The glyoxalase system was discovered over a hundred years ago and since then it has been claimed to provide the role of an indispensable enzyme system in order to protect cells from a toxic byproduct of glycolysis. This review gives a broad overview of what has been postulated in the last 30 years of glyoxalase research, but within this context it also challenges the concept that the glyoxalase system is an exclusive tool of detoxification and that its substrate, methylglyoxal, is solely a detrimental burden for every living cell due to its toxicity. An overview of consequences of a complete loss of the glyoxalase system in various model organisms is presented with an emphasis on the role of alternative detoxification pathways of methylglyoxal. Furthermore, this review focuses on the overlooked posttranslational modification of Glyoxalase 1 and its possible implications for cellular maintenance under various (patho-)physiological conditions. As a final note, an intriguing point of view for the substrate methylglyoxal is offered, the concept of methylglyoxal (MG)-mediated hormesis.
- Glyoxalase 1
- methylglyoxal
- glycation
1. Introduction
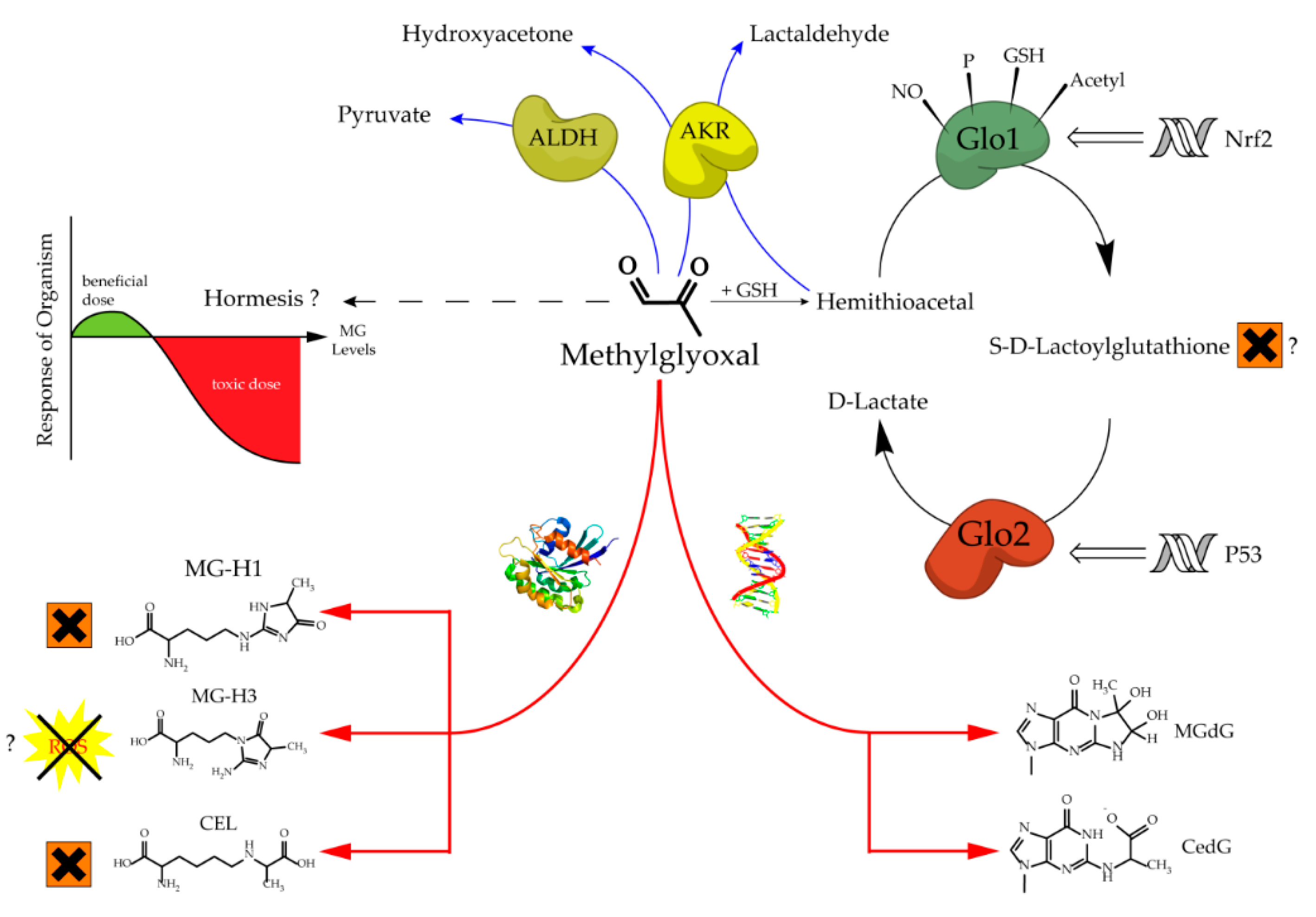
The glyoxalase system, which was first described over a hundred years ago, consists of two cooperating enzymes named Glyoxalase 1 (Glo1) and Glyoxalase 2 (Glo2) [1][2][1,2]. It is a highly conserved enzyme system, which exists in every living cell from crude single prokaryotes up to complex mammalian organisms [3][4][3,4]. The initial enzyme of the glyoxalase system is glyoxalase 1 and its substrate, hemithioacetal, is formed by a spontaneous reaction of methylglyoxal (MG) and glutathione (GSH) (Figure 1). MG is highly reactive and therefore a toxic compound, but also an inevitable by-product of glycolysis and gluconeogenesis during the conversion of triose phosphate isomers (dihydroxyacetone phosphate and glyceraldehyde 3-phosphate) [5]. Interestingly, the glyoxalase system has an extremely narrow substrate specificity among dicarbonyl metabolites. In fact, it detoxifies mostly MG via the hemithioacetal, which is converted to S-D-lactoylglutathione and then hydrolyzed by Glo2 to D-lactate (Figure 2). Within this context, Glo1 represents the rate-limiting step for the detoxification of MG and is therefore of highest interest because it is believed to maintain the intracellular concentration of harmful MG in a low range. As the prevention of increased intracellular MG concentration is mandatory for the viability of a cell, in order to maintain physiological functions, it is no surprise that in eukaryotic organisms, the abundancy of Glo1 is in the top 10% of cytosolic proteins [6][7][6,7].
Glo2 is encoded by the hydroxyacylglutathione hydrolase gene and, like Glo1, it is expressed in nearly all living cells in order to hydrolyze S-D-Lactoylglutathione, the intermediate product of the glyoxalase cycle, into D-Lactate and (recycled) GSH [8][9][8,9]. Interestingly, S-D-Lactoylglutathione has been reported to be potentially cytotoxic, inducing growth arrest, and decreasing cellular viability, but further studies are needed to confirm this, because of the non-physiological doses used in this study [10]. In comparison to Glo1, Glo2 has not been located solely in the cytosol, but also in the mitochondria [8]. The amount of research conducted and therefore, available literature regarding Glo2 is far less compared to its counterpart Glo1. This may be the result of the fact that Glo1 is the rate-limiting enzyme of the glyoxalase system and seems to be more important [6]. Nevertheless, it is surprising that Glo2 is regulated by members of the tumor suppressor gene p53. In fact, Glo2 is up-regulated by p63 and p73, which are members of the p53 family, and therefore Glo2 is believed to have a role as a pro-survival factor [11]. Fortunately, recent investigations about the role of mitochondrial Glo2 have revealed new and very interesting content. Antognelli et al. suggested a pro-apoptotic role of Glo2 in non-small-cell lung cancers, and this effect is mediated by a bioactive plant compound called Oleuropein. Another study from the same group revealed that Glo2 can be viewed as a driving force of prostate tumorigenesis and therefore, may represent a novel marker of progression in prostate cancer diagnosis [12][13][12,13]. It is a nice illustration, showing that Glo2 may have been overlooked in the context of malignancies and that there is still a lot of space for further studies.
Increased levels of MG can lead to an intracellular accumulation of advanced glycation end products (AGEs) and to increased amounts of glycated DNA adducts. Both events are associated with many pathological events in humans, such as obesity and diabetes, atherosclerosis, various cancer types and neurodegenerative diseases [14][15][16][17][18][19][20][14–20]. Regarding protein glycation, the preferred targets of MG are the amino acids arginine and, to a much lesser extent, lysine. This mainly results in MG-derived hydroimidazolones called MG-H1, MG-H2 and MG-H3 (structure displayed in Figure 1). Based upon quantitative measurements, MG-H1 is the dominant type of all AGEs found within a physiological context [21][22][23][21–23]. Two other modifications should be mentioned herein, the arginine modification argpyrimidine and the most abundant lysine modification N-(1-carboxyethyl)lysine (CEL) [24][25][24,25]. Regarding the modifications of nucleic acids the most reactive nucleotide is deoxyguanosine, resulting in N2-carboxyethyl-2’-deoxyguanosine (CedG) and 3-(2’-deoxyribosyl)-6,7-dihydro-6,7-dihydroxy-6/7-methylimidazo-[2,3-b]purin-9(8)one (MG-dG) [26]. CedG has been found to be elevated in animal models and in human tissue samples, especially within the context of diabetes, but further studies are still needed to confirm its relevance (Figure 1) [27].
