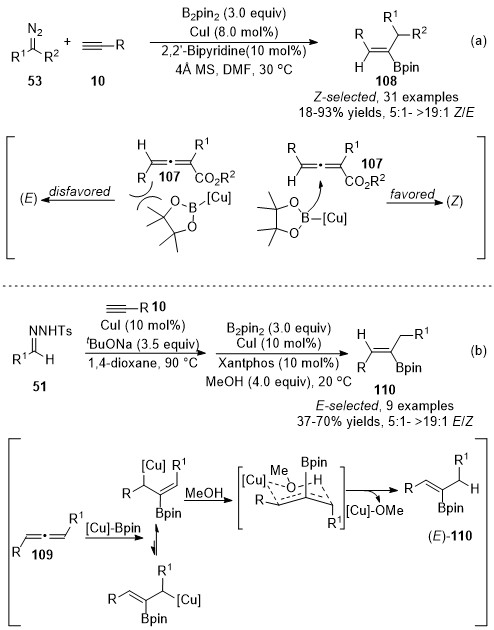
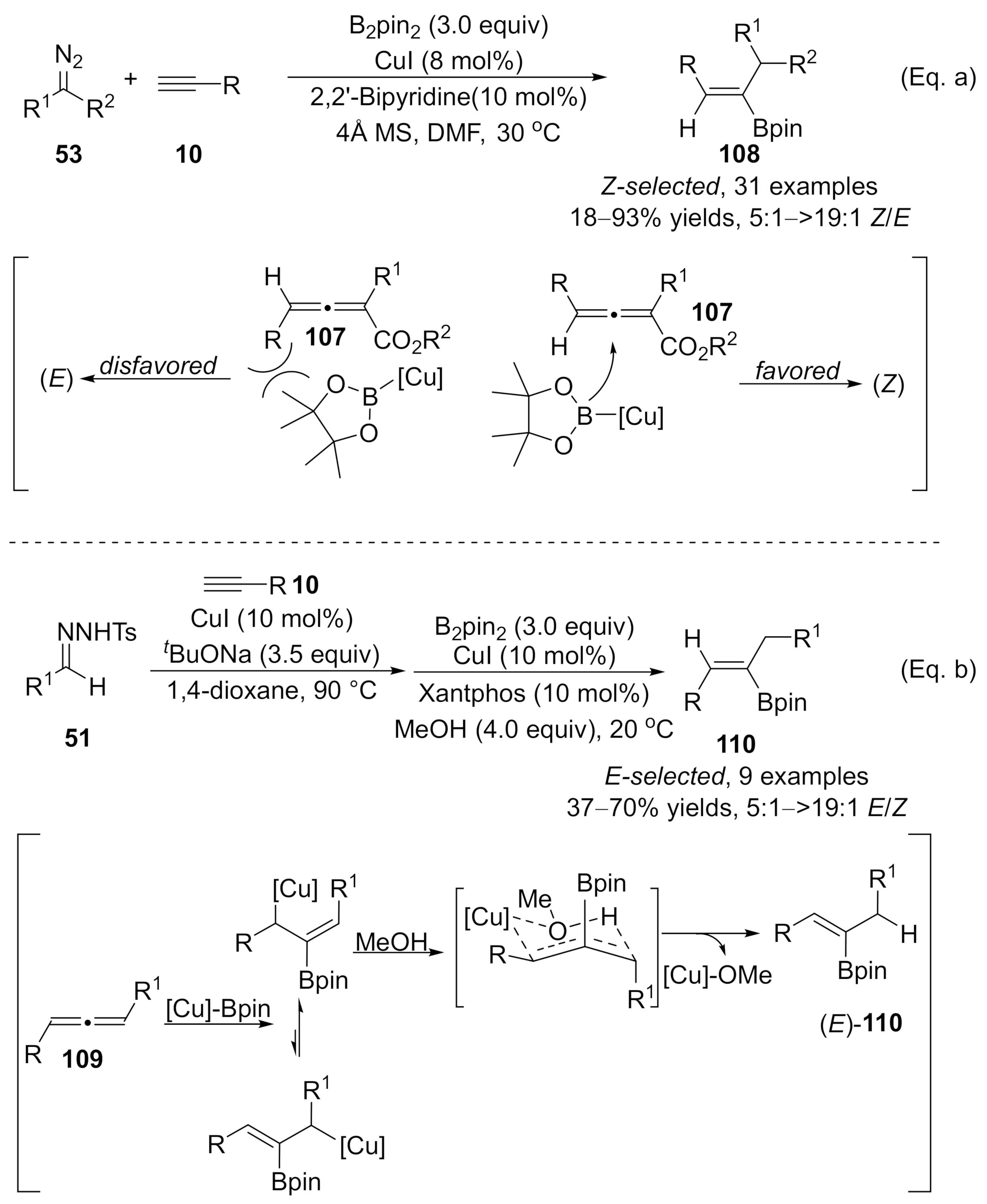
Scheme 27. Copper-catalyzed allenylation followed by boroalkylation.
3. Copper Carbene Intermediate Addition onto C-C Triple Bond
3.1 Cyclopropenation
Cyclopropenation is a well-known reaction of metal carbene intermediate with alkynes. This widely used reaction could be catalyzed by rhodium
, cobalt
[50]
, gold
[51]
, silver
and many others
. Herein, selected examples related to copper catalysis will be discussed.
In 2010, a new tridentate coordination copper complex, Cu[Ms(CH
2
SCN)
3
]BAr'
4
(BAr'4 = tetra(3,5-bis(trifluoromethylphenyl)borate), was designed by Miguel and co-workers by using [Cu(OTf)]
2
•C
6
H
6
and an alkylthiocyanate ligand
[57]
. This catalyst promoted the cyclopropenation of ethyl diazoacetate
34
(EDA) with a wide range of internal alkynes
111
, providing cyclopropenes
112
in moderate yields (Scheme 28). The same cyclopropenation work was achieved by Dias, unique bis(pyrazolyl)borate ligand supported [(CF
3
)
2
Bp]Cu(NCMe) catalyst was used, yielding cyclopropene products in moderate to high yields
[58]
.
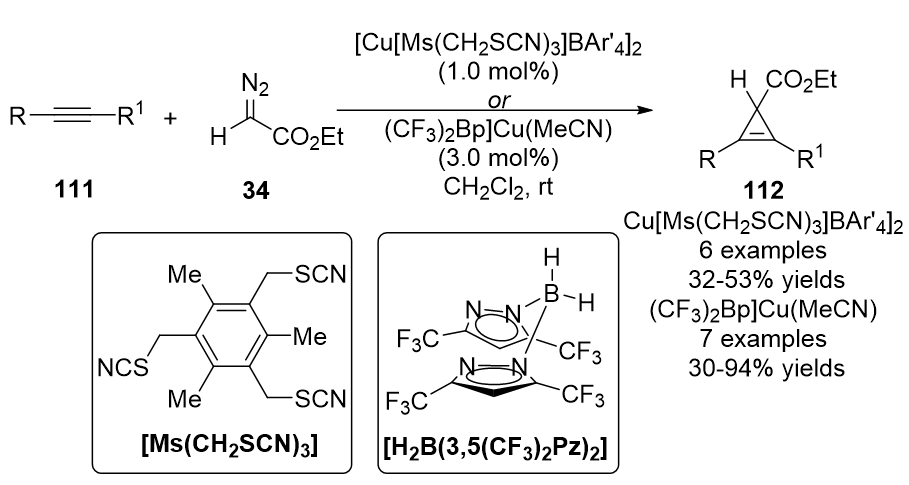
Scheme 28. Copper/alkylthiocyanate complex catalyzed cyclopropenation.
