TBX1, located on chromosome 22q11.21, encodes a T-box transcription factor and is a candidate gene for DiGeorge syndrome (DGS) and velocardiofacial syndrome (VCFS). Studies of Tbx1-mutant mice have provided insights into the underlying pathogenesis of DGS/VCFS and the knowledge to diagnose patients with DGS/VCFS. Genes, miRNAs, and epigenetics could change Tbx1 expression. Polymorphisms, variations, and mutations in TBX1 may induce the penetrance and severity of DGS/VCFS-like craniofacial phenotypes. The molecular basis of the variant sequence of TBX1 will further define how TBX1 contributes to the craniofacial and other phenotypes of DGS/VCFS. Since interactions with TBX1 and other molecules in transcriptional complexes or chromatin remodeling are crucial for TBX1 function, identifying and understanding these genetic and epigenetic modifiers individually for each patient may direct therapeutics to minimize the severity.
- 22q11.2 deletion syndrome
- DiGeorge syndrome
- velocardiofacial syndrome
- cleft palate
- skull base
1. Introduction
2. Craniofacial Phenotypes of Patients with DGS/VCFS
| Phenotypes | Features | Frequency | |||||
|---|---|---|---|---|---|---|---|
| Palatal anomalies | Overt cleft palate | 7–11% | |||||
| Submucous cleft palate | 5–23% | ||||||
| Bifid uvula | 5–10% | ||||||
| Velopharyngeal insufficiency | 27–92% | ||||||
| Dental anomalies | Tooth agenesis | 15% | |||||
| Hypoplasia of primary teeth | 32% | ||||||
| Hypoplasia of permanent teeth | 10% | ||||||
| Enamel hypomineralization of primary teeth | 39% | ||||||
| Enamel hypomineralization of permanent teeth | 41% | ||||||
| [ | 5 | ] | Ear-nose-throat abnormalities | Hearing loss | 33–39% | ||
| c.967_977dup AACCCCGTGGC | C-terminal | Thymic hypoplasia Postaxial polydactyly of the right fifth toe |
No | [4038] | Otitis media with effusion | 2% | |
| c.1158_1159delinsT | C-terminal | Hypoparathyroidism and hypocalcemia | Tracheomalacia/laryngomalacia | 2% | |||
| Laryngeal web | 1% | ||||||
| Ocular abnormalities | Hooding of the upper lid | 41% | |||||
| Ptosis | 9% | ||||||
| Hooding of the lower lid | 6% | ||||||
| Epicanthal folds | 3% | ||||||
| Distichiasis | 3% | ||||||
| Cranial base anomalies | Platybasia | 50–91% | |||||
| Basilar impression | 3% | ||||||
| Cervical spine anomalies | Atlas (C1) anomalies | 75% | |||||
| Axis (C2) anomalies | 59% | ||||||
| Fusion of C2–C3 | 34% |
| DGS/VCFS | Tbx1-Null Mice | |||
|---|---|---|---|---|
| Cranium | Dolichocephaly | Small cranium | ||
| Abnormal skull morphology | Hypoplastic parietal bone | |||
| Malar flattening | Hypoplastic interparietal bone | |||
| Long face | Unfused cranial sutures between frontal and parietal bones | |||
| Temporal bone hypoplasia | ||||
| Absent zygomatic arch | ||||
| Abnormal zygomatic arch morphology | ||||
| Cranial Base | Platybasia | Abnormal fusion of the basioccipital and basisphenoid bones | ||
| Basilar impression | Abnormal presphenoid bone morphology | |||
| Abnormal basioccipital bone morphology | ||||
| Palate | Cleft palate | Cleft palate | ||
| Facial asymmetry | Deafness | Yes | [4139] | |
| c.1223delC | C-terminal | Conotruncal anomaly face syndrome Velocardiofacial syndrome |
Yes | [5] |
| c.1253delA | C-terminal | DiGeorge syndrome | Yes | [4240] |
| c.1320-1342del23bp | C-terminal | Velocardiofacial syndrome | Yes/No | [4341] |
| c.1399-1428dup30 | C-terminal | Tetralogy of Fallot Scoliosis Facial asymmetry Upslanting palpebral fissures Absent pulmonary valve Isolated left pulmonary artery |
Yes | [4442] |
3.2. DiGeorge Syndrome Critical Region (DGCR)
3.3. MicroRNAs
4. Current Insightaniofacial Phenotypes of DGS/VCFS Mouse Models