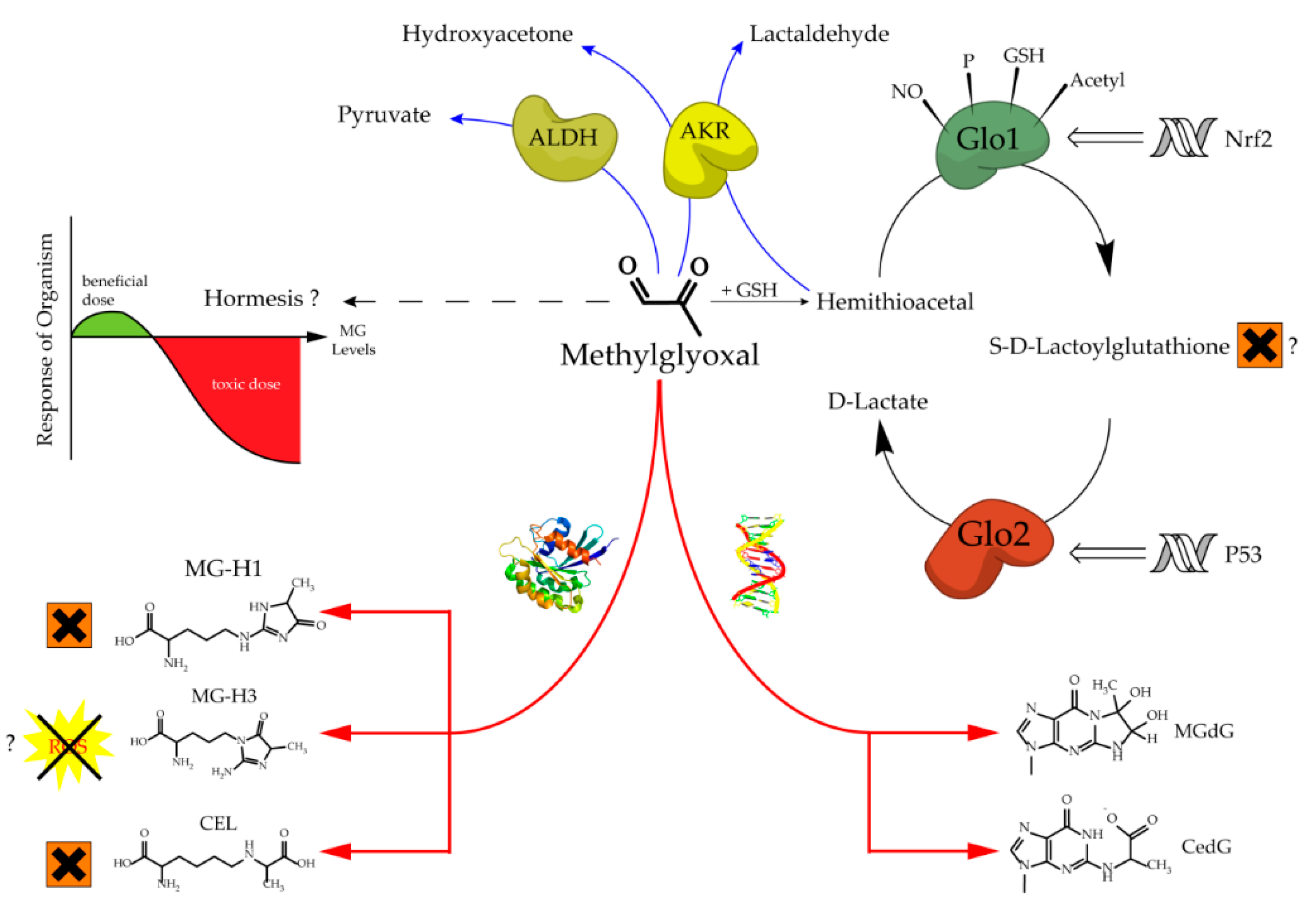
Figure 1.
Aspects of methylglyoxal metabolism and its possible implications.
The regulation of Glo1 activity and expression is complex and still not well understood. However, what we know is that Glo1 has a metal responsive element, an insulin-responsive element, an antioxidant responsive element, and it is a hotspot of copy number variation [28][29][30][28–30]. Downregulation of Glo1 via hyperglycemic or hypoxic conditions induces MG stress, whereas an upregulation via Nrf2 increases Glo1 activity and therefore can alleviate intracellular MG stress [29][31][29,31]. Many experimental data suggest that the modulation of Glo1 has a high impact towards the phenotype of healthy cells exposed to cellular stress, and also of malignant cells with an increased energy demand [7][15][17][32][33][34][35][7,15,17,32–35]. In cruder model organisms for instance, such as Caenorhabditis elegans (C. elegans), an overexpression of Glo1 results in an increase in mean and maximum lifespan by ~40% [36]. In bovine endothelial cells, the overexpression of Glo1 reduces the intracellular accumulation of AGEs under hyperglycemic conditions [37]. Consistently, overexpression in rat models of diabetes was reported to be protective regarding ateriogenesis and renal impairment driven by microvascular alterations [35][38][35,38]. On the other hand, the partial loss of Glo1 is associated with detrimental kidney damage in a murine Glo1 knock-down model [39]. Hyperglycemic episodes and therefore diabetes related late complications, such as nephro-, retino- and neuropathy, have been frequently linked to increased MG levels due to a lower Glo1 expression in humans [14][17][18][40][14,17,18,40]. Furthermore, in a recent clinical trial, a Glo1 inducer (resveratrol-hesperetin) improved metabolic and vascular health in a small cohort of overweight and obese humans [41]. Last but not least, the naturally occurring decrease in Glo1 during aging seems to be associated with an increased risk of age-related cardiovascular diseases [42].
As already mentioned, in many types of cancer the situation is quite different. Due to the vast amounts of glucose consumption resulting from the increased proliferation rates of most tumors, Glo1 is necessary to protect the cell from harmful MG. In malignant cells with a high proliferation rate, it is undoubtedly proven that Glo1 protects those cells from the increased formation of MG due to a high glycolytic flux within the context of the Warburg effect [43][44][45][43–45]. Therefore, it is no surprise that Glo1 is generally overexpressed in numerous cancers potentially as a part of a survival strategy [15]. This is the case in urological malignancies, different breast cancer types, gastric cancer cells, bladder, colon and hepatocellular carcinomas as well as leukemia [46][47][48][49][50][51][52][53].[46–53]
In prostate cancer, Glo1 is also linked to the maintenance of the metastatic phenotype by controlling the epithelial to mesenchymal transition [54]. The association of Glo1 with tumor growth could suggest its role as an oncogene, but this seems to be more an adaption to protect the tumor proteome against an increased intracellular flux of MG formation [55]. The inhibition of Glo1, therefore, may represent a potential target for anticancer therapeutics, although compounds such as bromobenzylglutathione cyclopentyl ester, which has been used in vitro, have to prove their therapeutic capacity in humans also [56].
2. Glo1 is not Indispensable for Crude and Complex Organisms
Due to its highly conserved role, the glyoxalase system has been viewed as a crucial enzyme system to maintain cellular viability [6][8][14][6,8,14]. This is a consequence of the necessity to detoxify the highly reactive dicarbonyl MG as fast as possible, in order to prevent glycation of proteins and DNA [7]. This has been proven in plants, crude prokaryotic microorganisms and many animals such as nematodes, arthropods, as well as chordates [4][5][36][57][58][59][4,5,36,57–59].
Unfortunately, the first attempts to abolish Glo1 activity failed, due to a high amount of copy number variants using the approach of gene trapping mutations [67]. The first global Glo1 KO in mice was established in 2017 [68]. Surprisingly, the effect of the complete global Glo1 KO was rather mild, at least in fish and mammals. Obviously, compensatory pathways for the glyoxalase system were underestimated in earlier studies. This is supported by the findings that in mammalian models a complete loss of Glo1 does not result in basal MG and MG-H1 elevations, even under high glucose conditions [72][74][72,74]. It has to be pointed out that most of the in vitro studies about MG-derived AGEs and DNA adducts had to use exogenously added MG in a supraphysiological range in order to achieve obvious molecular effects [64][72][75][76][77][64,72,75–77]. Certainly, Glo1 KO studies can be contradictory and provoking to the scientific dogma that silencing or inhibiting Glo1 has detrimental effects. Previously, this merged into the assumption of a cause-and-effect model between Glo1 activity and the levels of MG. If this is true, diseases associated with a complete inherited loss of Glo1 and therefore increased MG levels would have been described. As of yet, no known diseases have been attributed to Glo1 mutations in humans.
One reason for the development of late diabetic complications is the increase in MG-derived AGEs and DNA modifications, which is mainly due to the fact that Glo1 is downregulated in diabetes. Consequently, MG cannot be detoxified efficiently [14][20][21][34][77][14,20,21,34,77]. If this were true, then a diabetic Glo1 KO model would reflect a heavy damage of tissues which are prone to hyperglycemic conditions, such as kidneys, nerves and the eyes. However, recent data support the idea that the development of late diabetic complications due to increased AGEs and MG-derived DNA damage cannot be a result of one single downregulated enzyme. The data suggest that it is a multifactorial enzymatic system of high complexity, which is responsible for the detoxification of increased amounts of MG.
In summary, and to put the new findings into context, various in vivo studies of Glo1 KO models show that Glo1 is not an indispensable enzyme and thus, the glyoxalase system is neither. It is also a fascinating example that with increasing complexity and advanced evolution, nature may establish a backup system, which can compensate and substitute genetic mutations of highly conserved enzymes. This may not be perfectly the case in crude organisms, such as yeast and worm, but to some degree in flies and fish, and finally with perfection in a mammalian organism (Figure 2). This leads consequently to the question of why the glyoxalase system is not mandatory for a complex organism, despite it being a highly conserved and abundant system. The next part of this review tries to find answers to that essential question.