In 2016, a Cu(I)/
N
-heterocyclic carbene (CuNHC) complex catalyzed cyclopropenation of internal alkynylsilanes
113
with diazoacetate
15
was reported by Coleman’s group
[59]
. A series of 1,2,3-trisubstituted and 1,2,3,3-tetrasubstituted cyclopropenylsilane compounds
114
were isolated in moderate to good yields (Scheme 29). An interesting regioselective and chemodivergent reaction pathway occurred furnished a
tetra-substituted furan through an intramolecular cyclopropene ring-opening transformation in the case of electron-rich diazoacetate.
-substituted furan through an intramolecular cyclopropene ring-opening transformation in the case of electron-rich diazoacetate.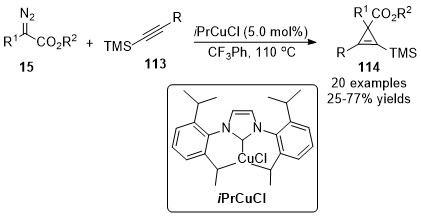
Scheme 29. Copper/N-heterocyclic carbene (CuNHC) complex catalyzed cyclopropenation.
3.2 Cascade Reaction Involving Carbene/Alkyne Metathesis Process
Carbene/alkyne metathesis (CAM) refers to the processes where a metal carbene reacts with an alkyne, generating a new vinyl metal carbene intermediate, which was difficult to access with other carbene precursors
. This
in situ
generated vinyl metal carbene intermediate could be involved in typical metal carbene reactions, such as [3+2]-cycloaddition
[63]
, cyclopropanation
, C-H bond insertion
, and others
. Herein, we summarized recent works on the copper-mediated cascade transformations involving carbene/alkyne metathesis.
It’s a general protocol for the synthesis of furan derivatives through transition metal-catalyzed formal [3+2] cycloaddition of
α
-diazocarbonyl compounds with alkynes
. However, the cases under copper carbenes mediated were limited. In 2014, Wang’s group developed a copper-catalyzed formal [3+2] cycloaddition reaction of terminal alkynes with
β
-keto
α
-diazoesters
115
(X = O), offering an operationally simple and applicable method for the synthesis of trisubstituted furans
116
(X = O) with a wide substrate scope (Scheme 30a)
[78]
. This reaction has also been applied to ethyl (
E
)-2-diazo-3-(methoxyimino)butanoate
115
(X = NOMe) for the synthesis of 2,3,5-trisubstituted
N
-methoxypyrroles (X = NOMe). Later in 2016, a Cu(I)-catalyzed cycloaddition of diazoacetates
15
with electron-rich internal aryl alkynes
117
was discovered by Coleman and co-workers
[79]
. Tetra-substituted furans
118 were generated in moderate isolated yields with high chemoselectivities and regioselectivities (Scheme 30b).
were generated in moderate isolated yields with high chemoselectivities and regioselectivities (Scheme 30b).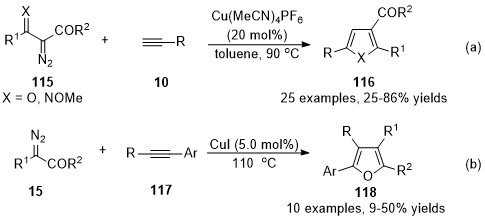
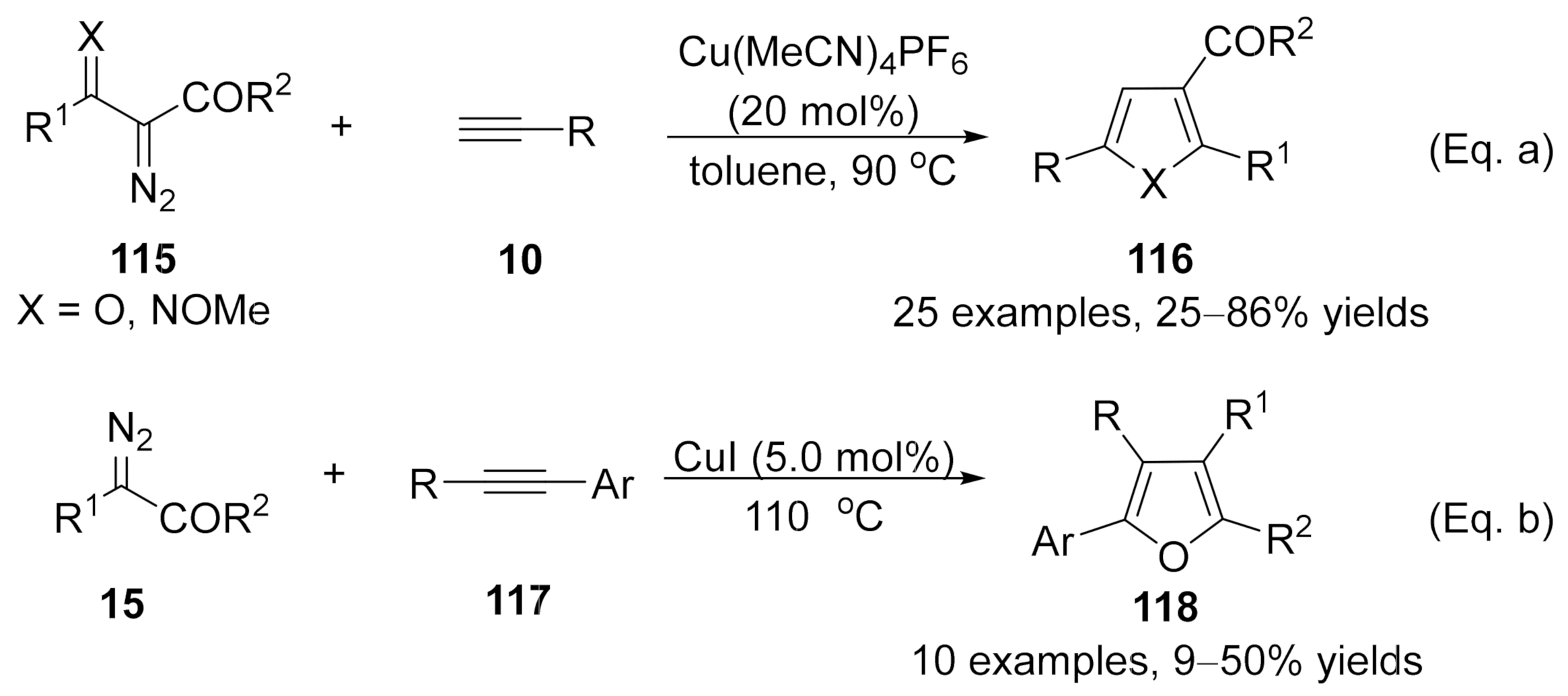
Scheme 30. Copper-catalyzed carbene/alkyne metathesis for the synthesis of furan derivatives.
In 2016, Xu’s group developed a chemo-divergent copper-catalyzed cascade reaction of alkynyl-tethered
α
-iminodiazoacetates
119
, providing polycyclic and multi-substituted pyrroles in high yields with a broad substrate scope
[80]
. Especially, the
tetra
-substituted 3-formylpyrroles
124
, which were difficult to access by alternate approaches. Mechanistic studies indicated that the
α
-imino carbene
120
is the key common intermediate in this divergent reaction, which was generated by metal-catalyzed carbene/alkyne metathesis of the alkynyl-tethered diazo compounds
121
. When R
1
, R
2
was imbedded with an aromatic ring, polycyclic pyrroles
122
were formed as the major products through a [3+2]-cyclization and aromatization process. Whereas, substrates with a methoxy group on the nitrogen (R
2
= OMe), the carbene intermediate underwent an
N
–O insertion/alkoxy migration/alcoholysis sequence, giving the 3-formylpyrrole products
124
in generally good to excellent yields (Scheme 31).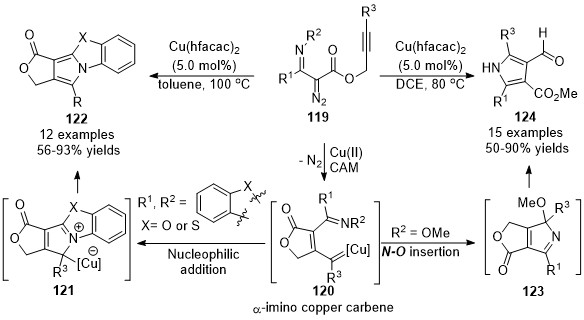
Scheme 31. Copper-catalyzed carbene/alkyne metathesis for the synthesis of pyrroles.