ThMouse penetrance and severity of congenital anomalies are related to genetic and environmental factors. Recent studies have revealed the function of TBX1 and models with DGS/VCFS help identify additional candidate genes or modifier genes that impact the severity and nfluence the penetrance of DGS/VCFS. Studiesand/or severity of DGS/VCFS mouse models have provided insights into signaling pathways and genes that interact with TBX1-related phenotypes. According to the mouse genome informatics (MGI) database (http://www.informatics.jax.org anccessed/or affect the on 3 August 2021), DGS/VCFS phenotypes. In addition, mouse models with DGS/VCFS may help the researchers to identify additional DGS/VCFS-related-related anomalies concerning Tbx1, Chrd, Tgfbr2, Vegfa, Fgf8, Crkl, Aldh1a2/Raldh2, Hoxa3, Kat6a/Moz/Myst3, Dicer1, Plxnd1, Dock1, Ndst1, Prickle1, Trappc10, Zfp366, and Foxn1 have been reported in genetically altered mice (Table 4). pWhenotypes. For example, there is potential to examine the phenotypes of these genes were analyzed according to biological process, “heart morphogenesis” and “cranial synchondroses, cranium, zygomatkeletal system development” were enriched. Our enrichment analysis using ToppCluster [49] indic arches, and pharyngeal muscles inated that genes associated with DGS/VCFS patients. The researchers also noted that ihenotypes in mice are specifically enriched in the morphogenesis of craniofacial tissues and heart (Figure 2A). Infotermation about ocular phenotypes inestingly, among these genes, only Tbx1-mut antd Chrd were specifically menrice is limited, although theshed in the morphogenesis of cricoid and thyroid cartilages (Figure 2A). Ge anomalies in patientses associated with DGS/VCFS have been reporphenotypes in mice also indicated [16][17].that DGS/VCrosstalk with key embryonicFS-related phenotypes involve the interaction of several signals, especiallying pathways, including bone morphogenetic protein (BMP), transforming growth factor (TGF)β, vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), Rand retinoic Acid, and Sonic Hedgehog (SHH), critically regulates DGS/VCFS-related pharyngeal developmentacid signaling pathways (Figure 2B). Genes involved in these signalinggenetic pathways may modify of theTbx1 phenotypic spectrum of DGS/VCFS. Given the broad spectrum of DGS/VCFS disease phenotypes, other genes essential to craniofacial development could modify the phenotypic spectrum. Genetically engineered mice are useful for studying disease re likely to induce phenotypes; however, ablation of essential genes involved in cardiovascular similar to developmeTbx1-nt may cause early embryonic lethalityll mice (Figure 2B, whichTable 4). would prevent observation of craniofacial pThenotypes. For example, ablation of Ufd1, whose human ose artholog has been mapped to the 1.5 Mb region, causes early embryonic lethality before organogenesis in mice described below.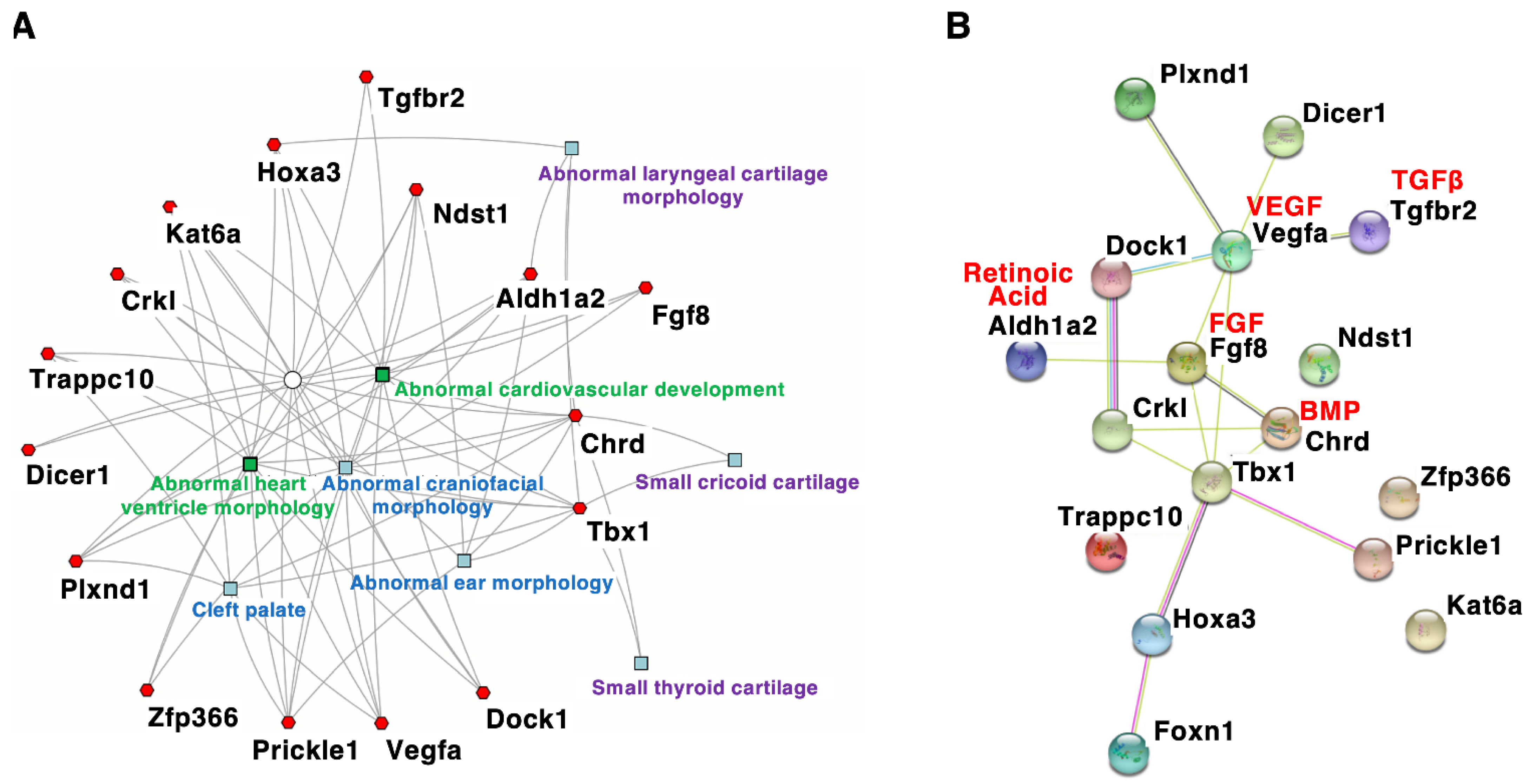
| Gene Symbol | Induced Mutation Type | Cranium | Palate | Teeth | Muscles | Ear-Nose-Throat | Hyoid Bones | Cardio-Vascular | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tbx1 | Null | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| Chrd | Null | Yes | Yes | nr | nr | Yes | Yes | Yes | |||
| Tgfbr2 | Deletion (Wnt1-Cre) | Yes | Yes | nr | nr | nr | nr | Yes | |||
| Vegfa | Null | Yes | Yes | Yes | nr | nr | nr | Yes | |||
| Fgf8 | Hypomorphic allele | Yes | Yes | Yes | nr | Yes | Yes | Yes | |||
| Crkl | Null | Yes | nr | nr | nr | Yes | nr | Yes | |||
| Aldh1a2 | Hypomorphic allele | nr | nr | nr | nr | Yes | Yes | Yes | |||
| Hoxa3 | Null | nr | Yes | nr | Yes | Yes | Yes | Yes | |||
| Kat6a | Null | nr | Yes | nr | nr | Yes | nr | Yes | |||