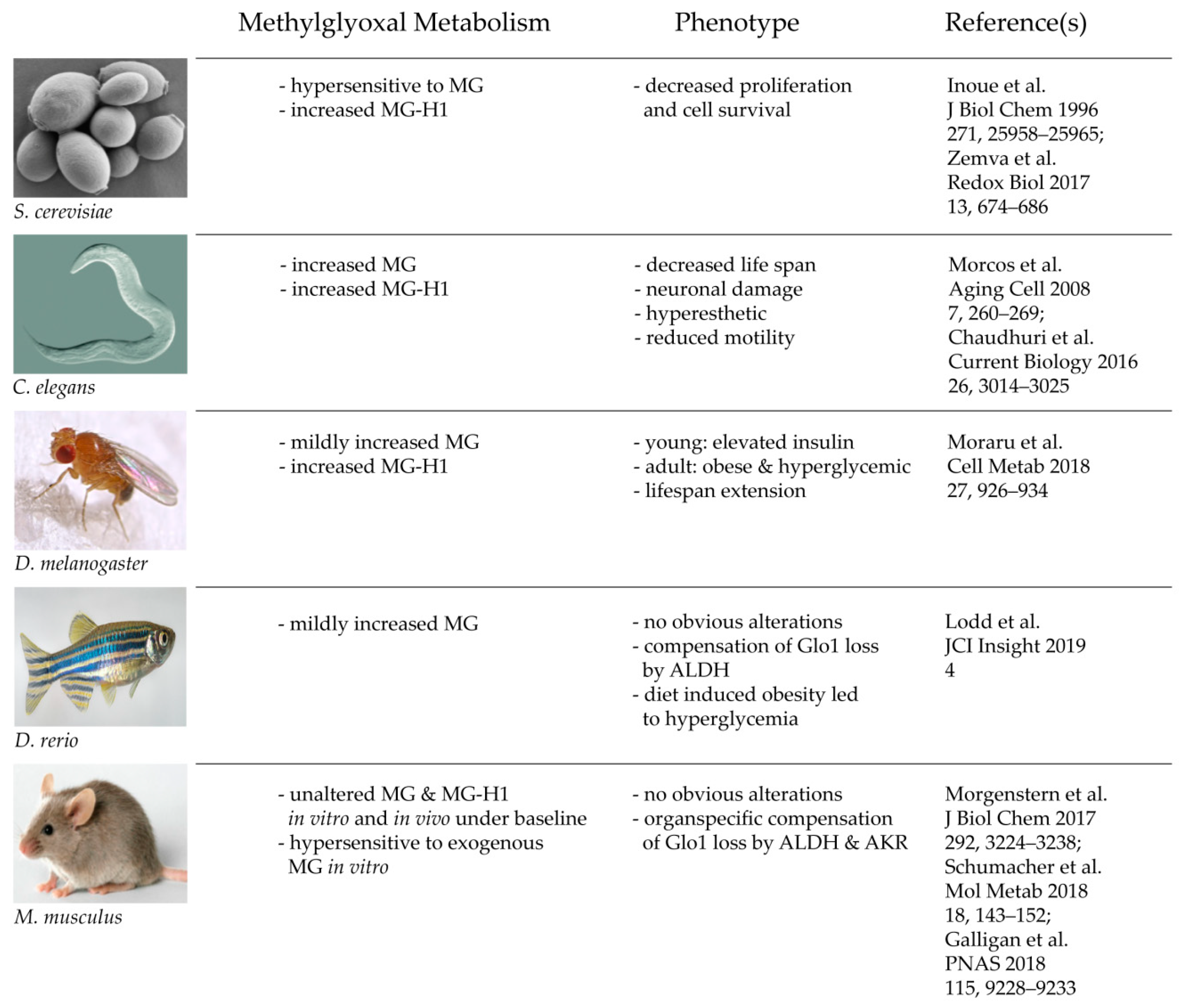
Figure 2. Consequences of a total loss of glyoxalase 1 in various model organisms.
Consequences of a total loss of glyoxalase 1 in various model organisms.
3. Modifications of Glo1 and Other Possible Intracellular Implications
Despite the extensive research on the glyoxalase system, and in particular Glo1, investigating PTMs of Glo1 in mammals have not been a focus in the field. Studies regarding the PTMs of Glo1 are rare and the consequences of those are unfortunately not well understood [77]. However, PTMs of Glo1 have been described in yeast and plants, which suggest that such regulatory mechanisms are as highly conserved as the glyoxalase system itself.
The most studied PTM of Glo1 is the phosphorylation. There are five different putative phosphorylation sites identified for Glo1, whereas Thr107 is the only one which has been identified as the one with physiological consequences [6][84][6,84]. A recent study was able to shed more light on the molecular consequences of the phosphorylation of Thr107. It was revealed by a pharmacological and genetic approach that the driving kinase is Ca2+/Calmodulin-dependent Kinase II delta (CamKIIδ) [87]. Moreover, phosphorylated Glo1 has an elevated catalytic efficiency (lower Km; higher Vmax) and it is also protected from rapid proteasomal degradation by ubiquitination [87]. Given the background that other studies have shown that an acetylation enforces the proteasomal degradation, it is a valid hypothesis to claim that the phosphoryl group could prevent the acetylation of Glo1 in a competitive way [85]. Interestingly, the reduced state of Glo1-phosphorylation, and therefore reduced Glo1 activity in vitro and in vivo, was associated with only mild changes regarding the phenotype. Only MG-specific DNA adducts (MG-dG) were increased and this was linked to increased nuclear damage markers such as p53 and γH2Ax. This effect was more pronounced in isolated murine endothelial cells as compared to the in vivo mouse model [87]. An important finding of this recent study was the association of phosphorylated Glo1 with diabetes, ageing and malignant cells. In fact, decreased Glo1 phosphorylation has been associated with a decline in Glo1 activity in diabetes or ageing, whereas the opposite effect seems to take place in human tumor cells, in which Glo1 activity is usually upregulated [87]. This could provide an explanation for the altered Glo1 status in different intracellular environments, described by many other studies [7][15][16][17][27][28][33][34][41][7,15,16,17,27,28,33,34,41]. Although these recent findings regarding the driving kinase of a Glo1 phosphorylation only scratch the surface of the underlying mechanisms, it might help to clarify the precise role of the glyoxalase system and Glo1 in particular. At this point it cannot be excluded that there are other kinases too, besides CamKIIδ, which are able to phosphorylate Glo1 efficiently. For instance, it has been shown that TNFα can induce multiple phosphorylation events at Glo1 [88].
4. Alternative Physiological Functions of Methylglyoxal
The intracellular concentrations of MG must be maintained at low levels in order to prevent cellular damage [5,7,16,17,18]. Although it has been speculated for some years that MG has important intracellular roles, there is little support for the idea that the production of MG in eukaryotes is a regulated process. An MG synthase has only been found in microorganisms supporting this concept, but no similar structures have been described in eukaryotes or even mammalian cells [93,94].
However, recent studies draw a slightly different picture. Sub-toxic doses of MG seem to be linked to a beneficial effect called hormesis. Interestingly, the benefit of physical exercises and the associated positive effects in aging driven by the increase in reactive oxygen species (ROS), may display the strongest “hormetin” available in the human body. The concept of the so called “mitohormesis” is able to challenge this common belief, with studies showing that the mitochondria need ROS in order to adapt and to prevent long-term overload of ROS under certain circumstances [99]. Metaphorically, one can phrase it as a “ROS training”, which occurs due to physical exercises or also starvation in the mitochondria and this is eventually a life-extending and health-promoting event.
Undoubtedly, MG increases ROS even at low levels, because of its high reactivity towards amino acids and nucleotides [77].In yeast it has been shown that low doses of MG induce a tolerance towards toxic concentrations of MG and H2O2 [100]. Interestingly, this MG-triggered tolerance is independent of Glo1 and secondly the finding could be translated into a mammalian in vitro system, in murine endothelial cells [100]. The effect is mostly driven by an increased expression of the protein quality system. Going higher on the evolutionary ladder, an interesting study by Ristow et al. was performed in C. elegans. By interfering with the threonine catabolism, the formation of MG was promoted, which unexpectedly increased lifespan in worms [101]. It is a highly provocative study, since other studies have reported that an overexpression of Glo1, which reduced MG levels, prolongs lifespan in C. elegans [36]. In contrast, the knock-down of Glo1 in C. elegans revealed a decrease in lifespan and that was linked to elevated MG as well as MG-H1 levels [102]. When looking into the first Glo1 KO in Drosophila fly, which recapitulates the progression of type 2 diabetes, the authors expected reduced longevity. Instead of that, it was reported that the Glo1 KO and potentially the mild increase in MG are associated with a significant lifespan extension [71]. This result suggests an evolutionary conserved role for this mechanism [101].
The nature of a biphasic dose–response relationship (hormesis) has been assessed in human tumor cell lines as well. The hormetic potential of MG in cancer cells has been discovered recently in different breast cancer and glioblastoma cell lines where the maintenance of tolerable MG stress at subtoxic levels was beneficial regarding cancer growth and resistance to apoptosis [103]. Such responses seem to be mainly driven by the upregulation of Nrf2 [104]. Another recent study in a very aggressive and lethal form of thyroid cancer reported comparable effects. In this in vitro approach, it was not MG itself that was responsible for the hormetic effect, but the accumulation of MG adducts that turned out to enhance the migration and invasion of anaplastic thyroid cancer. Interestingly, using resveratrol, which has been proven as an inducer of Glo1, the hormetic effect of increased MG adducts was lost [105]. The glycation of HSPs by MG in cancer cells, such as HSP27 or HSP90, could explain partly the metabolic advantage of cancer cells, which are reflected by a resistance towards cisplatin [15]. Investigating hormetic effects in cancer cells is a new field of research, which should be investigated thoroughly, in order to understand the interplay of MG and the glyoxalase system better in highly proliferating cells.
5. Conclusions
This review showed that the glyoxalase system is surprisingly not an indispensable system to maintain cellular viability. Especially in mammalian organisms, the loss of the glyoxalase system is compensated efficiently by enzymes of the AKR and ALDH family. Those enzymes can play a pivotal role within the context of MG mediated damage in healthy cells or malignant cells. Undoubtedly, and this has been shown herein, there is room for studying the glyoxalase metabolism from alternative points of view. This includes PTMs of Glo1, hormetic effects of MG and MG-derived AGEs, or even the intriguing concept that the glyoxalase system may reflect a regulator of cell division. It is a pity that many studies have focused relatively blindly on the cause-and-effect model between Glo1 and MG, which has been described in detail in the introduction of this review. This is further supported by the idea that there is so little literature published on Glo2 in the last 10 years (123 hits for Glo1 vs. eight hits for Glo2; [107]). This review wants to polarize and may provoke a bit in order to emphasize and encourage all researchers in the field to look beyond the obvious and investigate the glyoxalase system without prejudice.