At the same time, Xu and co-workers have also developed a copper-catalyzed carbene/alkyne metathesis cascade reaction with alkyne-tethered diazo compounds
125 [81]
. This transformation provided rapid access for the construction of multi-substituted 4-carboxyl quinoline derivatives
127
in high to excellent yields. In this cascade reaction, one C═N and one C═C bond were formed with the assistance of the copper catalyst under mild reaction conditions (Scheme 32a). Later in 2017, Ye’s group reported an analogous protocol by using ynamides
128
as carbene precursor
[82]
. In this work, a copper carbene was generated in situ through a catalytic oxidation process in the presence of quinoline
N
-oxide, followed by a CAM process and terminated by carbene reaction with an embedded azide group, providing a wide range of pyrrolo[3,4-
c
]quinolin-1-ones
130 in good yields. Those works represented practical methods for the dual functionalization of alkynes (Scheme 32b).
in good yields. Those works represented practical methods for the dual functionalization of alkynes (Scheme 32b).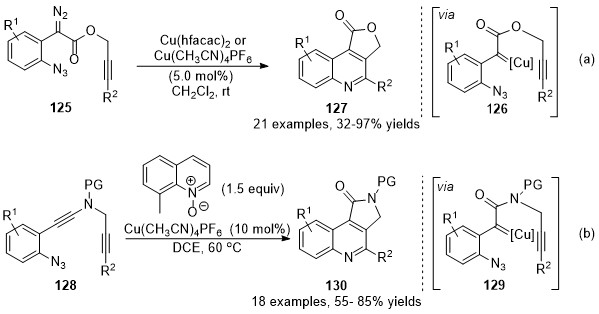
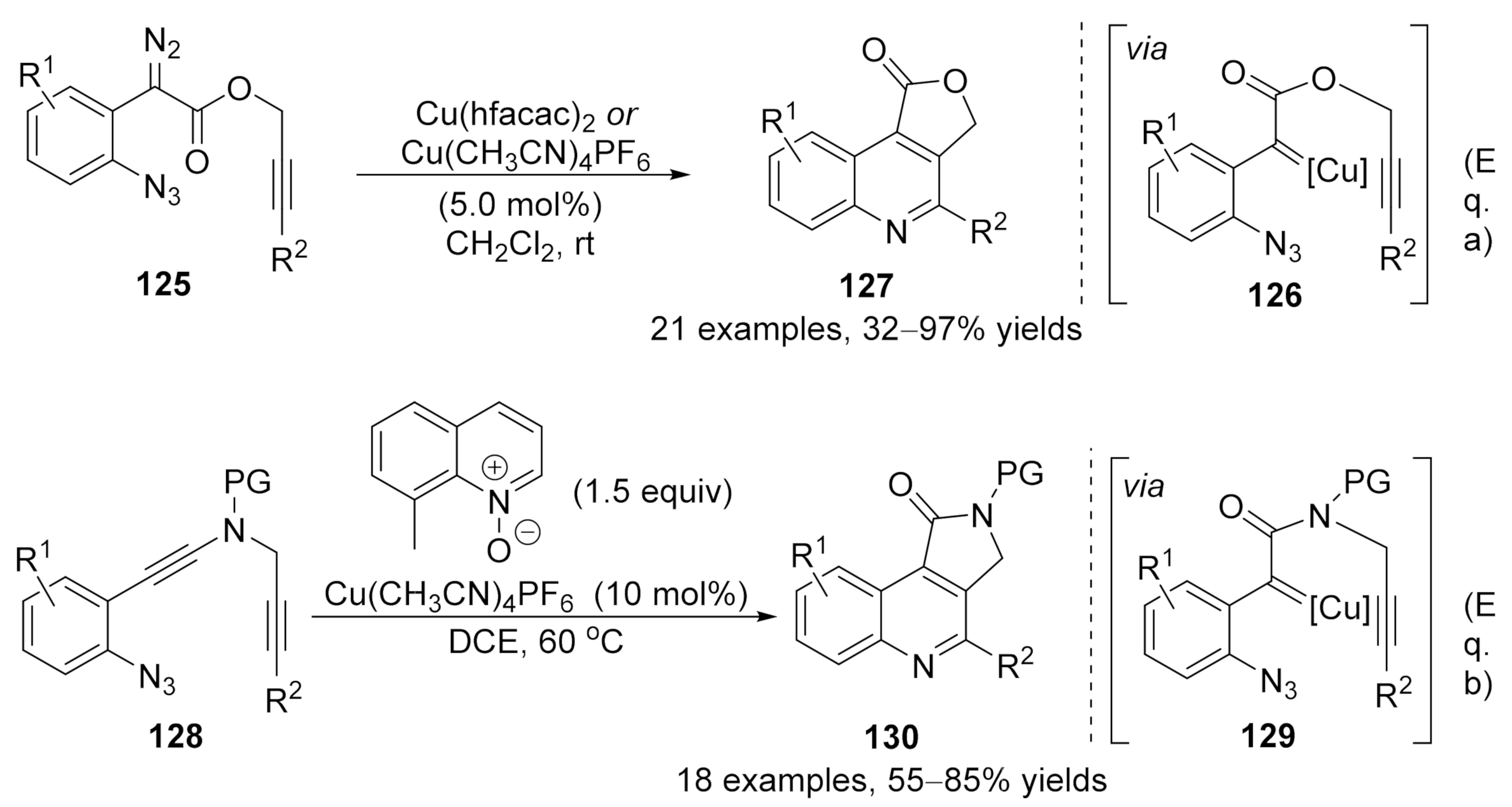
Scheme 32. Copper-catalyzed carbene/alkyne metathesis for the synthesis of quinolines.
In addition to the nucleophilic addition of the
in situ
formed copper carbene intermediates, electrophilic aromatic substitution or C(sp
2
)–H bond functionalization is another useful terminating transformation for the direct construction of polycyclic fused frameworks. In 2017, Doyle’s group reported a copper-catalyzed intramolecular cascade reaction of diazo compounds
131
, this transformation went through a CAM process followed by a carbene C(sp
2
)–H bond functionalization cascade, yielding the fused indeno-furanone derivatives
133
in excellent yields under mild reaction conditions (Scheme 33a)
[83]
. Instead of terminating reaction in C-H functionalization, a selective Buchner insertion reaction occurred as the terminating step in Xu’s work when the
ortho
-aniline substituted propargyl diazoacetates
134
were employed, selectively affording the dihydrocyclohepta[
b
]indole derivatives
136
in moderate to high yields (Scheme 33b). Notably, this reaction described a rare example of the Buchner reaction with donor/donor type metal carbene species
[84].
.
Scheme 33. Copper-catalyzed carbene/alkyne metathesis for the synthesis of tri-cyclic molecules.