| Dicer1 | Deletion (Wnt1-Cre) | Yes | nr | nr | nr | nr | nr | Yes | |||
| Plxnd1 | Single point mutation | nr | Yes | nr | nr | Yes | nr | Yes | Submucous cleft palate | Submucous cleft palate | |
| Dock1 | Undefined | nr | nr | nr | nr | Yes | nr | Yes | Bifid uvula | Bifid uvula | |
| Ndst1 | Single point mutation | nr | nr | nr | nr | Yes | nr | Yes | Highly arched palate | ||
| Prickle1 | Single point mutation | Yes | Yes | nr | nr | Yes | nr | Yes | Velopharyngeal insufficiency | ||
| Trappc10 | Undefined | Yes | Yes | nr | nr | nr | nr | Yes | Mandible | Retrognathia | Absent mandibular coronoid process |
| Zfp366 | Single point mutation | nr | nr | nr | nr | Yes | nr | Yes | Short mandible | Short mandible | |
| Foxn1 | Intragenic deletion | nr | nr | nr | nr | Yes | nr | Yes | Micrognathia | Micrognathia | |
| Teeth | Enamel hypoplasia | Abnormal upper incisor morphology | |||||||||
| Single central incisor | Absent upper incisors | ||||||||||
| Small teeth | |||||||||||
| Abnormality of the dentition | |||||||||||
| Carious teeth | |||||||||||
| Muscles | Pharyngeal hypotonia | Absent masseter muscle | |||||||||
| Absent pterygoid muscle | |||||||||||
| Absent temporalis muscle | |||||||||||
| Eyes | Hypertelorism/telecanthus | Hypertelorism | |||||||||
| Downslanted palpebral fissures | |||||||||||
| Proptosis | |||||||||||
| Strabismus | |||||||||||
| Abnormal eyelid morphology | |||||||||||
| Epicanthus | |||||||||||
| Microphthalmia | |||||||||||
| External Ears | Small earlobe | Ear lobe hypoplasia | |||||||||
| Low-set ears | Lowered ear position | ||||||||||
| Abnormally folded pinna | Abnormal ear shape | ||||||||||
| Preauricular pit | Absent outer ear | ||||||||||
| Anotia | |||||||||||
| Middle and Inner Ears | Chronic otitis media | Abnormal middle ear ossicle morphology | |||||||||
| Conductive hearing loss | Absent middle ear ossicles | ||||||||||
| Sensorineural hearing loss | Abnormal stapes morphology | ||||||||||
| Auditory canal stenosis | Abnormal incus morphology | ||||||||||
| Pulsatile tympanic membrane | Abnormal malleus morphology | ||||||||||
| Thickened tympanic membrane | Absent stapes | ||||||||||
| Tympanic membrane retraction | Abnormal external auditory canal morphology | ||||||||||
| Decreased tympanic ring size | |||||||||||
| Nose | Prominent nasal bridge | Short snout | |||||||||
| Abnormal nasal morphology | |||||||||||
| Underdeveloped nasal alae | |||||||||||
| Choanal atresia | |||||||||||
| Throat | Abnormal thorax morphology | Small thyroid cartilage | |||||||||
| Abnormality of the pharynx | Small cricoid cartilage | ||||||||||
| Abnormal thyroid cartilage morphology | |||||||||||
| Pharynx hypoplasia | |||||||||||
| Hyoid bones | Delayed development of the hyoid bone | Hyoid bone hypoplasia | |||||||||
| Invisible hyoid ossification center | Abnormal hyoid bone morphology | ||||||||||
| Cervical spine | Dysmorphic C1 | Abnormal cervical atlas (C1) morphology | |||||||||
| Anterior arch cleft of C1 | Absent arcus anterior of C1 | ||||||||||
| Open posterior arch C1 | |||||||||||
| Fusion of C1–C2 | |||||||||||
| Fusion of C2–C3 | |||||||||||
| Upswept C2 lamina | |||||||||||
| Platyspondyly | |||||||||||
| Others | Short clavicle | ||||||||||
| References | [14][15][16][17][18][19][20][21][22][23][24][25][26][27][28] | [6][7][8][9][10][2927][3028][3129][3230][3331][3432][3533][3634] |
3. Genetics of DGS/VCFS
3.1. TBX1 Gene
| Mutation | Domain | Condition | Craniofacial Anomalies | References |
|---|---|---|---|---|
| c.89_284del | N-terminal | DiGeorge syndrome | Yes | ClinVar Variant: 971780 |
| c.199_224del | N-terminal | DiGeorge syndrome | Yes | ClinVar Variant: 949172 |
| c.292A>T | N-terminal | DiGeorge syndrome | Yes | ClinVar Variant: 526036 |