References
- Dakin, H.D.; Dudley, H.W. AN ENZYME CONCERNED WITH THE FORMATION OF HYDROXY ACIDS FROM KETONIC ALDEHYDES. J. Biol. Chem. 1913, 14, 155–157.Dakin, H.D.; Dudley, H.W. An enzyme concerned with the formation OF hydroxy acids from ketonic aldehydes. J. Biol. Chem. 1913, 14, 155–157. [Google Scholar]
- Thornalley, P.J. The glyoxalase system in health and disease. Mol. Asp. Med. 1993, 14, 287–371, doi:10.1016/0098-2997(93)90002-U.Thornalley, P.J. The glyoxalase system in health and disease. Mol. Asp. Med. 1993, 14, 287–371. [Google Scholar] [CrossRef]
- Kaur, C.; Sharma, S.; Hasan, M.; Pareek, A.; Singla-Pareek, S.; Sopory, S. Characteristic variations and similarities in biochemical, molecular, and functional properties of glyoxalases across prokaryotes and eukaryotes. Int. J. Mol. Sci. 2017, 18, 250, doi:10.3390/ijms18040250.Kaur, C.; Sharma, S.; Hasan, M.; Pareek, A.; Singla-Pareek, S.; Sopory, S. Characteristic variations and similarities in biochemical, molecular, and functional properties of glyoxalases across prokaryotes and eukaryotes. Int. J. Mol. Sci. 2017, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Batth, R.; Kumari, S.; Mustafiz, A. Arabidopsis thaliana contains both Ni2+ and Zn2+ dependent glyoxalase I enzymes and ectopic expression of the latter contributes more towards abiotic stress tolerance in E. coli. PLoS ONE 2016, 11, e0159348, doi:10.1371/journal.pone.0159348.Jain, M.; Batth, R.; Kumari, S.; Mustafiz, A. Arabidopsis thaliana contains both Ni2+ and Zn2+ dependent glyoxalase I enzymes and ectopic expression of the latter contributes more towards abiotic stress tolerance in E. coli. PLoS ONE 2016, 11, e0159348. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23, doi:10.3389/fnins.2015.00023.Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Glyoxalase I—Structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348, doi:10.1042/bst0311343.Thornalley, P.J. Glyoxalase I—Structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef]
- Nigro, C.; Leone, A.; Raciti, G.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-glyoxalase 1 balance: The root of vascular damage. Int. J. Mol. Sci. 2017, 18, 188, doi:10.3390/ijms18010188.Nigro, C.; Leone, A.; Raciti, G.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-glyoxalase 1 balance: The root of vascular damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef]
- Silva, M.S.; Gomes, R.; Ferreira, A.; Freire, A.P.; Cordeiro, C.A. The glyoxalase pathway: The first hundred years… and beyond. Biochem. J. 2013, 453, 1–15, doi:10.1042/BJ20121743.Silva, M.S.; Gomes, R.; Ferreira, A.; Freire, A.P.; Cordeiro, C.A. The glyoxalase pathway: The first hundred years… and beyond. Biochem. J. 2013, 453, 1–15. [Google Scholar] [CrossRef]
- Cameron, A.D.; Ridderström, M.; Olin, B.; Mannervik, B. Crystal structure of human glyoxalase II and its complex with a glutathione thiolester substrate analogue. Structure 1999, 7, 1067–1078, doi:10.1016/s0969-2126(99)80174-9.Cameron, A.D.; Ridderström, M.; Olin, B.; Mannervik, B. Crystal structure of human glyoxalase II and its complex with a glutathione thiolester substrate analogue. Structure 1999, 7, 1067–1078. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Tisdale, M.J. Inhibition of proliferation of human promyelocytic leukaemia HL60 cells by S-D-lactoylglutathione in vitro. Leuk. Res. 1988, 12, 897–904, doi:10.1016/0145-2126(88)90016-1.Thornalley, P.J.; Tisdale, M.J. Inhibition of proliferation of human promyelocytic leukaemia HL60 cells by S-D-lactoylglutathione in vitro. Leuk. Res. 1988, 12, 897–904. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X. Glyoxalase II, a detoxifying enzyme of glycolysis byproduct methylglyoxal and a target of p63 and p73, is a pro-survival factor of the p53 family. J. Biol. Chem. 2006, 281, 26702–26713, doi:10.1074/jbc.M604758200.Xu, Y.; Chen, X. Glyoxalase II, a detoxifying enzyme of glycolysis byproduct methylglyoxal and a target of p63 and p73, is a pro-survival factor of the p53 family. J. Biol. Chem. 2006, 281, 26702–26713. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Frosini, R.; Santolla, M.F.; Peirce, M.J.; Talesa, V.N. Oleuropein-induced apoptosis is mediated by mitochondrial glyoxalase 2 in NSCLC A549 cells: A mechanistic inside and a possible novel nonenzymatic role for an ancient enzyme. Oxidative Med. Cell. Longev. 2019, 2019, 8576961, doi:10.1155/2019/8576961.Antognelli, C.; Frosini, R.; Santolla, M.F.; Peirce, M.J.; Talesa, V.N. Oleuropein-induced apoptosis is mediated by mitochondrial glyoxalase 2 in NSCLC A549 cells: A mechanistic inside and a possible novel nonenzymatic role for an ancient enzyme. Oxidative Med. Cell. Longev. 2019, 2019, 8576961. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Ferri, I.; Bellezza, G.; Siccu, P.; Love, H.D.; Talesa, V.N.; Sidoni, A. Glyoxalase 2 drives tumorigenesis in human prostate cells in a mechanism involving androgen receptor and p53-p21 axis. Mol. Carcinog. 2017, 56, 2112–2126, doi:10.1002/mc.22668.Antognelli, C.; Ferri, I.; Bellezza, G.; Siccu, P.; Love, H.D.; Talesa, V.N.; Sidoni, A. Glyoxalase 2 drives tumorigenesis in human prostate cells in a mechanism involving androgen receptor and p53-p21 axis. Mol. Carcinog. 2017, 56, 2112–2126. [Google Scholar] [CrossRef] [PubMed]
- Hidmark, A.; Fleming, T.; Vittas, S.; Mendler, M.; Deshpande, D.; Groener, J.B.; Müller, B.P.; Reeh, P.W.; Sauer, S.K.; Pham, M.; et al. A new paradigm to understand and treat diabetic neuropathy. Exp. Clin. Endocrinol. Diabetes 2014, 122, 201–207, doi:10.1055/s-0034-1367023.Hidmark, A.; Fleming, T.; Vittas, S.; Mendler, M.; Deshpande, D.; Groener, J.B.; Müller, B.P.; Reeh, P.W.; Sauer, S.K.; Pham, M.; et al. A new paradigm to understand and treat diabetic neuropathy. Exp. Clin. Endocrinol. Diabetes 2014, 122, 201–207. [Google Scholar] [CrossRef]
- Bellahcène, A.; Nokin, M.-J.; Castronovo, V.; Schalkwijk, C. Methylglyoxal-derived stress: An emerging biological factor involved in the onset and progression of cancer. Semin. Cancer Biol. 2018, 49, 64–74, doi:10.1016/j.semcancer.2017.05.010.Bellahcène, A.; Nokin, M.-J.; Castronovo, V.; Schalkwijk, C. Methylglyoxal-derived stress: An emerging biological factor involved in the onset and progression of cancer. Semin. Cancer Biol. 2018, 49, 64–74. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. Methylglyoxal and glyoxalase I in atherosclerosis. Biochem. Soc. Trans. 2014, 42, 443–449, doi:10.1042/BST20140001.Hanssen, N.M.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. Methylglyoxal and glyoxalase I in atherosclerosis. Biochem. Soc. Trans. 2014, 42, 443–449. [Google Scholar] [CrossRef]
- Maessen, D.E.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861, doi:10.1042/CS20140683.Maessen, D.E.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef]
- Matafome, P.; Sena, C.; Seiça, R. Methylglyoxal, obesity, and diabetes. Endocrine 2013, 43, 472–484, doi:10.1007/s12020-012-9795-8.Matafome, P.; Sena, C.; Seiça, R. Methylglyoxal, obesity, and diabetes. Endocrine 2013, 43, 472–484. [Google Scholar] [CrossRef]
- Srikanth, V.; Westcott, B.; Forbes, J.; Phan, T.G.; Beare, R.; Venn, A.; Pearson, S.; Greenaway, T.; Parameswaran, V.; Münch, G. Methylglyoxal, cognitive function and cerebral atrophy in older people. J. Gerontol. Ser. A 2013, 68, 68–73, doi:10.1093/gerona/gls100.Srikanth, V.; Westcott, B.; Forbes, J.; Phan, T.G.; Beare, R.; Venn, A.; Pearson, S.; Greenaway, T.; Parameswaran, V.; Münch, G. Methylglyoxal, cognitive function and cerebral atrophy in older people. J. Gerontol. Ser. A 2013, 68, 68–73. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116, doi:10.1042/bj3440109.Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592, doi:10.1042/BJ20030763.Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J.; Dawczynski, J.; Franke, S.; Strobel, J.; Stein, G.; Haik, G.M. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Investig. Opthalmol. Vis. Sci. 2003, 44, 5287, doi:10.1167/iovs.03-0573.Ahmed, N.; Thornalley, P.J.; Dawczynski, J.; Franke, S.; Strobel, J.; Stein, G.; Haik, G.M. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Investig. Opthalmol. Vis. Sci. 2003, 44, 5287. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Dobler, D.; Dean, M.; Thornalley, P.J. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J. Biol. Chem. 2005, 280, 5724–5732, doi:10.1074/jbc.M410973200.Ahmed, N.; Dobler, D.; Dean, M.; Thornalley, P.J. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J. Biol. Chem. 2005, 280, 5724–5732. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Argirov, O.K.; Minhas, H.S.; Cordeiro, C.A.A.; Thornalley, P.J. Assay of advanced glycation endproducts (AGEs): Surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to N∊-carboxymethyl-lysine- and N∊-(1-carboxyethyl)lysine-modified albumin. Biochem. J. 2002, 364, 1–14, doi:10.1042/bj3640001.Ahmed, N.; Argirov, O.K.; Minhas, H.S.; Cordeiro, C.A.A.; Thornalley, P.J. Assay of advanced glycation endproducts (AGEs): Surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to N∊-carboxymethyl-lysine- and N∊-(1-carboxyethyl)lysine-modified albumin. Biochem. J. 2002, 364, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K. The reactions of phenylglyoxal and related reagents with amino acids. J. Biochem. 1977, 81, 395–402, doi:10.1093/oxfordjournals.jbchem.a131471.Takahashi, K. The reactions of phenylglyoxal and related reagents with amino acids. J. Biochem. 1977, 81, 395–402. [Google Scholar] [CrossRef]