In 2018, Xu and co-workers developed an intermolecular copper-catalyzed formal CAM process
[85]
, which underwent a copper promoted [3+2] cycloaddition/dinitrogen exclusion/nucleophilic addition process, providing a direct and effective access to 2
H
-chromene derivatives
139
in generally good to high yields. Mechanistic studies indicated that the 3
H
-pyrazole
138 is the key intermediate in this cascade transformation, and this critical intermediate was isolated and confirmed by single-crystal X-ray diffraction and spectroscopy analysis for the first time (Scheme 34).
is the key intermediate in this cascade transformation, and this critical intermediate was isolated and confirmed by single-crystal X-ray diffraction and spectroscopy analysis for the first time (Scheme 34).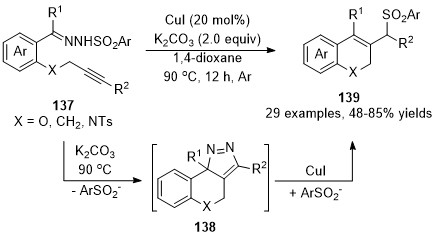
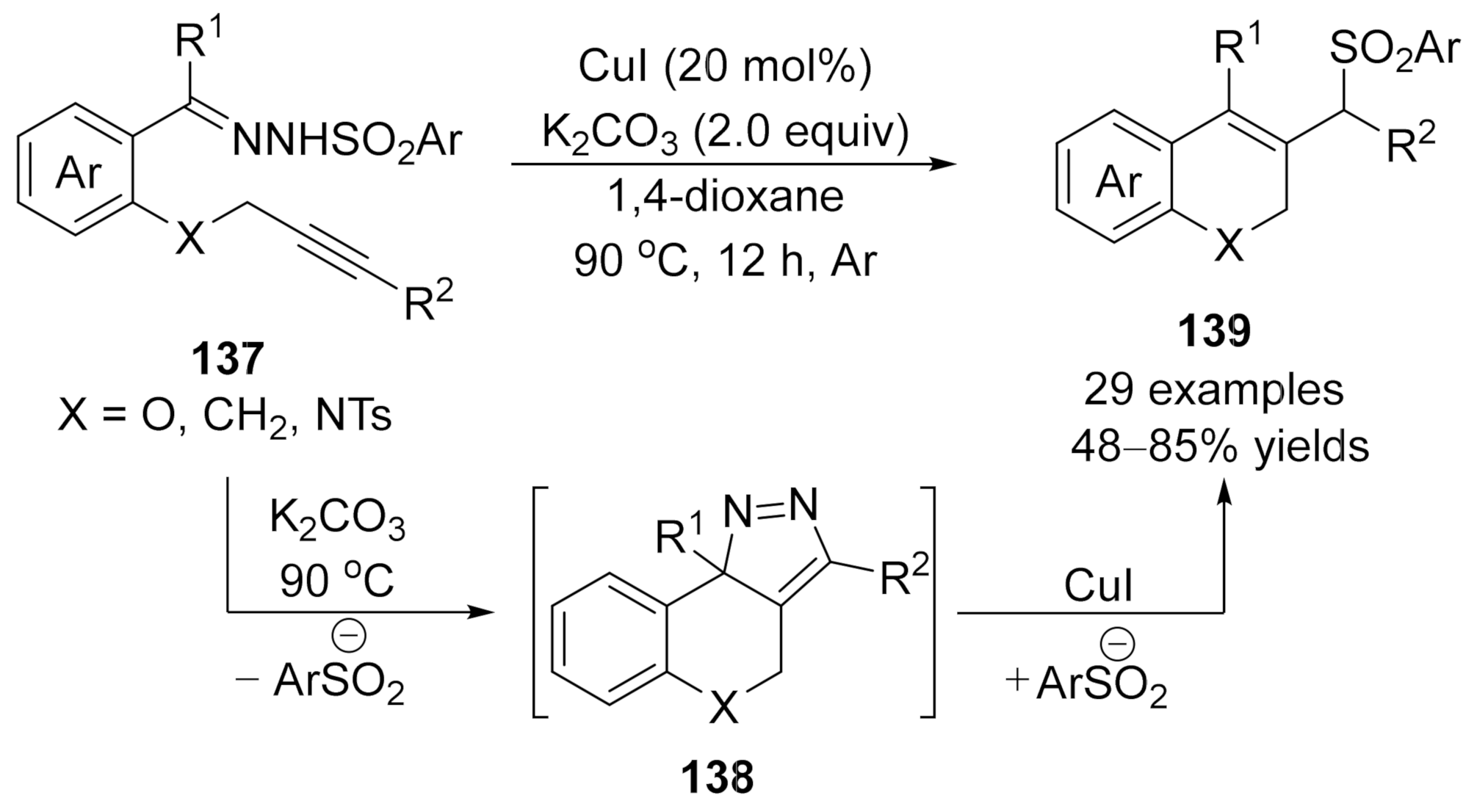
Scheme 34. Copper-catalyzed formal carbene/alkyne metathesis for the synthesis of 2H-chromene derivatives.
Based on a similar protocol, a copper-catalyzed formal [1+2+2]-annulation of alkyne-tethered diazo compounds
140
with pyridines
141
has been reported by Xu’s group recently
[86]
. In contrast to the previously reported cascade reaction that was terminated the copper carbene intermediate on the carbonic center, a vinylogous addition of vinyl carbene intermediate with pyridine derivatives was occurred in this reaction, followed by an intramolecular annulation to form cycloadducts
146
, which underwent a decarboxylative aromatization process to form the desired polycyclic fused indolizine derivatives
147
in good to high yields (Scheme 35, path a), although direct formal [3+2]-cycloaddition
via pyridinium ylide pathway could not be ruled out so far (Scheme 35, path b).
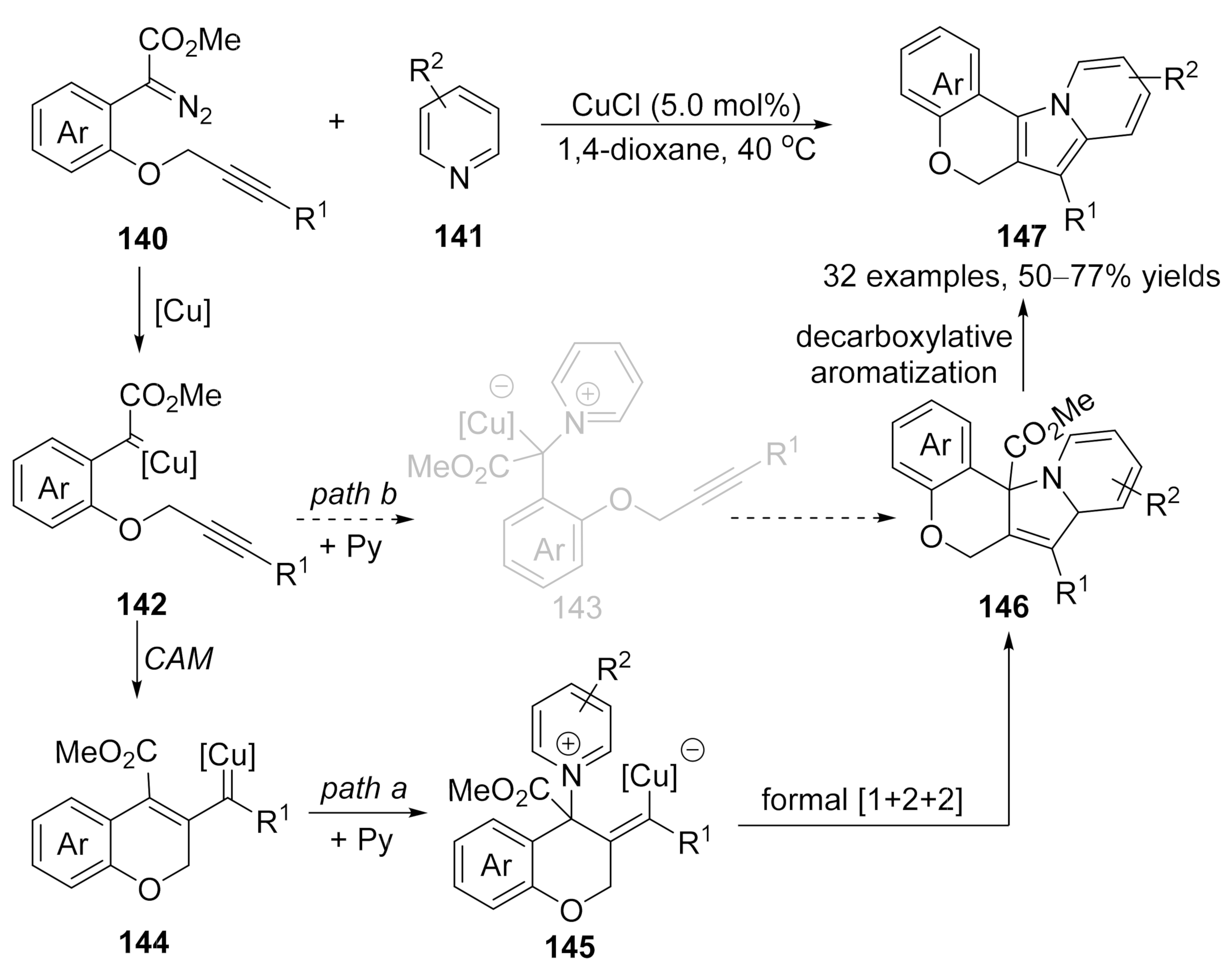 pyridinium ylide pathway could not be ruled out so far (Scheme 35, path b).