| c.385G>A | T-box | Tetralogy of Fallot | No | ClinVar Variant: 488618 |
| c.443T>A (F148Y) | T-box | Conotruncal anomaly face syndrome | Yes | [5] |
| c.503T>C | T-box | DiGeorge syndrome Velocardiofacial syndrome (Shprintzen syndrome) Tetralogy of Fallot |
Yes | ClinVar Variant: 973222 |
| c.569C > A (P190Q) | T-box | Congenital heart defects | No | [3836] |
| c.582C>G (H194Q) | T-box | Velocardiofacial syndrome | Yes | [3937] |
| c.928G>A (G310S) | C-terminal | DiGeorge syndrome | Yes |
References
- Tézenas Du Montcel, S.; Mendizabai, H.; Aymé, S.; Lévy, A.; Philip, N. Prevalence of 22q11 microdeletion. J. Med. Genet. 1996, 33, 719. Kaimal, V.; Bardes, E.E.; Tabar, S.C.; Jegga, A.G.; Aronow, B.J. ToppCluster: A multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res. 2010, 38, W96-102.
- Lopez-Rivera, E.; Liu, Y.P.; Verbitsky, M.; Anderson, B.R.; Capone, V.P.; Otto, E.A.; Yan, Z.; Mitrotti, A.; Martino, J.; Steers, N.J.; et al. Genetic Drivers of Kidney Defects in the DiGeorge Syndrome. N. Engl. J. Med. 2017, 376, 742–754. Jerome, L.A.; Papaioannou, V.E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet. 2001, 27, 286–291.
- Du, Q.; de la Morena, M.T.; van Oers, N.S.C. The Genetics and Epigenetics of 22q11.2 Deletion Syndrome. Front. Genet. 2019, 10, 1365. Lindsay, E.A.; Vitelli, F.; Su, H.; Morishima, M.; Huynh, T.; Pramparo, T.; Jurecic, V.; Ogunrinu, G.; Sutherland, H.F.; Scambler, P.J.; et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 2001, 410, 97–101.
- Gorlin, R.J.; Cohen, M.M., Jr.; Hennekam, R.C.M. Syndromes of the Head and the Neck; Oxford University Press: New York, NY, USA, 2001; pp. 850–853. Merscher, S.; Funke, B.; Epstein, J.A.; Heyer, J.; Puech, A.; Lu, M.M.; Xavier, R.J.; Demay, M.B.; Russell, R.G.; Factor, S.; et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell 2001, 104, 619–629.
- Yagi, H.; Furutani, Y.; Hamada, H.; Sasaki, T.; Asakawa, S.; Minoshima, S.; Ichida, F.; Joo, K.; Kimura, M.; Imamura, S.; et al. Role of TBX1 in human del22q11.2 syndrome. Lancet 2003, 362, 1366–1373. Hu, T.; Yamagishi, H.; Maeda, J.; McAnally, J.; Yamagishi, C.; Srivastava, D. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development 2004, 131, 5491–5502.
- Jerome, L.A.; Papaioannou, V.E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet. 2001, 27, 286–291. Liao, J.; Kochilas, L.; Nowotschin, S.; Arnold, J.S.; Aggarwal, V.S.; Epstein, J.A.; Brown, M.C.; Adams, J.; Morrow, B.E. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum. Mol. Genet. 2004, 13, 1577–1585.
- Lindsay, E.A.; Vitelli, F.; Su, H.; Morishima, M.; Huynh, T.; Pramparo, T.; Jurecic, V.; Ogunrinu, G.; Sutherland, H.F.; Scambler, P.J.; et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 2001, 410, 97–101. Zhang, Z.; Huynh, T.; Baldini, A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development 2006, 133, 3587–3595.
- Merscher, S.; Funke, B.; Epstein, J.A.; Heyer, J.; Puech, A.; Lu, M.M.; Xavier, R.J.; Demay, M.B.; Russell, R.G.; Factor, S.; et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell 2001, 104, 619–629. Arnold, J.S.; Werling, U.; Braunstein, E.M.; Liao, J.; Nowotschin, S.; Edelmann, W.; Hebert, J.M.; Morrow, B.E. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development 2006, 133, 977–987.
- Hu, T.; Yamagishi, H.; Maeda, J.; McAnally, J.; Yamagishi, C.; Srivastava, D. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development 2004, 131, 5491–5502. Arnold, J.S.; Braunstein, E.M.; Ohyama, T.; Groves, A.K.; Adams, J.C.; Brown, M.C.; Morrow, B.E. Tissue-specific roles of Tbx1 in the development of the outer, middle and inner ear, defective in 22q11DS patients. Hum. Mol. Genet. 2006, 15, 1629–1639.