- Schneider, M.; Thoss, G.; Hübner-Parajsz, C.; Kientsch-Engel, R.; Stahl, P.; Pischetsrieder, M. Determination of glycated nucleobases in human urine by a new monoclonal antibody specific for N 2 -Carboxyethyl-2‘-deoxyguanosine. Chem. Res. Toxicol. 2004, 17, 1385–1390, doi:10.1021/tx049929d.Schneider, M.; Thoss, G.; Hübner-Parajsz, C.; Kientsch-Engel, R.; Stahl, P.; Pischetsrieder, M. Determination of glycated nucleobases in human urine by a new monoclonal antibody specific for N 2 -Carboxyethyl-2‘-deoxyguanosine. Chem. Res. Toxicol. 2004, 17, 1385–1390. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Waris, S.; Fleming, T.; Santarius, T.; Larkin, S.J.; Winklhofer-Roob, B.M.; Stratton, M.R.; Rabbani, N. Imidazopurinones are markers of physiological genomic damage linked to DNA instability and glyoxalase 1-associated tumour multidrug resistance. Nucleic Acids Res. 2010, 38, 5432–5442, doi:10.1093/nar/gkq306.Thornalley, P.J.; Waris, S.; Fleming, T.; Santarius, T.; Larkin, S.J.; Winklhofer-Roob, B.M.; Stratton, M.R.; Rabbani, N. Imidazopurinones are markers of physiological genomic damage linked to DNA instability and glyoxalase 1-associated tumour multidrug resistance. Nucleic Acids Res. 2010, 38, 5432–5442. [Google Scholar] [CrossRef]
- Ranganathan, S.; Ciaccio, P.J.; Walsh, E.S.; Tew, K.D. Genomic sequence of human glyoxalase-I: Analysis of promoter activity and its regulation. Gene 1999, 240, 149–155, doi:10.1016/S0378-1119(99)00420-5.Ranganathan, S.; Ciaccio, P.J.; Walsh, E.S.; Tew, K.D. Genomic sequence of human glyoxalase-I: Analysis of promoter activity and its regulation. Gene 1999, 240, 149–155. [Google Scholar] [CrossRef]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M.; et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222, doi:10.1042/BJ20111648.Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M.; et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef]
- Shafie, A.; Xue, M.; Thornalley, P.J.; Rabbani, N. Copy number variation of glyoxalase I. Biochem. Soc. Trans. 2014, 42, 500–503, doi:10.1042/BST20140011.Shafie, A.; Xue, M.; Thornalley, P.J.; Rabbani, N. Copy number variation of glyoxalase I. Biochem. Soc. Trans. 2014, 42, 500–503. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Xi, H.S.; Li, S. HIF1α is required for survival maintenance of chronic myeloid leukemia stem cells. Blood 2012, 119, 2595–2607, doi:10.1182/blood-2011-10-387381.Zhang, H.; Li, H.; Xi, H.S.; Li, S. HIF1α is required for survival maintenance of chronic myeloid leukemia stem cells. Blood 2012, 119, 2595–2607. [Google Scholar] [CrossRef]
- Hutschenreuther, A.; Bigl, M.; Hemdan, N.; Debebe, T.; Gaunitz, F.; Birkenmeier, G. Modulation of GLO1 expression affects malignant properties of cells. Int. J. Mol. Sci. 2016, 17, 2133, doi:10.3390/ijms17122133.Hutschenreuther, A.; Bigl, M.; Hemdan, N.; Debebe, T.; Gaunitz, F.; Birkenmeier, G. Modulation of GLO1 expression affects malignant properties of cells. Int. J. Mol. Sci. 2016, 17, 2133. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.S.; Lercher, M.; Fleming, T.H.; Nawroth, P.P.; Bischoff, M.S.; Dihlmann, S.; Böckler, D.; Hakimi, M. Reduced glyoxalase 1 activity in carotid artery plaques of nondiabetic patients with increased hemoglobin A1c level. J. Vasc. Surg. 2016, 64, 990–994, doi:10.1016/j.jvs.2016.04.025.Peters, A.S.; Lercher, M.; Fleming, T.H.; Nawroth, P.P.; Bischoff, M.S.; Dihlmann, S.; Böckler, D.; Hakimi, M. Reduced glyoxalase 1 activity in carotid artery plaques of nondiabetic patients with increased hemoglobin A1c level. J. Vasc. Surg. 2016, 64, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Glyoxalase 1 modulation in obesity and diabetes. Antioxid. Redox Signal. 2019, 30, 354–374, doi:10.1089/ars.2017.7424.Rabbani, N.; Thornalley, P.J. Glyoxalase 1 modulation in obesity and diabetes. Antioxid. Redox Signal. 2019, 30, 354–374. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011, 286, 1374–1380, doi:10.1074/jbc.M110.144097.Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011, 286, 1374–1380. [Google Scholar] [CrossRef]
- Morcos, M.; Du, X.; Pfisterer, F.; Hutter, H.; Sayed, A.A.R.; Thornalley, P.; Ahmed, N.; Baynes, J.; Thorpe, S.; Kukudov, G.; et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell 2008, 7, 260–269, doi:10.1111/j.1474-9726.2008.00371.x.Morcos, M.; Du, X.; Pfisterer, F.; Hutter, H.; Sayed, A.A.R.; Thornalley, P.; Ahmed, N.; Baynes, J.; Thorpe, S.; Kukudov, G.; et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell 2008, 7, 260–269. [Google Scholar] [CrossRef]
- Shinohara, M.; Thornalley, P.J.; Giardino, I.; Beisswenger, P.; Thorpe, S.R.; Onorato, J.; Brownlee, M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J. Clin. Investig. 1998, 101, 1142–1147, doi:10.1172/JCI119885.Shinohara, M.; Thornalley, P.J.; Giardino, I.; Beisswenger, P.; Thorpe, S.R.; Onorato, J.; Brownlee, M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J. Clin. Investig. 1998, 101, 1142–1147. [Google Scholar] [CrossRef]
- Brouwers, O.; Yu, L.; Niessen, P.; Slenter, J.; Jaspers, K.; Wagenaar, A.; Post, M.; Miyata, T.; Backes, W.; Stehouwer, C.; et al. Glyoxalase-1 overexpression partially prevents diabetes-induced impaired arteriogenesis in a rat hindlimb ligation model. Glycoconj. J. 2016, 33, 627–630, doi:10.1007/s10719-016-9681-3.Brouwers, O.; Yu, L.; Niessen, P.; Slenter, J.; Jaspers, K.; Wagenaar, A.; Post, M.; Miyata, T.; Backes, W.; Stehouwer, C.; et al. Glyoxalase-1 overexpression partially prevents diabetes-induced impaired arteriogenesis in a rat hindlimb ligation model. Glycoconj. J. 2016, 33, 627–630. [Google Scholar] [CrossRef]
- Giacco, F.; Du, X.; D’Agati, V.D.; Milne, R.; Sui, G.; Geoffrion, M.; Brownlee, M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 2014, 63, 291–299, doi:10.2337/db13-0316.Giacco, F.; Du, X.; D’Agati, V.D.; Milne, R.; Sui, G.; Geoffrion, M.; Brownlee, M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes 2014, 63, 50–52, doi:10.2337/db13-1606.Rabbani, N.; Thornalley, P.J. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes 2014, 63, 50–52. [Google Scholar] [CrossRef]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.-B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes 2016, 65, 2282–2294, doi:10.2337/db16-0153.Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.-B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes 2016, 65, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Rabbani, N.; Thornalley, P.J. Glyoxalase in ageing. Semin. Cell Dev. Biol. 2011, 22, 293–301, doi:10.1016/j.semcdb.2011.02.013.Xue, M.; Rabbani, N.; Thornalley, P.J. Glyoxalase in ageing. Semin. Cell Dev. Biol. 2011, 22, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Santarius, T.; Bignell, G.R.; Greenman, C.D.; Widaa, S.; Chen, L.; Mahoney, C.L.; Butler, A.; Edkins, S.; Waris, S.; Thornalley, P.J.; et al. GLO1-A novel amplified gene in human cancer. Genes Chromosomes Cancer 2010, 49, 711–725, doi:10.1002/gcc.20784.Santarius, T.; Bignell, G.R.; Greenman, C.D.; Widaa, S.; Chen, L.; Mahoney, C.L.; Butler, A.; Edkins, S.; Waris, S.; Thornalley, P.J.; et al. GLO1-A novel amplified gene in human cancer. Genes Chromosomes Cancer 2010, 49, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033, doi:10.1126/science.1160809.Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308, doi:10.1016/j.ccr.2012.02.014.Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef]