pyridinium ylide pathway could not be ruled out so far (Scheme 35, path b).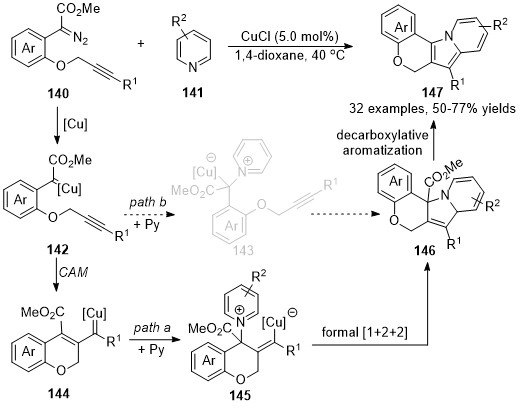
Scheme 35. Copper-catalyzed formal carbene/alkyne metathesis for the synthesis of polycyclic indolizines.
4.4 Conclusions
This review has summarized the recent progress of catalytic alkyne functionalization involving copper carbene intermediates. Copper carbene species derived from different carbene precursors have been introduced to react with alkynes through two distinguished reaction models: cross-coupling reaction of copper carbene intermediates with terminal alkynes and addition of copper carbene intermediates onto the C-C triple bond. The former reaction involves alkynoate or allenoate copper intermediates, followed by protonation, nucleophilic substitution, electrophilic addition, or elimination process, yielding functionalized alkynes and allenes, respectively. The latter version includes cyclopropenation and cascade reaction via carbene/alkyne metathesis process. Although substantial progress has been realized in this field, challenges remain, e.g., the carbene precursors are still mainly limited to the diazo compounds; the catalytic efficiency could be further improved, especially in the asymmetric catalysis; synthetic applications of this chemistry are still under exploration. Therefore, the synthetic potential of this chemistry could be envisioned through the introduction of a variety of readily accessible carbene precursors and with the development of robust copper catalysts/ligands, including novel methodology discovery for the catalytic alkyne functionalization, and expeditious assembly of molecules with structural complexity and diversity for the leading compound development.
References
- Fei Ye; Xiaoshen Ma; Qing Xiao; Huan Li; Yan Zhang; Jianbo Wang; ChemInform Abstract: C(sp)-C(sp3) Bond Formation Through Cu-Catalyzed Cross-Coupling of N-Tosylhydrazones and Trialkylsilylethynes.. ChemInform 2012, 43, no-no, 10.1002/chin.201237181.
- Tiebo Xiao; Ping Zhang; Yang Xie; Jun Wang; Lei Zhou; CuI-catalyzed cross-coupling of terminal alkynes with dialkoxycarbenes: a general method for the synthesis of unsymmetrical propargylic acetals. Organic & Biomolecular Chemistry 2014, 12, 6215-6222, 10.1039/c4ob00614c.
- Yasuhiro Uozumi; Takao Osako; Makoto Nagaosa; Go Hamasaka; Asymmetric Copper-Catalyzed C(sp)–H Bond Insertion of Carbenoids Derived from N-Tosylhydrazones. Synlett 2018, 29, 2251-2256, 10.1055/s-0037-1611003.
- Chengpeng Wang; Fei Ye; Chenggui Wu; Yan Zhang; Jianbo Wang; Construction of All-Carbon Quaternary Centers through Cu-Catalyzed Sequential Carbene Migratory Insertion and Nucleophilic Substitution/Michael Addition. The Journal of Organic Chemistry 2015, 80, 8748-8757, 10.1021/acs.joc.5b01592.
- Xinxin Lv; Zhenghui Kang; Dong Xing; Wenhao Hu; Cu(I)-Catalyzed Three-Component Reaction of Diazo Compound with Terminal Alkyne and Nitrosobenzene for the Synthesis of Trifluoromethyl Dihydroisoxazoles. Organic Letters 2018, 20, 4843-4847, 10.1021/acs.orglett.8b01981.
- Jiuwei Che; Alavala Gopi Krishna Reddy; Li Niu; Dong Xing; Wenhao Hu; Cu(I)-Catalyzed Three-Component Reaction of α-Diazo Amide with Terminal Alkyne and Isatin Ketimine via Electrophilic Trapping of Active Alkynoate-Copper Intermediate. Organic Letters 2019, 21, 4571-4574, 10.1021/acs.orglett.9b01470.
- Zhikun Zhang; Qi Zhou; Weizhi Yu; Tianjiao Li; Guojiao Wu; Yan Zhang; Jianbo Wang; Cu(I)-Catalyzed Cross-Coupling of Terminal Alkynes with Trifluoromethyl Ketone N-Tosylhydrazones: Access to 1,1-Difluoro-1,3-enynes. Organic Letters 2015, 17, 2474-2477, 10.1021/acs.orglett.5b00980.
- Chenggui Wu; Zhenxing Liu; Zhikun Zhang; Fei Ye; Guisheng Deng; Yan Zhang; Jianbo Wang; Copper(I)-Catalyzed Stereoselective Synthesis of (E )-α-Alkynyl-α,β-unsaturated Esters from a Terminal Alkyne, Diazoesters and Aldehydes. Advanced Synthesis & Catalysis 2016, 358, 2480-2488, 10.1002/adsc.201600374.
- Yujing Zhou; Fei Ye; Qi Zhou; Yan Zhang; Jianbo Wang; Cu(I)-Catalyzed Tandem Reaction of Carbene Coupling and Horner–Wadsworth–Emmons Type Olefination: Access toward Enynes. Organic Letters 2016, 18, 2024-2027, 10.1021/acs.orglett.6b00631.
- Andrés Suárez; Gregory C. Fu; A Straightforward and Mild Synthesis of Functionalized 3-Alkynoates. Angewandte Chemie 2004, 116, 3664-3666, 10.1002/ange.200454070.
- Matthew Hassink; Xiaozhong Liu; Joseph M. Fox; Copper-Catalyzed Synthesis of 2,4-Disubstituted Allenoates from α-Diazoesters. Organic Letters 2011, 13, 2388-2391, 10.1021/ol2006242.
- Jian Li; Dong Ding; Li Liu; Jiangtao Sun; CuI-catalyzed cross-coupling of diazoacetamide with terminal alkynes: an approach to synthesizing substituted dienamides and 3-butynamides. RSC Advances 2013, 3, 21260-21266, 10.1039/c3ra44801k.
- Mohammad Lokman Hossain; Fei Ye; Yan Zhang; Jianbo Wang; CuI-Catalyzed Cross-Coupling of N-Tosylhydrazones with Terminal Alkynes: Synthesis of 1,3-Disubstituted Allenes. The Journal of Organic Chemistry 2013, 78, 1236-1241, 10.1021/jo3024686.
- Chenggui Wu; Fangdong Hu; Zhenxing Liu; Guisheng Deng; Fei Ye; Yan Zhang; Jianbo Wang; Cu(I)-catalyzed coupling of diaryldiazomethanes with terminal alkynes: an efficient synthesis of tri-aryl-substituted allenes. Tetrahedron 2015, 71, 9196-9201, 10.1016/j.tet.2015.10.043.