- Liao, J.; Kochilas, L.; Nowotschin, S.; Arnold, J.S.; Aggarwal, V.S.; Epstein, J.A.; Brown, M.C.; Adams, J.; Morrow, B.E. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum. Mol. Genet. 2004, 13, 1577–1585. Choi, M.; Klingensmith, J. Chordin is a modifier of tbx1 for the craniofacial malformations of 22q11 deletion syndrome phenotypes in mouse. PLoS Genet. 2009, 5, e1000395.
- Baldini, A. Dissecting contiguous gene defects: TBX1. Curr. Opin. Genet. Dev. 2005, 15, 279–284. Moraes, F.; Novoa, A.; Jerome-Majewska, L.A.; Papaioannou, V.E.; Mallo, M. Tbx1 is required for proper neural crest migration and to stabilize spatial patterns during middle and inner ear development. Mech. Dev. 2005, 122, 199–212.
- Aggarwal, V.S.; Morrow, B.E. Genetic modifiers of the physical malformations in velo-cardio-facial syndrome/DiGeorge syndrome. Dev. Disabil. Res. Rev. 2008, 14, 19–25. Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Tbx1 regulates oral epithelial adhesion and palatal development. Hum. Mol. Genet. 2012, 21, 2524–2537.
- Papangeli, I.; Scambler, P. The 22q11 deletion: DiGeorge and velocardiofacial syndromes and the role of TBX1. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 393–403. Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Loss of Tbx1 induces bone phenotypes similar to cleidocranial dysplasia. Hum. Mol. Genet. 2015, 24, 424–435.
- Heliovaara, A.; Ranta, R.; Rautio, J. Pharyngeal morphology in children with submucous cleft palate with and without surgery. Eur. Arch. Oto-Rhino-Laryngol. Head Neck 2005, 262, 534–538. Funato, N.; Srivastava, D.; Shibata, S.; Yanagisawa, H. TBX1 Regulates Chondrocyte Maturation in the Spheno-occipital Synchondrosis. J. Dent. Res. 2020, 99, 1182–1191.
- Heliövaara, A.; Hurmerinta, K. Craniofacial cephalometric morphology in children with CATCH 22 syndrome. Orthod. Craniofac. Res. 2006, 9, 186–192. Bachiller, D.; Klingensmith, J.; Shneyder, N.; Tran, U.; Anderson, R.; Rossant, J.; De Robertis, E.M. The role of chordin/Bmp signals in mammalian pharyngeal development and DiGeorge syndrome. Development 2003, 130, 3567–3578.
- Ryan, A.K.; Goodship, J.A.; Wilson, D.I.; Philip, N.; Levy, A.; Seidel, H.; Schuffenhauer, S.; Oechsler, H.; Belohradsky, B.; Prieur, M.; et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: A European collaborative study. J. Med. Genet. 1997, 34, 798–804. Wurdak, H.; Ittner, L.M.; Lang, K.S.; Leveen, P.; Suter, U.; Fischer, J.A.; Karlsson, S.; Born, W.; Sommer, L. Inactivation of TGFβ signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev. 2005, 19, 530–535.
- McDonald-McGinn, D.M.; Kirschner, R.; Goldmuntz, E.; Sullivan, K.; Eicher, P.; Gerdes, M.; Moss, E.; Solot, C.; Wang, P.; Jacobs, I.; et al. The Philadelphia story: The 22q11.2 deletion: Report on 250 patients. Genet. Couns. 1999, 10, 11–24. Stalmans, I.; Lambrechts, D.; De Smet, F.; Jansen, S.; Wang, J.; Maity, S.; Kneer, P.; von der Ohe, M.; Swillen, A.; Maes, C.; et al. VEGF: A modifier of the del22q11 (DiGeorge) syndrome? Nat. Med. 2003, 9, 173–182.
- Klingberg, G.; Oskarsdóttir, S.; Johannesson, E.L.; Norén, J.G. Oral manifestations in 22q11 deletion syndrome. Int. J. Paediatr. Dent. 2002, 12, 14–23. Abu-Issa, R.; Smyth, G.; Smoak, I.; Yamamura, K.; Meyers, E.N. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 2002, 129, 4613–4625.
- Ricchetti, E.T.; States, L.; Hosalkar, H.S.; Tamai, J.; Maisenbacher, M.; McDonald-McGinn, D.M.; Zackai, E.H.; Drummond, D.S. Radiographic study of the upper cervical spine in the 22q11.2 deletion syndrome. J. Bone Joint Surg. Am. 2004, 86, 1751–1760. Guris, D.L.; Fantes, J.; Tara, D.; Druker, B.J.; Imamoto, A. Mice lacking the homologue of the human 22q11.2 gene CRLK phenocopy neurocristopathies of DiGeorge syndrome. Nat. Genet. 2001, 27, 293–298.
- Herman, S.B.; Guo, T.; McGinn, D.M.M.; Bolsa, A.; Shanske, A.L.; Bassett, A.S.; Chow, E.W.C.; Bowser, M.; Sheridan, M.; Beemer, F.; et al. Overt cleft palate phenotype and TBX1 genotype correlations in velo-cardio-facial/DiGeorge/22q11.2 deletion syndrome patients. Am. J. Med. Genet. A 2012, 158A, 2781–2787. Vermot, J.; Niederreither, K.; Garnier, J.M.; Chambon, P.; Dollé, P. Decreased embryonic retinoic acid synthesis results in a DiGeorge syndrome phenotype in newborn mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1763–1768.