- Antognelli, C.; Talesa, V.N. Glyoxalases in urological malignancies. Int. J. Mol. Sci. 2018, 19, 415, doi:10.3390/ijms19020415.Antognelli, C.; Talesa, V.N. Glyoxalases in urological malignancies. Int. J. Mol. Sci. 2018, 19, 415. [Google Scholar] [CrossRef]
- Yang, Y.-X.; Chen, Z.-C.; Zhang, G.-Y.; Yi, H.; Xiao, Z.-Q. A subcelluar proteomic investigation into vincristine-resistant gastric cancer cell line. J. Cell. Biochem. 2008, 104, 1010–1021, doi:10.1002/jcb.21687.Yang, Y.-X.; Chen, Z.-C.; Zhang, G.-Y.; Yi, H.; Xiao, Z.-Q. A subcelluar proteomic investigation into vincristine-resistant gastric cancer cell line. J. Cell. Biochem. 2008, 104, 1010–1021. [Google Scholar] [CrossRef]
- Nakamura, T.; Furukawa, Y.; Nakagawa, H.; Tsunoda, T.; Ohigashi, H.; Murata, K.; Ishikawa, O.; Ohgaki, K.; Kashimura, N.; Miyamoto, M.; et al. Genome-wide cDNA microarray analysis of gene expression profiles in pancreatic cancers using populations of tumor cells and normal ductal epithelial cells selected for purity by laser microdissection. Oncogene 2004, 23, 2385–2400, doi:10.1038/sj.onc.1207392.Nakamura, T.; Furukawa, Y.; Nakagawa, H.; Tsunoda, T.; Ohigashi, H.; Murata, K.; Ishikawa, O.; Ohgaki, K.; Kashimura, N.; Miyamoto, M.; et al. Genome-wide cDNA microarray analysis of gene expression profiles in pancreatic cancers using populations of tumor cells and normal ductal epithelial cells selected for purity by laser microdissection. Oncogene 2004, 23, 2385–2400. [Google Scholar] [CrossRef]
- Rulli, A.; Carli, L.; Romani, R.; Baroni, T.; Giovannini, E.; Rosi, G.; Talesa, V. Expression of glyoxalase I and II in normal and breast cancer tissues. Breast Cancer Res. Treat. 2001, 66, 67–72, doi:10.1023/A:1010632919129.Rulli, A.; Carli, L.; Romani, R.; Baroni, T.; Giovannini, E.; Rosi, G.; Talesa, V. Expression of glyoxalase I and II in normal and breast cancer tissues. Breast Cancer Res. Treat. 2001, 66, 67–72. [Google Scholar] [CrossRef]
- Zhang, D.; Tai, L.K.; Wong, L.L.; Chiu, L.-L.; Sethi, S.K.; Koay, E.S.C. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu -positive breast cancer*. Mol. Cell. Proteom. 2005, 4, 1686–1696, doi:10.1074/mcp.M400221-MCP200.Zhang, D.; Tai, L.K.; Wong, L.L.; Chiu, L.-L.; Sethi, S.K.; Koay, E.S.C. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu -positive breast cancer*. Mol. Cell. Proteom. 2005, 4, 1686–1696. [Google Scholar] [CrossRef]
- Mearini, E.; Romani, R.; Mearini, L.; Antognelli, C.; Zucchi, A.; Baroni, T.; Porena, M.; Talesa, V.. Differing expression of enzymes of the glyoxalase system in superficial and invasive bladder carcinomas. Eur. J. Cancer 2002, 38, 1946–1950, doi:10.1016/S0959-8049(02)00236-8.Mearini, E.; Romani, R.; Mearini, L.; Antognelli, C.; Zucchi, A.; Baroni, T.; Porena, M.; Talesa, V. Differing expression of enzymes of the glyoxalase system in superficial and invasive bladder carcinomas. Eur. J. Cancer 2002, 38, 1946–1950. [Google Scholar] [CrossRef]
- Song, H.-Y.; Liu, Y.-K.; Feng, J.-T.; Cui, J.-F.; Dai, Z.; Zhang, L.-J.; Feng, J.-X.; Shen, H.-L.; Tang, Z.-Y. Proteomic analysis on metastasis-associated proteins of human hepatocellular carcinoma tissues. J. Cancer Res. Clin. Oncol. 2006, 132, 92–98, doi:10.1007/s00432-005-0044-x.Song, H.-Y.; Liu, Y.-K.; Feng, J.-T.; Cui, J.-F.; Dai, Z.; Zhang, L.-J.; Feng, J.-X.; Shen, H.-L.; Tang, Z.-Y. Proteomic analysis on metastasis-associated proteins of human hepatocellular carcinoma tissues. J. Cancer Res. Clin. Oncol. 2006, 132, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.-W. Proteomic analysis of human acute leukemia cells: Insight into their classification. Clin. Cancer Res. 2004, 10, 6887–6896, doi:10.1158/1078-0432.CCR-04-0307.Cui, J.-W. Proteomic analysis of human acute leukemia cells: Insight into their classification. Clin. Cancer Res. 2004, 10, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Mezzasoma, L.; Mearini, E.; Talesa, V.N. Glyoxalase 1-419C>A variant is associated with oxidative stress: Implications in prostate cancer progression. PLoS ONE 2013, 8, e74014, doi:10.1371/journal.pone.0074014.Antognelli, C.; Mezzasoma, L.; Mearini, E.; Talesa, V.N. Glyoxalase 1-419C>A variant is associated with oxidative stress: Implications in prostate cancer progression. PLoS ONE 2013, 8, e74014. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Multiple roles of glyoxalase 1-mediated suppression of methylglyoxal glycation in cancer biology-Involvement in tumour suppression, tumour growth, multidrug resistance and target for chemotherapy. Semin. Cancer Biol. 2018, 49, 83–93, doi:10.1016/j.semcancer.2017.05.006.Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Multiple roles of glyoxalase 1-mediated suppression of methylglyoxal glycation in cancer biology-Involvement in tumour suppression, tumour growth, multidrug resistance and target for chemotherapy. Semin. Cancer Biol. 2018, 49, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Edwards, L.G.; Kang, Y.; Wyatt, C.; Davies, N.; Ladan, M.J.; Double, J. Antitumour activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and induction of apoptosis. Biochem. Pharmacol. 1996, 51, 1365–1372, doi:10.1016/0006-2952(96)00059-7.Thornalley, P.J.; Edwards, L.G.; Kang, Y.; Wyatt, C.; Davies, N.; Ladan, M.J.; Double, J. Antitumour activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and induction of apoptosis. Biochem. Pharmacol. 1996, 51, 1365–1372. [Google Scholar] [CrossRef]
- Jörgens, K.; Stoll, S.J.; Pohl, J.; Fleming, T.H.; Sticht, C.; Nawroth, P.P.; Hammes, H.-P.; Kroll, J. High tissue glucose alters intersomitic blood vessels in zebrafish via methylglyoxal targeting the VEGF receptor signaling cascade. Diabetes 2015, 64, 213–225, doi:10.2337/db14-0352.Jörgens, K.; Stoll, S.J.; Pohl, J.; Fleming, T.H.; Sticht, C.; Nawroth, P.P.; Hammes, H.-P.; Kroll, J. High tissue glucose alters intersomitic blood vessels in zebrafish via methylglyoxal targeting the VEGF receptor signaling cascade. Diabetes 2015, 64, 213–225. [Google Scholar] [CrossRef]
- Urscher, M.; Alisch, R.; Deponte, M. The glyoxalase system of malaria parasites—Implications for cell biology and general glyoxalase research. Semin. Cell Dev. Biol. 2011, 22, 262–270, doi:10.1016/j.semcdb.2011.02.003.Urscher, M.; Alisch, R.; Deponte, M. The glyoxalase system of malaria parasites—Implications for cell biology and general glyoxalase research. Semin. Cell Dev. Biol. 2011, 22, 262–270. [Google Scholar] [CrossRef]
- Garrido, D.; Rubin, T.; Poidevin, M.; Maroni, B.; Le Rouzic, A.; Parvy, J.-P.; Montagne, J. Fatty acid synthase cooperates with glyoxalase 1 to protect against sugar toxicity. PLoS Genet. 2015, 11, e1004995, doi:10.1371/journal.pgen.1004995.Garrido, D.; Rubin, T.; Poidevin, M.; Maroni, B.; Le Rouzic, A.; Parvy, J.-P.; Montagne, J. Fatty acid synthase cooperates with glyoxalase 1 to protect against sugar toxicity. PLoS Genet. 2015, 11, e1004995. [Google Scholar] [CrossRef]
- Ghosh, A.; Kushwaha, H.R.; Hasan, M.R.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Presence of unique glyoxalase III proteins in plants indicates the existence of shorter route for methylglyoxal detoxification. Sci. Rep. 2016, 6, 18358, doi:10.1038/srep18358.Ghosh, A.; Kushwaha, H.R.; Hasan, M.R.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Presence of unique glyoxalase III proteins in plants indicates the existence of shorter route for methylglyoxal detoxification. Sci. Rep. 2016, 6, 18358. [Google Scholar] [CrossRef]
- Jagt, D.L.V.; A Hunsaker, L. Methylglyoxal metabolism and diabetic complications: Roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem. Biol. Interact. 2003, 143–144, 341–351.Jagt, D.L.V.; A Hunsaker, L. Methylglyoxal metabolism and diabetic complications: Roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem. Biol. Interact. 2003, 143–144, 341–351. [Google Scholar] [CrossRef]