- Fei Ye; Chengpeng Wang; Xiaoshen Ma; Mohammad Lokman Hossain; Ying Xia; Yan Zhang; Jianbo Wang; Synthesis of Terminal Allenes through Copper-Mediated Cross-Coupling of Ethyne withN-Tosylhydrazones or α-Diazoesters. The Journal of Organic Chemistry 2014, 80, 647-652, 10.1021/jo502316q.
- Shuai Xu; Ri Chen; Zihao Fu; Yunpeng Gao; Jianbo Wang; Cu(I)-Catalyzed Coupling of Bis(trimethylsilyl)diazomethane with Terminal Alkynes: A Synthesis of 1,1-Disilyl Allenes. The Journal of Organic Chemistry 2018, 83, 6186-6192, 10.1021/acs.joc.8b00651.
- Jian-Siang Poh; Duc N. Tran; Claudio Battilocchio; Joel M. Hawkins; Steven V. Ley; A Versatile Room-Temperature Route to Di- and Trisubstituted Allenes Using Flow-Generated Diazo Compounds. Angewandte Chemie International Edition 2015, 54, 7920-7923, 10.1002/anie.201501538.
- Fangdong Hu; Ying Xia; Chen Ma; Yan Zhang; Jianbo Wang; Cu(I)-Catalyzed Synthesis of Furan-Substituted Allenes by Use of Conjugated Ene-yne Ketones as Carbene Precursors. The Journal of Organic Chemistry 2016, 81, 3275-3285, 10.1021/acs.joc.6b00236.
- Yu Tang; Quangang Chen; Dr. Xiaohua Liu; Gang Wang; Lili Lin; Dr. Xiaoming Feng; Direct Synthesis of Chiral Allenoates from the Asymmetric C-H Insertion of α-Diazoesters into Terminal Alkynes. Angewandte Chemie 2015, 127, 9648-9652, 10.1002/ange.201501918.
- Wen-Dao Chu; Lei Zhang; Zhikun Zhang; Qi Zhou; Fanyang Mo; Yan Zhang; Jianbo Wang; Enantioselective Synthesis of Trisubstituted Allenes via Cu(I)-Catalyzed Coupling of Diazoalkanes with Terminal Alkynes. Journal of the American Chemical Society 2016, 138, 14558-14561, 10.1021/jacs.6b09674.
- Jian-Siang Poh; Szabolcs Makai; Timo Von Keutz; Duc N. Tran; Claudio Battilocchio; Patrick Pasau; Steven V. Ley; Rapid Asymmetric Synthesis of Disubstituted Allenes by Coupling of Flow-Generated Diazo Compounds and Propargylated Amines. Angewandte Chemie 2017, 129, 1890-1894, 10.1002/ange.201611067.
- Yu Tang; Jian Xu; Jian Yang; Lili Lin; XiaoMing Feng; Xiaohua Liu; Asymmetric Three-Component Reaction for the Synthesis of Tetrasubstituted Allenoates via Allenoate-Copper Intermediates. Chem 2018, 4, 1658-1672, 10.1016/j.chempr.2018.04.012.
- Guangyang Xu; Zhen Wang; Ying Shao; Jiangtao Sun; Copper-Catalyzed Tandem Cross-Coupling and Alkynylogous Aldol Reaction: Access to Chiral Exocyclic α-Allenols. Organic Letters 2021, 23, 5175-5179, 10.1021/acs.orglett.1c01712.
- Shengming Ma; Electrophilic Addition and Cyclization Reactions of Allenes. Accounts of Chemical Research 2009, 42, 1679-1688, 10.1021/ar900153r.
- Marcus A. Tius; Cationic Cyclopentannelation of Allene Ethers. Accounts of Chemical Research 2003, 36, 284-290, 10.1021/ar0200394.
- Shengming Ma; Transition Metal-Catalyzed/Mediated Reaction of Allenes with a Nucleophilic Functionality Connected to the α-Carbon Atom. Accounts of Chemical Research 2003, 36, 701-712, 10.1021/ar020133k.
- José L. Mascareñas; Iván Varela; Fernando López; Allenes and Derivatives in Gold(I)- and Platinum(II)-Catalyzed Formal Cycloadditions. Accounts of Chemical Research 2019, 52, 465-479, 10.1021/acs.accounts.8b00567.
- Rémi Blieck; Marc Taillefer; Florian Monnier; Metal-Catalyzed Intermolecular Hydrofunctionalization of Allenes: Easy Access to Allylic Structures via the Selective Formation of C–N, C–C, and C–O Bonds. Chemical Reviews 2020, 120, 13545-13598, 10.1021/acs.chemrev.0c00803.
- Shengming Ma; Some Typical Advances in the Synthetic Applications of Allenes. Chemical Reviews 2005, 105, 2829-2872, 10.1021/cr020024j.
- José M. Alonso; Pedro Almendros; Deciphering the Chameleonic Chemistry of Allenols: Breaking the Taboo of a Onetime Esoteric Functionality. Chemical Reviews 2021, 121, 4193-4252, 10.1021/acs.chemrev.0c00986.
- Zhiming Wang; Xingzhu Xu; Ohyun Kwon; Phosphine catalysis of allenes with electrophiles. Chemical Society Reviews 2014, 43, 2927-2940, 10.1039/c4cs00054d.
- C. S. Adams; C. D. Weatherly; E. G. Burke; J. M. Schomaker; The conversion of allenes to strained three-membered heterocycles. Chemical Society Reviews 2014, 43, 3136-3163, 10.1039/c3cs60416k.
- Fei Ye; Yi Shi; Lei Zhou; Qing Xiao; Yan Zhang; Jianbo Wang; Expeditious Synthesis of Phenanthrenes via CuBr2-Catalyzed Coupling of Terminal Alkynes and N-Tosylhydrazones Derived from O-Formyl Biphenyls. Organic Letters 2011, 13, 5020-5023, 10.1021/ol201788v.
- Lei Zhou; Yi Shi; Qing Xiao; Yizhou Liu; Fei Ye; Yan Zhang; Jianbo Wang; CuBr-Catalyzed Coupling of N-Tosylhydrazones and Terminal Alkynes: Synthesis of Benzofurans and Indoles. Organic Letters 2011, 13, 968-971, 10.1021/ol103009n.
- Gullapalli Kumaraswamy; Neerasa Jayaprakash; Guniganti Balakishan; Cu(i)-catalyzed tandem benzyldiazoester coupling with terminal alkyne–allene formation–Michael reaction: Application to the syntheses of oxa and azacycles. Organic & Biomolecular Chemistry 2011, 9, 7913-7920, 10.1039/c1ob06128c.
- Kai Liu; Chenghao Zhu; Junxiang Min; Shiyong Peng; Guangyang Xu; Jiangtao Sun; Stereodivergent Synthesis of N-Heterocycles by Catalyst-Controlled, Activity-Directed Tandem Annulation of Diazo Compounds with Amino Alkynes. Angewandte Chemie International Edition 2015, 54, 12962-12967, 10.1002/anie.201507122.
- Danqing Ji; Kai Liu; Jiangtao Sun; Tandem Reaction of Allenoate Formation and Cyclization: Divergent Synthesis of Four- to Six-Membered Heterocycles. Organic Letters 2018, 20, 7708-7711, 10.1021/acs.orglett.8b03435.
- Chen-Liang Deng; Ren-Jie Song; Sheng-Mei Guo; Zhi-Qiang Wang; Jin-Heng Li; Copper/Silver-Cocatalyzed Conia-Ene Reaction of Linear β-Alkynic β-Ketoesters. Organic Letters 2007, 9, 5111-5114, 10.1021/ol7023289.
- Ting Yang; Alessandro Ferrali; Filippo Sladojevich; Leonie Campbell; Darren J. Dixon; Brønsted Base/Lewis Acid Cooperative Catalysis in the Enantioselective Conia-Ene Reaction. Journal of the American Chemical Society 2009, 131, 9140-9141, 10.1021/ja9004859.