- Hamidi, M.; Nabi, S.; Husein, M.; Mohamed, M.E.; Tay, K.Y.; McKillop, S. Cervical spine abnormalities in 22q11.2 deletion syndrome. Cleft Palate-Craniofacial J. 2014, 51, 230–233. Chisaka, O.; Capecchi, M.R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature 1991, 350, 473–479.
- Jackson, O.; Crowley, T.B.; Sharkus, R.; Smith, R.; Jeong, S.; Solot, C.; McDonald-Mcginn, D. Palatal evaluation and treatment in 22q11.2 deletion syndrome. Am. J. Med. Genet. Part A 2019, 179, 1184–1195. Voss, A.K.; Vanyai, H.K.; Collin, C.; Dixon, M.P.; McLennan, T.J.; Sheikh, B.N.; Scambler, P.; Thomas, T. MOZ Regulates the Tbx1 Locus, and Moz Mutation Partially Phenocopies DiGeorge Syndrome. Dev. Cell 2012, 23, 652–663.
- Bassett, A.S.; Chow, E.W.C.; Husted, J.; Weksberg, R.; Caluseriu, O.; Webb, G.D.; Gatzoulis, M.A. Clinical features of 78 adults with 22q11 Deletion Syndrome. Am. J. Med. Genet. A 2005, 138, 307–313. Sheehy, N.T.; Cordes, K.R.; White, M.P.; Ivey, K.N.; Srivastava, D. The neural crest-enriched microRNA miR-452 regulates epithelial-mesenchymal signaling in the first pharyngeal arch. Development 2010, 137, 4307–4316.
- Loos, E.; Verhaert, N.; Willaert, A.; Devriendt, K.; Swillen, A.; Hermans, R.; Op de Beeck, K.; Hens, G. Malformations of the middle and inner ear on CT imaging in 22q11 deletion syndrome. Am. J. Med. Genet. Part A 2016, 170, 2975–2983. Gershwin, M.E. DiGeorge syndrome: Congenital thymic hypoplasia. Animal model: Congenitally athymic (nude) mouse. Am. J. Pathol. 1977, 89, 809–812.
- Verheij, E.; Kist, A.L.; Mink van der Molen, A.B.; Stegeman, I.; van Zanten, G.A.; Grolman, W.; Thomeer, H.G.X.M. Otologic and audiologic findings in 22q11.2 deletion syndrome. Eur. Arch. Oto-Rhino-Laryngol. Head Neck 2017, 274, 765–771. Kobrynski, L.J.; Sullivan, K.E. Velocardiofacial syndrome, DiGeorge syndrome: The chromosome 22q11.2 deletion syndromes. Lancet 2007, 370, 1443–1452.
- Ford, L.C.; Sulprizio, S.L.; Rasgon, B.M. Otolaryngological manifestations of velocardiofacial syndrome: A retrospective review of 35 patients. Laryngoscope 2000, 110, 362–367. Oberoi, S.; Vargervik, K. Velocardiofacial syndrome with single central incisor. Am. J. Med. Genet. A 2005, 132A, 194–197.
- Kobrynski, L.J.; Sullivan, K.E. Velocardiofacial syndrome, DiGeorge syndrome: The chromosome 22q11.2 deletion syndromes. Lancet 2007, 370, 1443–1452. Zhang, Z.; Huynh, T.; Baldini, A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development 2006, 133, 3587–3595.
- Oberoi, S.; Vargervik, K. Velocardiofacial syndrome with single central incisor. Am. J. Med. Genet. A 2005, 132A, 194–197. Arnold, J.S.; Werling, U.; Braunstein, E.M.; Liao, J.; Nowotschin, S.; Edelmann, W.; Hebert, J.M.; Morrow, B.E. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development 2006, 133, 977–987.
- Zhang, Z.; Huynh, T.; Baldini, A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development 2006, 133, 3587–3595. Arnold, J.S.; Braunstein, E.M.; Ohyama, T.; Groves, A.K.; Adams, J.C.; Brown, M.C.; Morrow, B.E. Tissue-specific roles of Tbx1 in the development of the outer, middle and inner ear, defective in 22q11DS patients. Hum. Mol. Genet. 2006, 15, 1629–1639.
- Arnold, J.S.; Werling, U.; Braunstein, E.M.; Liao, J.; Nowotschin, S.; Edelmann, W.; Hebert, J.M.; Morrow, B.E. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development 2006, 133, 977–987. Choi, M.; Klingensmith, J. Chordin is a modifier of tbx1 for the craniofacial malformations of 22q11 deletion syndrome phenotypes in mouse. PLoS Genet. 2009, 5, e1000395.
- Arnold, J.S.; Braunstein, E.M.; Ohyama, T.; Groves, A.K.; Adams, J.C.; Brown, M.C.; Morrow, B.E. Tissue-specific roles of Tbx1 in the development of the outer, middle and inner ear, defective in 22q11DS patients. Hum. Mol. Genet. 2006, 15, 1629–1639. Moraes, F.; Novoa, A.; Jerome-Majewska, L.A.; Papaioannou, V.E.; Mallo, M. Tbx1 is required for proper neural crest migration and to stabilize spatial patterns during middle and inner ear development. Mech. Dev. 2005, 122, 199–212.