- Jagt, D.L.V.; Hassebrook, R.K.; Hunsaker, L.A.; Brown, W.M.; Royer, R.E. Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase-I: Roles for glutathione in both enzymes and implications for diabetic complications. Chem. Biol. Interact. 2001, 130–132, 549–562, doi:10.1016/S0009-2797(00)00298-2.Jagt, D.L.V.; Hassebrook, R.K.; Hunsaker, L.A.; Brown, W.M.; Royer, R.E. Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase-I: Roles for glutathione in both enzymes and implications for diabetic complications. Chem. Biol. Interact. 2001, 130–132, 549–562. [Google Scholar] [CrossRef]
- Richarme, G.; Mihoub, M.; Dairou, J.; Bui, L.C.; Leger, T.; Lamouri, A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 2015, 290, 1885–1897, doi:10.1074/jbc.M114.597815.Richarme, G.; Mihoub, M.; Dairou, J.; Bui, L.C.; Leger, T.; Lamouri, A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 2015, 290, 1885–1897. [Google Scholar] [CrossRef]
- Galligan, J.J.; Wepy, J.A.; Streeter, M.D.; Kingsley, P.J.; Mitchener, M.M.; Wauchope, O.R.; Beavers, W.N.; Rose, K.L.; Wang, T.; Spiegel, D.A.; et al. Methylglyoxal-derived posttranslational arginine modifications are abundant histone marks. Proc. Natl. Acad. Sci. USA 2018, 115, 9228–9233, doi:10.1073/pnas.1802901115.Galligan, J.J.; Wepy, J.A.; Streeter, M.D.; Kingsley, P.J.; Mitchener, M.M.; Wauchope, O.R.; Beavers, W.N.; Rose, K.L.; Wang, T.; Spiegel, D.A.; et al. Methylglyoxal-derived posttranslational arginine modifications are abundant histone marks. Proc. Natl. Acad. Sci. USA 2018, 115, 9228–9233. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.P.; Barski, O.A.; Ahmed, Y.; O’Toole, T.E.; Conklin, D.J.; Bhatnagar, A.; Srivastava, S. Reductive metabolism of AGE precursors: A metabolic route for preventing AGE accumulation in cardiovascular tissue. Diabetes 2009, 58, 2486–2497, doi:10.2337/db09-0375.Baba, S.P.; Barski, O.A.; Ahmed, Y.; O’Toole, T.E.; Conklin, D.J.; Bhatnagar, A.; Srivastava, S. Reductive metabolism of AGE precursors: A metabolic route for preventing AGE accumulation in cardiovascular tissue. Diabetes 2009, 58, 2486–2497. [Google Scholar] [CrossRef]
- Wortmann, M.; Hakimi, M.; Fleming, T.; Peters, A.S.; Sijmonsma, T.P.; Herzig, S.; Nawroth, P.P.; Böckler, D.; Dihlmann, S.A. Glyoxalase-1 knockdown does not have major short term effects on energy expenditure and atherosclerosis in mice. J. Diabetes Res. 2016, 2016, 1–8, doi:10.1155/2016/2981639.Wortmann, M.; Hakimi, M.; Fleming, T.; Peters, A.S.; Sijmonsma, T.P.; Herzig, S.; Nawroth, P.P.; Böckler, D.; Dihlmann, S.A. Glyoxalase-1 knockdown does not have major short term effects on energy expenditure and atherosclerosis in mice. J. Diabetes Res. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Shafie, A.; Xue, M.; Barker, G.; Zehnder, D.; Thornalley, P.J.; Rabbani, N. Reappraisal of putative glyoxalase 1-deficient mouse and dicarbonyl stress on embryonic stem cells in vitro. Biochem. J. 2016, 473, 4255–4270, doi:10.1042/BCJ20160691.Shafie, A.; Xue, M.; Barker, G.; Zehnder, D.; Thornalley, P.J.; Rabbani, N. Reappraisal of putative glyoxalase 1-deficient mouse and dicarbonyl stress on embryonic stem cells in vitro. Biochem. J. 2016, 473, 4255–4270. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, D.M.; Kwon, K.; Park, C. Generation and characterization of mouse knockout for glyoxalase 1. Biochem. Biophys. Res. Commun. 2017, 490, 460–465, doi:10.1016/j.bbrc.2017.06.063.Jang, S.; Kwon, D.M.; Kwon, K.; Park, C. Generation and characterization of mouse knockout for glyoxalase 1. Biochem. Biophys. Res. Commun. 2017, 490, 460–465. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308, doi:10.1038/nprot.2013.143.Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Lodd, E.; Wiggenhauser, L.M.; Morgenstern, J.; Fleming, T.H.; Poschet, G.; Büttner, M.; Tabler, C.T.; Wohlfart, D.P.; Nawroth, P.P.; Kroll, J. The combination of loss of glyoxalase1 and obesity results in hyperglycemia. JCI Insight 2019, 4, doi:10.1172/jci.insight.126154.Lodd, E.; Wiggenhauser, L.M.; Morgenstern, J.; Fleming, T.H.; Poschet, G.; Büttner, M.; Tabler, C.T.; Wohlfart, D.P.; Nawroth, P.P.; Kroll, J. The combination of loss of glyoxalase1 and obesity results in hyperglycemia. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated levels of the reactive metabolite methylglyoxal recapitulate progression of type 2 diabetes. Cell Metab. 2018, 27, 926–934.e8, doi:10.1016/j.cmet.2018.02.003.Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated levels of the reactive metabolite methylglyoxal recapitulate progression of type 2 diabetes. Cell Metab. 2018, 27, 926–934.e8. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, J.; Fleming, T.; Schumacher, D.; Eckstein, V.; Freichel, M.; Herzig, S.; Nawroth, P. Loss of glyoxalase 1 induces compensatory mechanism to achieve dicarbonyl detoxification in mammalian schwann cells. J. Biol. Chem. 2017, 292, 3224–3238, doi:10.1074/jbc.M116.760132.Morgenstern, J.; Fleming, T.; Schumacher, D.; Eckstein, V.; Freichel, M.; Herzig, S.; Nawroth, P. Loss of glyoxalase 1 induces compensatory mechanism to achieve dicarbonyl detoxification in mammalian schwann cells. J. Biol. Chem. 2017, 292, 3224–3238. [Google Scholar] [CrossRef] [PubMed]
- Schemmel, K.E.; Padiyara, R.S.; D’Souza, J.J. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: A review. J. Diabetes Its Complicat. 2010, 24, 354–360, doi:10.1016/j.jdiacomp.2009.07.005.Schemmel, K.E.; Padiyara, R.S.; D’Souza, J.J. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: A review. J. Diabetes Its Complicat. 2010, 24, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Morgenstern, J.; Oguchi, Y.; Volk, N.; Kopf, S.; Groener, J.B.; Nawroth, P.P.; Fleming, T.; Freichel, M. Compensatory mechanisms for methylglyoxal detoxification in experimental & clinical diabetes. Mol. Metab. 2018, 18, 143–152, doi:10.1016/j.molmet.2018.09.005.Schumacher, D.; Morgenstern, J.; Oguchi, Y.; Volk, N.; Kopf, S.; Groener, J.B.; Nawroth, P.P.; Fleming, T.; Freichel, M. Compensatory mechanisms for methylglyoxal detoxification in experimental & clinical diabetes. Mol. Metab. 2018, 18, 143–152. [Google Scholar] [CrossRef]
- Di Emidio, G.; Santini, S.J.; D’Alessandro, A.M.; Vetuschi, A.; Sferra, R.; Artini, P.G.; Carta, G.; Falone, S.; Amicarelli, F.; Tatone, C. SIRT1 participates in the response to methylglyoxal-dependent glycative stress in mouse oocytes and ovary. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 1389–1401, doi:10.1016/j.bbadis.2019.02.011.Di Emidio, G.; Santini, S.J.; D’Alessandro, A.M.; Vetuschi, A.; Sferra, R.; Artini, P.G.; Carta, G.; Falone, S.; Amicarelli, F.; Tatone, C. SIRT1 participates in the response to methylglyoxal-dependent glycative stress in mouse oocytes and ovary. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 1389–1401. [Google Scholar] [CrossRef]
- Roy, A.; Sarker, S.; Upadhyay, P.; Pal, A.; Adhikary, A.; Jana, K.; Ray, M. Methylglyoxal at metronomic doses sensitizes breast cancer cells to doxorubicin and cisplatin causing synergistic induction of programmed cell death and inhibition of stemness. Biochem. Pharmacol. 2018, 156, 322–339, doi:10.1016/j.bcp.2018.08.041.Roy, A.; Sarker, S.; Upadhyay, P.; Pal, A.; Adhikary, A.; Jana, K.; Ray, M. Methylglyoxal at metronomic doses sensitizes breast cancer cells to doxorubicin and cisplatin causing synergistic induction of programmed cell death and inhibition of stemness. Biochem. Pharmacol. 2018, 156, 322–339. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461, doi:10.1152/physrev.00001.2019.Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Shin, R.; Alvarez, S.; Burch, A.Y.; Jez, J.M.; Schachtman, D.P. Phosphoproteomic identification of targets of the Arabidopsis sucrose nonfermenting-like kinase SnRK2.8 reveals a connection to metabolic processes. Proc. Natl. Acad. Sci. USA 2007, 104, 6460–6465, doi:10.1073/pnas.0610208104.Shin, R.; Alvarez, S.; Burch, A.Y.; Jez, J.M.; Schachtman, D.P. Phosphoproteomic identification of targets of the Arabidopsis sucrose nonfermenting-like kinase SnRK2.8 reveals a connection to metabolic processes. Proc. Natl. Acad. Sci. USA 2007, 104, 6460–6465. [Google Scholar] [CrossRef]