- Gullapalli Kumaraswamy; Guniganti Balakishan; Copper(I)-Catalysed Domino Coupling and Cyclisation Reaction: A Mild, Expedient Route for the Synthesis of Indene and Dihydronaphthalene Derivatives. European Journal of Organic Chemistry 2015, 2015, 3141-3146, 10.1002/ejoc.201500284.
- Junxiang Min; Guangyang Xu; Jiangtao Sun; Synthesis of Six-Membered Carbo-/Heterocycles via Cascade Reaction of Alkynes and Diazo Compounds. The Journal of Organic Chemistry 2017, 82, 5492-5498, 10.1021/acs.joc.7b00758.
- V. Helan; A. V. Gulevich; V. Gevorgyan; Cu-catalyzed transannulation reaction of pyridotriazoles with terminal alkynes under aerobic conditions: efficient synthesis of indolizines. Chemical Science 2015, 6, 1928-1931, 10.1039/c4sc03358b.
- Qi Sun; Linge Li; Liyan Liu; Qianqian Guan; Yu Yang; Zhenggen Zha; Zhiyong Wang; Copper-Catalyzed Geminal Difunctionalization of Terminal Alkynes by Splitting Sulfonyl Hydrazones into Two Parts. Organic Letters 2018, 20, 5592-5596, 10.1021/acs.orglett.8b02268.
- Ziyong Li; Jiangtao Sun; Copper-Catalyzed 1,1-Boroalkylation of Terminal Alkynes: Access to Alkenylboronates via a Three-Component Reaction. Organic Letters 2021, 23, 3706-3711, 10.1021/acs.orglett.1c01081.
- Vincent N. G. Lindsay; Dominic Fiset; Philipp J. Gritsch; Soula Azzi; André B. Charette; Stereoselective Rh2(S-IBAZ)4-Catalyzed Cyclopropanation of Alkenes, Alkynes, and Allenes: Asymmetric Synthesis of Diacceptor Cyclopropylphosphonates and Alkylidenecyclopropanes. Journal of the American Chemical Society 2013, 135, 1463-1470, 10.1021/ja3099728.
- Takayuki Goto; Koji Takeda; Naoyuki Shimada; Hisanori Nambu; Masahiro Anada; Motoo Shiro; Dr. Kaori Ando; Dr. Shunichi Hashimoto; Highly Enantioselective Cyclopropenation Reaction of 1-Alkynes with α-Alkyl-α-Diazoesters Catalyzed by Dirhodium(II) Carboxylates. Angewandte Chemie 2011, 123, 6935-6940, 10.1002/ange.201101905.
- Jin Gong; Zhensheng Zhao; Fuming Zhang; Songyun Wu; Guangqi Yan; Yingjie Quan; Baochun Ma; One-Pot Novel Regioselective Cycloisomerization Synthesis of 2-Substituted or 3-Substituted 4H-Furo[3,2-c]chromene through the Intermediate Cyclopropenes of 3-Diazochroman-4-one and Phenylacetylene. Organic Letters 2014, 16, 5524-5527, 10.1021/ol502854y.
- John F. Briones; Huw M.L. Davies; Rh2(S-PTAD)4-catalyzed asymmetric cyclopropenation of aryl alkynes. Tetrahedron 2011, 67, 4313-4317, 10.1016/j.tet.2011.04.029.
- Zhi-Qi Zhang; Meng-Meng Zheng; Xiao-Song Xue; Ilan Marek; Fa-Guang Zhang; Jun-An Ma; Catalytic Enantioselective Cyclopropenation of Internal Alkynes: Access to Difluoromethylated Three‐Membered Carbocycles. Angewandte Chemie International Edition 2019, 58, 18191-18196, 10.1002/anie.201911701.
- Xin Cui; Xue Xu; Hongjian Lu; Shifa Zhu; Lukasz Wojtas; X. Peter Zhang; Enantioselective Cyclopropenation of Alkynes with Acceptor/Acceptor-Substituted Diazo Reagents via Co(II)-Based Metalloradical Catalysis. Journal of the American Chemical Society 2011, 133, 3304-3307, 10.1021/ja111334j.
- John F. Briones; Huw M. L. Davies; Gold(I)-Catalyzed Asymmetric Cyclopropenation of Internal Alkynes. Journal of the American Chemical Society 2012, 134, 11916-11919, 10.1021/ja304506g.
- John F. Briones; Huw M. L. Davies; Silver Triflate-Catalyzed Cyclopropenation of Internal Alkynes with Donor-/Acceptor-Substituted Diazo Compounds. Organic Letters 2011, 13, 3984-3987, 10.1021/ol201503j.
- Zhaohong Liu; Qiangqiang Li; Peiqiu Liao; Xihe Bi; Silver-Catalyzed [2+1] Cyclopropenation of Alkynes with Unstable Diazoalkanes:N-Nosylhydrazones as Room-Temperature Decomposable Diazo Surrogates. Chemistry – A European Journal 2017, 23, 4756-4760, 10.1002/chem.201605335.
- Longrui Chen; Devonna Leslie; Michael G. Coleman; James Mack; Recyclable heterogeneous metal foil-catalyzed cyclopropenation of alkynes and diazoacetates under solvent-free mechanochemical reaction conditions. Chemical Science 2018, 9, 4650-4661, 10.1039/c8sc00443a.
- Misaki Uehara; Hidehiro Suematsu; Yoichi Yasutomi; Tsutomu Katsuki; Enantioenriched Synthesis of Cyclopropenes with a Quaternary Stereocenter, Versatile Building Blocks. Journal of the American Chemical Society 2010, 133, 170-171, 10.1021/ja1089217.
- María J. González; Luis A. López; Rubén Vicente; Zinc-Catalyzed Cyclopropenation of Alkynes via 2-Furylcarbenoids. Organic Letters 2014, 16, 5780-5783, 10.1021/ol502848n.
- Héctor Martínez-García; Dolores Morales; Julio Pérez; Marcos Puerto; Daniel Miguel; 1,3,5-Tris(thiocyanatomethyl)mesitylene as a Ligand. Pseudooctahedral Molybdenum, Manganese, and Rhenium Carbonyl Complexes and Copper and Silver Dimers. Copper-Catalyzed Carbene- and Nitrene-Transfer Reactions. Inorganic Chemistry 2010, 49, 6974-6985, 10.1021/ic1006082.
- Anurag Noonikara-Poyil; Shawn G. Ridlen; H. V. Rasika Dias; Isolable Copper(I) η2-Cyclopropene Complexes. Inorganic Chemistry 2020, 59, 17860-17865, 10.1021/acs.inorgchem.0c02886.
- Thomas J. Thomas; Benjamin A. Merritt; Betsegaw E. Lemma; Adina M. McKoy; Tri Nguyen; Andrew K. Swenson; Jeffrey L. Mills; Michael G. Coleman; Cyclopropenation of internal alkynylsilanes and diazoacetates catalyzed by copper(i) N-heterocyclic carbene complexes. Organic & Biomolecular Chemistry 2016, 14, 1742-1747, 10.1039/c5ob02259b.
- Carlos Saá; Damián Padín; Jesús A. Varela; Recent Advances in Ruthenium-Catalyzed Carbene/Alkyne Metathesis (CAM) Transformations. Synlett 2020, 31, 1147-1157, 10.1055/s-0039-1690861.
- Soumen Dey; Suman De Sarkar; Synthetic Applications of Vinyl Ruthenium Carbenes Derived from Diazoalkanes and Alkynes. Advanced Synthesis & Catalysis 2017, 359, 2709-2722, 10.1002/adsc.201700622.
- Chao Pei; Cheng Zhang; Yu Qian; Xinfang Xu; Catalytic carbene/alkyne metathesis (CAM): a versatile strategy for alkyne bifunctionalization. Organic & Biomolecular Chemistry 2018, 16, 8677-8685, 10.1039/c8ob02420k.