- Choi, M.; Klingensmith, J. Chordin is a modifier of tbx1 for the craniofacial malformations of 22q11 deletion syndrome phenotypes in mouse. PLoS Genet. 2009, 5, e1000395. Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Tbx1 regulates oral epithelial adhesion and palatal development. Hum. Mol. Genet. 2012, 21, 2524–2537.
- Moraes, F.; Novoa, A.; Jerome-Majewska, L.A.; Papaioannou, V.E.; Mallo, M. Tbx1 is required for proper neural crest migration and to stabilize spatial patterns during middle and inner ear development. Mech. Dev. 2005, 122, 199–212. Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Loss of Tbx1 induces bone phenotypes similar to cleidocranial dysplasia. Hum. Mol. Genet. 2015, 24, 424–435.
- Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Tbx1 regulates oral epithelial adhesion and palatal development. Hum. Mol. Genet. 2012, 21, 2524–2537. Funato, N.; Srivastava, D.; Shibata, S.; Yanagisawa, H. TBX1 Regulates Chondrocyte Maturation in the Spheno-occipital Synchondrosis. J. Dent. Res. 2020, 99, 1182–1191.
- Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Loss of Tbx1 induces bone phenotypes similar to cleidocranial dysplasia. Hum. Mol. Genet. 2015, 24, 424–435. Solot, C.B.; Sell, D.; Mayne, A.; Baylis, A.L.; Persson, C.; Jackson, O.; McDonald-McGinn, D.M. Speech-Language Disorders in 22q11.2 Deletion Syndrome: Best Practices for Diagnosis and Management. Am. J. Speech-Lang. Pathol. 2019, 28, 984–999.
- Funato, N.; Srivastava, D.; Shibata, S.; Yanagisawa, H. TBX1 Regulates Chondrocyte Maturation in the Spheno-occipital Synchondrosis. J. Dent. Res. 2020, 99, 1182–1191. Jaouadi, A.; Tabebi, M.; Abdelhedi, F.; Abid, D.; Kamoun, F.; Chabchoub, I.; Maatoug, S.; Doukali, H.; Belghuith, N.; Ksentini, M.A.; et al. A novel TBX1 missense mutation in patients with syndromic congenital heart defects. Biochem. Biophys. Res. Commun. 2018, 499, 563–569.
- Solot, C.B.; Sell, D.; Mayne, A.; Baylis, A.L.; Persson, C.; Jackson, O.; McDonald-McGinn, D.M. Speech-Language Disorders in 22q11.2 Deletion Syndrome: Best Practices for Diagnosis and Management. Am. J. Speech-Lang. Pathol. 2019, 28, 984–999. Zweier, C.; Sticht, H.; Aydin-Yaylagül, I.; Campbell, C.E.; Rauch, A. Human TBX1 Missense Mutations Cause Gain of Function Resulting in the Same Phenotype as 22q11.2 Deletions. Am. J. Hum. Genet. 2007, 80, 510–517.
- Jaouadi, A.; Tabebi, M.; Abdelhedi, F.; Abid, D.; Kamoun, F.; Chabchoub, I.; Maatoug, S.; Doukali, H.; Belghuith, N.; Ksentini, M.A.; et al. A novel TBX1 missense mutation in patients with syndromic congenital heart defects. Biochem. Biophys. Res. Commun. 2018, 499, 563–569. Hasegawa, K.; Tanaka, H.; Higuchi, Y.; Hayashi, Y.; Kobayashi, K.; Tsukahara, H. Novel heterozygous mutation in TBX1 in an infant with hypocalcemic seizures. Clin. Pediatr. Endocrinol. 2018, 27, 159–164.
- Zweier, C.; Sticht, H.; Aydin-Yaylagül, I.; Campbell, C.E.; Rauch, A. Human TBX1 Missense Mutations Cause Gain of Function Resulting in the Same Phenotype as 22q11.2 Deletions. Am. J. Hum. Genet. 2007, 80, 510–517. Alghamdi, M.; Al Khalifah, R.; Al Homyani, D.K.; Alkhamis, W.H.; Arold, S.T.; Ekhzaimy, A.; El-Wetidy, M.; Kashour, T.; Halwani, R. A novel TBX1 variant causing hypoparathyroidism and deafness. J. Endocr. Soc. 2020, 4, bvz028.
- Hasegawa, K.; Tanaka, H.; Higuchi, Y.; Hayashi, Y.; Kobayashi, K.; Tsukahara, H. Novel heterozygous mutation in TBX1 in an infant with hypocalcemic seizures. Clin. Pediatr. Endocrinol. 2018, 27, 159–164. Ogata, T.; Niihori, T.; Tanaka, N.; Kawai, M.; Nagashima, T.; Funayama, R.; Nakayama, K.; Nakashima, S.; Kato, F.; Fukami, M.; et al. TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS ONE 2014, 9, e91598.
- Alghamdi, M.; Al Khalifah, R.; Al Homyani, D.K.; Alkhamis, W.H.; Arold, S.T.; Ekhzaimy, A.; El-Wetidy, M.; Kashour, T.; Halwani, R. A novel TBX1 variant causing hypoparathyroidism and deafness. J. Endocr. Soc. 2020, 4, bvz028. Paylor, R.; Glaser, B.; Mupo, A.; Ataliotis, P.; Spencer, C.; Sobotka, A.; Sparks, C.; Choi, C.H.; Oghalai, J.; Curran, S.; et al. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: Implications for 22q11 deletion syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 7729–7734.