- Khan, M.M.K.; Jan, A.; Karibe, H.; Komatsu, S. Identification of phosphoproteins regulated by gibberellin in rice leaf sheath. Plant Mol. Biol. 2005, 58, 27–40, doi:10.1007/s11103-005-4013-1.Khan, M.M.K.; Jan, A.; Karibe, H.; Komatsu, S. Identification of phosphoproteins regulated by gibberellin in rice leaf sheath. Plant Mol. Biol. 2005, 58, 27–40. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Jamshed, M.; Kumar, A.; Skori, L.; Scandola, S.; Wang, T.; Spiegel, D.; Samuel, M. Glyoxalase goes green: The expanding roles of glyoxalase in plants. Int. J. Mol. Sci. 2017, 18, 898, doi:10.3390/ijms18040898.Sankaranarayanan, S.; Jamshed, M.; Kumar, A.; Skori, L.; Scandola, S.; Wang, T.; Spiegel, D.; Samuel, M. Glyoxalase goes green: The expanding roles of glyoxalase in plants. Int. J. Mol. Sci. 2017, 18, 898. [Google Scholar] [CrossRef]
- Proietti, S.; Falconieri, G.S.; Bertini, L.; Baccelli, I.; Paccosi, E.; Belardo, A.; Timperio, A.M.; Caruso, C. GLYI4 plays A role in methylglyoxal detoxification and jasmonate-mediated stress responses in arabidopsis thaliana. Biomolecules 2019, 9, 635, doi:10.3390/biom9100635.Proietti, S.; Falconieri, G.S.; Bertini, L.; Baccelli, I.; Paccosi, E.; Belardo, A.; Timperio, A.M.; Caruso, C. GLYI4 plays A role in methylglyoxal detoxification and jasmonate-mediated stress responses in arabidopsis thaliana. Biomolecules 2019, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Choi, B.Y.; Murata, K.; Kimura, A. Sexual response of Saccharomyces cerevisiae: Phosphorylation of yeast glyoxalase I by a cell extract of mating factor-treated cells. J. Biochem. 1990, 108, 4–6, doi:10.1093/oxfordjournals.jbchem.a123159.Inoue, Y.; Choi, B.Y.; Murata, K.; Kimura, A. Sexual response of Saccharomyces cerevisiae: Phosphorylation of yeast glyoxalase I by a cell extract of mating factor-treated cells. J. Biochem. 1990, 108, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, A.; Kim, K.-R.; Oshima, G.; Kunimoto, M.; Okawa, K.; Iwamatsu, A.; Nakagawa, Y. Nitric oxide inactivates glyoxalase I in cooperation with glutathione. J. Biochem. 2000, 128, 647–654, doi:10.1093/oxfordjournals.jbchem.a022797.Mitsumoto, A.; Kim, K.-R.; Oshima, G.; Kunimoto, M.; Okawa, K.; Iwamatsu, A.; Nakagawa, Y. Nitric oxide inactivates glyoxalase I in cooperation with glutathione. J. Biochem. 2000, 128, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Birkenmeier, G.; Stegemann, C.; Hoffmann, R.; Günther, R.; Huse, K.; Birkemeyer, C. Posttranslational modification of human glyoxalase 1 indicates redox-dependent regulation. PLoS ONE 2010, 5, e10399, doi:10.1371/journal.pone.0010399.Birkenmeier, G.; Stegemann, C.; Hoffmann, R.; Günther, R.; Huse, K.; Birkemeyer, C. Posttranslational modification of human glyoxalase 1 indicates redox-dependent regulation. PLoS ONE 2010, 5, e10399. [Google Scholar] [CrossRef] [PubMed]
- Spanos, C.; Maldonado, E.M.; Fisher, C.P.; Leenutaphong, P.; Oviedo-Orta, E.; Windridge, D.; Salguero, F.J.; Bermúdez-Fajardo, A.; Weeks, M.E.; Evans, C.; et al. Proteomic identification and characterization of hepatic glyoxalase 1 dysregulation in non-alcoholic fatty liver disease. Proteome Sci. 2018, 16, 1–16, doi:10.1186/s12953-018-0131-y.Spanos, C.; Maldonado, E.M.; Fisher, C.P.; Leenutaphong, P.; Oviedo-Orta, E.; Windridge, D.; Salguero, F.J.; Bermúdez-Fajardo, A.; Weeks, M.E.; Evans, C.; et al. Proteomic identification and characterization of hepatic glyoxalase 1 dysregulation in non-alcoholic fatty liver disease. Proteome Sci. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- de Hemptinne, V.; Rondas, D.; Toepoel, M.; Vancompernolle, K. Phosphorylation on Thr-106 and NO-modification of glyoxalase I suppress the TNF-induced transcriptional activity of NF-κB. Mol. Cell. Biochem. 2009, 325, 169–178, doi:10.1007/s11010-009-0031-7.de Hemptinne, V.; Rondas, D.; Toepoel, M.; Vancompernolle, K. Phosphorylation on Thr-106 and NO-modification of glyoxalase I suppress the TNF-induced transcriptional activity of NF-κB. Mol. Cell. Biochem. 2009, 325, 169–178. [Google Scholar] [CrossRef]
- Morgenstern, J.; Katz, S.; Krebs-Haupenthal, J.; Chen, J.; Saadatmand, A.; Cortizo, F.G.; Moraru, A.; Zemva, J.; Campos, M.C.; Teleman, A.; et al. Phosphorylation of T107 by CamKIIδ regulates the detoxification efficiency and proteomic integrity of glyoxalase 1. Cell Rep. 2020, 32, 108160, doi:10.1016/j.celrep.2020.108160.Morgenstern, J.; Katz, S.; Krebs-Haupenthal, J.; Chen, J.; Saadatmand, A.; Cortizo, F.G.; Moraru, A.; Zemva, J.; Campos, M.C.; Teleman, A.; et al. Phosphorylation of T107 by CamKIIδ regulates the detoxification efficiency and proteomic integrity of glyoxalase 1. Cell Rep. 2020, 32, 108160. [Google Scholar] [CrossRef]
- de Hemptinne, V.; Rondas, D.; Vandekerckhove, J.; Vancompernolle, K. Tumour necrosis factor induces phosphorylation primarily of the nitric-oxide-responsive form of glyoxalase I. Biochem. J. 2007, 407, 121–128, doi:10.1042/BJ20070379.de Hemptinne, V.; Rondas, D.; Vandekerckhove, J.; Vancompernolle, K. Tumour necrosis factor induces phosphorylation primarily of the nitric-oxide-responsive form of glyoxalase I. Biochem. J. 2007, 407, 121–128. [Google Scholar] [CrossRef]
- Santos, A.L.; Lindner, A.B. Protein posttranslational modifications: Roles in aging and age-related disease. Oxidative Med. Cell. Longev. 2017, 2017, 1–19. [Google Scholar] [CrossRef]
- Egyud, L.G.; Szent-Gyorgyi, A. On the regulation of cell division. Proc. Natl. Acad. Sci. USA 1966, 56, 203–207. [Google Scholar] [CrossRef]
- Szent-Gyorgyi, A.; Egyud, L. On regulators of cell division. Science 1966, 152, 676–677. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M. On the promine/retine theory of cell division: Now and then. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1999, 1426, 1–16. [Google Scholar] [CrossRef]
- Huang, K.; Rudolph, F.B.; Bennett, G.N. Characterization of methylglyoxal synthase from Clostridium acetobutylicum ATCC 824 and its use in the formation of 1, 2-propanediol. Appl. Environ. Microbiol. 1999, 65, 3244–3247. [Google Scholar] [CrossRef] [PubMed]
- Jagt, D.L.V. Glyoxalase II: Molecular characteristics, kinetics and mechanism. Biochem. Soc. Trans. 1993, 21, 522–527. [Google Scholar] [CrossRef]
- Douglas, H. Science, hormesis and regulation. Hum. Exp. Toxicol. 2008, 27, 603–607. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Nakamura, H. Overview of biological, epidemiological, and clinical evidence of radiation hormesis. Int. J. Mol. Sci. 2018, 19, 2387. [Google Scholar] [CrossRef]
- Cook, R.; Calabrese, E.J. The importance of hormesis to public health. Environ. Health Perspect. 2006, 114, 1631–1635. [Google Scholar] [CrossRef]
- Rattan, S.I.S. Hormesis in aging. Ageing Res. Rev. 2008, 7, 63–78. [Google Scholar] [CrossRef]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef]
- Zemva, J.; Fink, C.A.; Fleming, T.H.; Schmidt, L.; Loft, A.; Herzig, S.; Knieß, R.A.; Mayer, M.; Bukau, B.; Nawroth, P.P.; et al. Hormesis enables cells to handle accumulating toxic metabolites during increased energy flux. Redox Biol. 2017, 13, 674–686. [Google Scholar] [CrossRef]
- Ravichandran, M.; Priebe, S.; Grigolon, G.; Rozanov, L.; Groth, M.; Laube, B.; Guthke, R.; Platzer, M.; Zarse, K.; Ristow, M. Impairing L-threonine catabolism promotes healthspan through methylglyoxal-mediated proteohormesis. Cell Metab. 2018, 27, 914–925.e5. [Google Scholar] [CrossRef] [PubMed]
- Schlotterer, A.; Kukudov, G.; Bozorgmehr, F.; Hutter, H.; Du, X.; Oikonomou, D.; Ibrahim, Y.; Pfisterer, F.; Rabbani, N.; Thornalley, P.; et al. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes 2009, 58, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Cancer biology and hormesis: Human tumor cell lines commonly display hormetic (Biphasic) dose responses. Crit. Rev. Toxicol. 2005, 35, 463–582. [Google Scholar] [CrossRef] [PubMed]
- Nokin, M.-J.; Durieux, F.; Bellier, J.; Peulen, O.; Uchida, K.; Spiegel, D.A.; Cochrane, J.R.; Hutton, C.A.; Castronovo, V.; Bellahcène, A. Hormetic potential of methylglyoxal, a side-product of glycolysis, in switching tumours from growth to death. Sci. Rep. 2017, 7, 11722. [Google Scholar] [CrossRef]
- Antognelli, C.; Moretti, S.; Frosini, R.; Puxeddu, E.; Sidoni, A.; Talesa, V.N. Methylglyoxal acts as a tumor-promoting factor in anaplastic thyroid cancer. Cells 2019, 8, 547. [Google Scholar] [CrossRef]
- Wang, T.; Kartika, R.; Spiegel, D.A. Exploring post-translational arginine modification using chemically synthesized methylglyoxal hydroimidazolones. J. Am. Chem. Soc. 2012, 134, 8958–8967. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=glyoxalase+%5BTitle%5D%29+AND+%281%5BTitle%5D&filter=years.2010-2020 (accessed on 3 September 2020).