- Yu Qian; Charles S. Shanahan; Michael P. Doyle; Templated Carbene Metathesis Reactions from the Modular Assembly of Enol-diazo Compounds and Propargyl Acetates. European Journal of Organic Chemistry 2013, 2013, 6032-6037, 10.1002/ejoc.201301000.
- Alexis Archambeau; Frédéric Miege; Christophe Meyer; Janine Cossy; Intramolecular Cyclopropanation and C–H Insertion Reactions with Metal Carbenoids Generated from Cyclopropenes. Accounts of Chemical Research 2015, 48, 1021-1031, 10.1021/acs.accounts.5b00016.
- Albert Padwa; Thomas J. Blacklock; Roman Loza; Silver-promoted isomerizations of some cyclopropene derivatives. Journal of the American Chemical Society 1981, 103, 2404-2405, 10.1021/ja00399a042.
- Albert Padwa; Simon L. Xu; A new phenol synthesis from the rhodium (I) catalyzed reaction of cyclopropenes and alkynes. Journal of the American Chemical Society 1992, 114, 5881-5882, 10.1021/ja00040a072.
- Fermín Cambeiro; Susana López; Jesús A. Varela; Carlos Saá; Cyclization by Catalytic Ruthenium Carbene Insertion into C sp 3-H Bonds. Angewandte Chemie International Edition 2011, 51, 723-727, 10.1002/anie.201107344.
- Thomas R. Hoye; Christopher J. Dinsmore; Rhodium(II) acetate catalyzed alkyne insertion reactions of .alpha.-diazo ketones: mechanistic inferences. Journal of the American Chemical Society 1991, 113, 4343-4345, 10.1021/ja00011a055.
- Kuiyong Dong; Chao Pei; Qian Zeng; Hanlin Wei; Michael P. Doyle; Xinfang Xu; Selective C(sp3)–H Bond Insertion in Carbene/Alkyne Metathesis Reactions. Enantioselective Construction of Dihydroindoles. ACS Catalysis 2018, 8, 9543-9549, 10.1021/acscatal.8b02822.
- Kuiyong Dong; Xing Fan; Chao Pei; Yang Zheng; Sailan Chang; Ju Cai; Lihua Qiu; Zhi-Xiang Yu; Xinfang Xu; Transient-axial-chirality controlled asymmetric rhodium-carbene C(sp2)-H functionalization for the synthesis of chiral fluorenes. Nature Communications 2020, 11, 1-10, 10.1038/s41467-020-16098-8.
- Patricia Panne; Joseph M. Fox; Rh-Catalyzed Intermolecular Reactions of Alkynes with α-Diazoesters That Possess β-Hydrogens: Ligand-Based Control over Divergent Pathways. Journal of the American Chemical Society 2006, 129, 22-23, 10.1021/ja0660195.
- Yike Ni And; John Montgomery; Synthetic Studies and Mechanistic Insight in Nickel-Catalyzed [4+2+1] Cycloadditions. Journal of the American Chemical Society 2006, 128, 2609-2614, 10.1021/ja057741q.
- Fermín Cambeiro; Susana López; Jesús A. Varela; Carlos Saá; Vinyl Dihydropyrans and Dihydrooxazines: Cyclizations of Catalytic Ruthenium Carbenes Derived from Alkynals and Alkynones. Angewandte Chemie International Edition 2014, 53, 5959-5963, 10.1002/anie.201400675.
- Likai Xia; Yong Rok Lee; ChemInform Abstract: Regioselective Synthesis of Highly Functionalized Furans Through the RuII-Catalyzed [3 + 2] Cycloaddition of Diazodicarbonyl Compounds.. ChemInform 2015, 46, 3430-3442, 10.1002/chin.201506028.
- Daria Kurandina; Vladimir Gevorgyan; Rhodium Thiavinyl Carbenes from 1,2,3-Thiadiazoles Enable Modular Synthesis of Multisubstituted Thiophenes. Organic Letters 2016, 18, 1804-1807, 10.1021/acs.orglett.6b00541.
- Xin Cui; Xue Xu; Lukasz Wojtas; Martin M. Kim; X. Peter Zhang; Regioselective Synthesis of Multisubstituted Furans via Metalloradical Cyclization of Alkynes with α-Diazocarbonyls: Construction of Functionalized α-Oligofurans. Journal of the American Chemical Society 2012, 134, 19981-19984, 10.1021/ja309446n.
- Anton V. Gulevich; Alexander S. Dudnik; Natalia Chernyak; Vladimir Gevorgyan; Transition Metal-Mediated Synthesis of Monocyclic Aromatic Heterocycles. Chemical Reviews 2013, 113, 3084-3213, 10.1021/cr300333u.
- Mohammad Lokman Hossain; Fei Ye; Yan Zhang; Jianbo Wang; Cu(I)-catalyzed reaction of diazo compounds with terminal alkynes: a direct synthesis of trisubstituted furans. Tetrahedron 2014, 70, 6957-6962, 10.1016/j.tet.2014.07.086.
- Andrew K. Swenson; Kate E. Higgins; Matthew G. Brewer; William W. Brennessel; Michael G. Coleman; Highly selective synthesis of tetra-substituted furans and cyclopropenes: copper(i)-catalyzed formal cycloadditions of internal aryl alkynes and diazoacetates. Organic & Biomolecular Chemistry 2012, 10, 7483-7486, 10.1039/c2ob26295a.
- Cheng Zhang; Sailan Chang; Lihua Qiu; Xinfang Xu; Chemodivergent synthesis of multi-substituted/fused pyrroles via copper-catalyzed carbene cascade reaction of propargyl α-iminodiazoacetates. Chemical Communications 2016, 52, 12470-12473, 10.1039/c6cc06864b.
- Ruwei Yao; Guangwei Rong; Bin Yan; Lihua Qiu; Xinfang Xu; Dual-Functionalization of Alkynes via Copper-Catalyzed Carbene/Alkyne Metathesis: A Direct Access to the 4-Carboxyl Quinolines. ACS Catalysis 2016, 6, 1024-1027, 10.1021/acscatal.5b02648.
- Wen-Bo Shen; Qing Sun; Long Li; Xin Liu; Bo Zhou; Juanzhu Yan; Long-Wu Ye; Divergent synthesis of N-heterocycles via controllable cyclization of azido-diynes catalyzed by copper and gold. Nature Communications 2017, 8, 1-9, 10.1038/s41467-017-01853-1.
- Huang Qiu; Yifan Deng; Kostiantyn O. Marichev; Michael P. Doyle; Diverse Pathways in Catalytic Reactions of Propargyl Aryldiazoacetates: Selectivity between Three Reaction Sites. The Journal of Organic Chemistry 2017, 82, 1584-1590, 10.1021/acs.joc.6b02770.
- Qian Zeng; Kuiyong Dong; Jingjing Huang; Lihua Qiu; Xinfang Xu; Copper-catalyzed carbene/alkyne metathesis terminated with the Buchner reaction: synthesis of dihydrocyclohepta[b]indoles. Organic & Biomolecular Chemistry 2019, 17, 2326-2330, 10.1039/c9ob00113a.
- Yang Zheng; Lihua Qiu; Kemiao Hong; Shanliang Dong; Xinfang Xu; Copper- or Thermally Induced Divergent Outcomes: Synthesis of 4-Methyl 2H -Chromenes and Spiro-4H -Pyrazoles. Chemistry - A European Journal 2017, 24, 6705-6711, 10.1002/chem.201704759.
- Shanliang Dong; Jingjing Huang; Hong-Kai Sha; Lihua Qiu; Wenhao Hu; Xinfang Xu; Copper-catalyzed formal [1 + 2 + 2]-annulation of alkyne-tethered diazoacetates and pyridines: access to polycyclic indolizines. Organic & Biomolecular Chemistry 2020, 18, 1926-1932, 10.1039/d0ob00222d.