- Ogata, T.; Niihori, T.; Tanaka, N.; Kawai, M.; Nagashima, T.; Funayama, R.; Nakayama, K.; Nakashima, S.; Kato, F.; Fukami, M.; et al. TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS ONE 2014, 9, e91598. Rauch, R.; Hofbeck, M.; Zweier, C.; Koch, A.; Zink, S.; Trautmann, U.; Hoyer, J.; Kaulitz, R.; Singer, H.; Rauch, A. Comprehensive genotype-phenotype analysis in 230 patients with tetralogy of Fallot. J. Med. Genet. 2010, 47, 321–331.
- Paylor, R.; Glaser, B.; Mupo, A.; Ataliotis, P.; Spencer, C.; Sobotka, A.; Sparks, C.; Choi, C.H.; Oghalai, J.; Curran, S.; et al. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: Implications for 22q11 deletion syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 7729–7734. Stark, K.L.; Xu, B.; Bagchi, A.; Lai, W.-S.; Liu, H.; Hsu, R.; Wan, X.; Pavlidis, P.; Mills, A.A.; Karayiorgou, M.; et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat. Genet. 2008, 40, 751–760.
- Rauch, R.; Hofbeck, M.; Zweier, C.; Koch, A.; Zink, S.; Trautmann, U.; Hoyer, J.; Kaulitz, R.; Singer, H.; Rauch, A. Comprehensive genotype-phenotype analysis in 230 patients with tetralogy of Fallot. J. Med. Genet. 2010, 47, 321–331. Edelmann, L.; Stankiewicz, P.; Spiteri, E.; Pandita, R.K.; Shaffer, L.; Lupski, J.R.; Morrow, B.E.; Lupski, J. Two functional copies of the DGCR6 gene are present on human chromosome 22q11 due to a duplication of an ancestral locus. Genome Res. 2001, 11, 208–217.
- Stark, K.L.; Xu, B.; Bagchi, A.; Lai, W.-S.; Liu, H.; Hsu, R.; Wan, X.; Pavlidis, P.; Mills, A.A.; Karayiorgou, M.; et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat. Genet. 2008, 40, 751–760. Hierck, B.P.; Molin, D.G.M.; Boot, M.J.; Poelmann, R.E.; Gittenberger-De Groot, A.C. A chicken model for DGCR6 as a modifier gene in the DiGeorge critical region. Pediatr. Res. 2004, 56, 440–448.
- Edelmann, L.; Stankiewicz, P.; Spiteri, E.; Pandita, R.K.; Shaffer, L.; Lupski, J.R.; Morrow, B.E.; Lupski, J. Two functional copies of the DGCR6 gene are present on human chromosome 22q11 due to a duplication of an ancestral locus. Genome Res. 2001, 11, 208–217. Gao, S.; Moreno, M.; Eliason, S.; Cao, H.; Li, X.; Yu, W.; Bidlack, F.B.; Margolis, H.C.; Baldini, A.; Amendt, B.A. TBX1 protein interactions and microRNA-96-5p regulation controls cell proliferation during craniofacial and dental development: Implications for 22q11.2 deletion syndrome. Hum. Mol. Genet. 2015, 24, 2330–2348.
- Hierck, B.P.; Molin, D.G.M.; Boot, M.J.; Poelmann, R.E.; Gittenberger-De Groot, A.C. A chicken model for DGCR6 as a modifier gene in the DiGeorge critical region. Pediatr. Res. 2004, 56, 440–448. Sun, H.; Jiang, P. MicroRNA-451a acts as tumor suppressor in cutaneous basal cell carcinoma. Mol. Genet. Genom. Med. 2018, 6, 1001–1009.
- Gao, S.; Moreno, M.; Eliason, S.; Cao, H.; Li, X.; Yu, W.; Bidlack, F.B.; Margolis, H.C.; Baldini, A.; Amendt, B.A. TBX1 protein interactions and microRNA-96-5p regulation controls cell proliferation during craniofacial and dental development: Implications for 22q11.2 deletion syndrome. Hum. Mol. Genet. 2015, 24, 2330–2348. Wang, J.; Bai, Y.; Li, H.; Greene, S.B.; Klysik, E.; Yu, W.; Schwartz, R.J.; Williams, T.J.; Martin, J.F. MicroRNA-17-92, a direct Ap-2alpha transcriptional target, modulates T-box factor activity in orofacial clefting. PLoS Genet. 2013, 9, e1003785.
- Sun, H.; Jiang, P. MicroRNA-451a acts as tumor suppressor in cutaneous basal cell carcinoma. Mol. Genet. Genom. Med. 2018, 6, 1001–1009.
- Wang, J.; Bai, Y.; Li, H.; Greene, S.B.; Klysik, E.; Yu, W.; Schwartz, R.J.; Williams, T.J.; Martin, J.F. MicroRNA-17-92, a direct Ap-2alpha transcriptional target, modulates T-box factor activity in orofacial clefting. PLoS Genet. 2013, 9, e1003785.
- Lindsay, E.A.; Botta, A.; Jurecic, V.; Carattini-Rivera, S.; Cheah, Y.C.; Rosenblatt, H.M.; Bradley, A.; Baldini, A. Congenital heart disease in mice deficient for the DiGeorge syndrome region. Nature 1999, 401, 379–383.
