Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Massoud Vosough and Version 2 by Catherine Yang.
Organ and tissue shortage are known as a crucially important public health problem as unfortunately a small percentage of patients receive transplants. Liver tissue engineering (TE) enables us to reproduce and restore liver functions, fully or partially, which could be used in the treatment of acute or chronic liver disorders and/or generate an appropriate functional organ which can be transplanted or employed as an extracorporeal device.
- tissue engineering
- regenerative medicine
- liver
1. Application of Tissue Engineering in Liver Disease
To restore liver functions using a functional implantable liver tissue, the following three components are essential: i) bio-compatible scaffolds, ii) functional cells which could be derived from adult tissues or from pluripotent stem cells [1][2] and iii) standardized growth factors and active bio-molecules.
The progress in implantable engineered hepatic tissues could be a promising strategy to overcome the current limitations of cell-based approaches. Limited cellular engraftment and short-term survival of implanted cells are the major challenges which are yet to be achieved [3]. Diverse methodologies can be employed to produce hepatic micro-tissues, such as cell encapsulation, 3D printing, microfluidic systems and decellularization/recellularization approaches [4]. A schematic representation of liver diseases, and possible application of technologies used in liver tissue engineering presented in Figure 1.
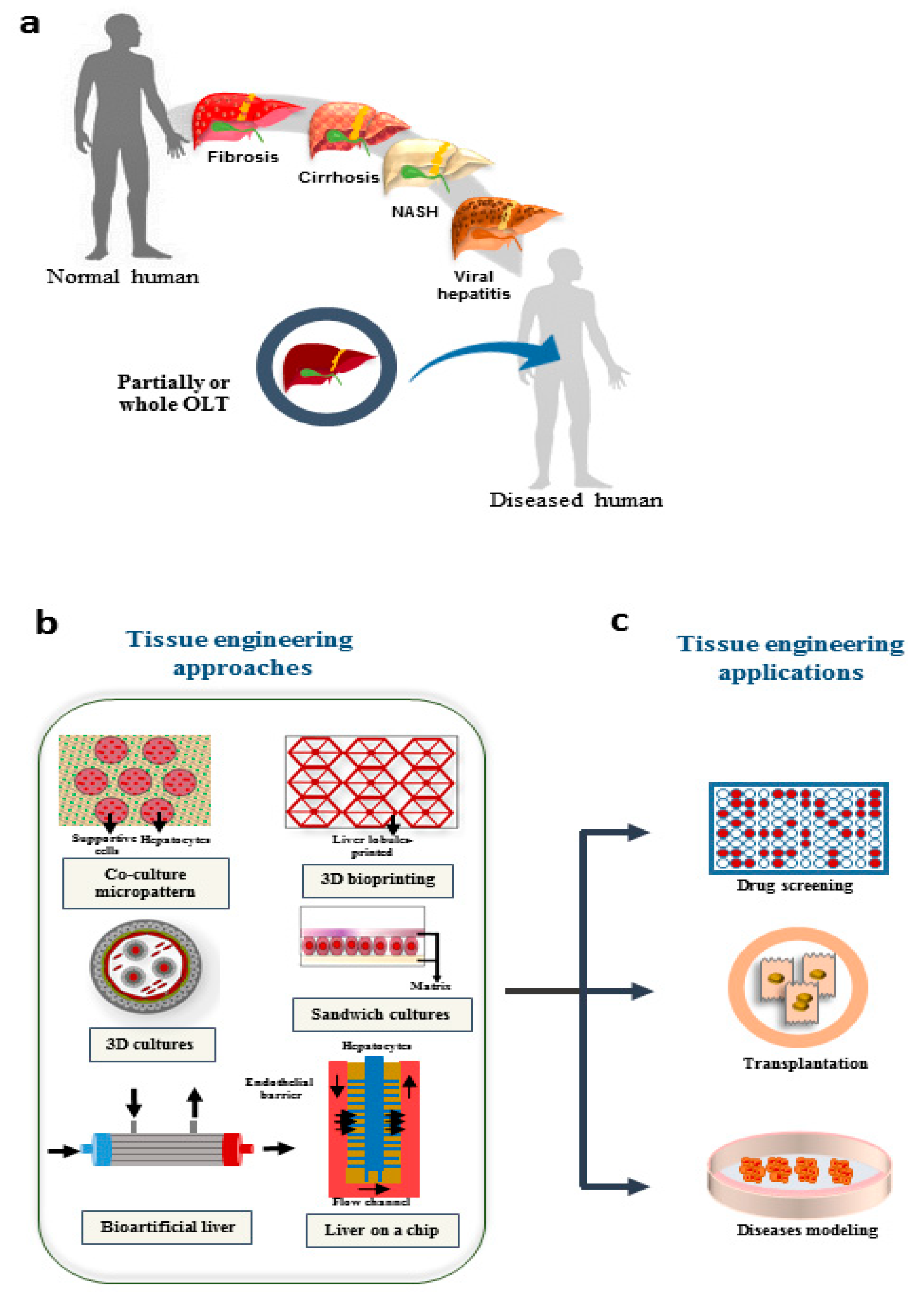
Figure 1. Possible applications of TE in treatment of liver diseases. (a) Different diseases that result in liver failure; the only approved approach for end stage diseases is liver transplantation. (b) Different engineering approaches are growing to overcome the limitations in treatment of organ failure, drug screening, and disease modeling. (c) The possible applications which are promising using tissue engineering approaches. NASH: Nonalcoholic steatohepatitis; OLT: orthotopic liver transplantation.
The progress in implantable engineered hepatic tissues could be a promising strategy to overcome the current limitations of cell-based approaches.
2. Different Scaffolds Applied in Liver TE
Biocompatibility was the significant feature of the first-generation biomaterials; however, second-generation ones are characterized by their bio-interactivity. While the first-generation biomaterials were passive, second generation components were specifically designed to induce tissue regeneration. In order to improve the mechanical features of polymers, to use their great properties and to enhance tissue interaction, ceramics and polymer composites have been proposed [5]. Nowadays, third-generation biomaterials are bio-responsive and capable to activate specific genes involved in cell differentiation, function and proliferation [6].
2.1. Physical and Biochemical Properties of Scaffolds Used in Liver TE
Safety and biocompatibility are principal features of crucial importance for biosynthetic liver scaffolds. Cells embedded in bio-artificial scaffolds should be capable to replicate, dig for extra space or even generate new extra cellular matrix (ECM) [7][8]. Thus, an ideal scaffold should mimic the physiologic properties of native liver ECM. However, the development of a scaffold, capable to support cell functions depends on parameters such as the surface features, underlying material, and characteristics of the selected cell line [7]. Biocompatibility of scaffold permits concurrent generation of new tissues along with matrix degradation [9]. The biological characteristics of the scaffolds influence their interactions with target organs and tissues. Furthermore, an optimized scaffold should circumvent immune system for incorporated cells. The immune-inert biomaterials with characteristic immune regulatory features (i.e., reduced activity of NK cells as well as B and T cells-mediated immunity) have been recently proposed [9].
The majority of scaffolds are typically made of hybrid materials, bio-ceramics and polymers, whether synthetic or natural [10].
In order to achieve better cell attachment and make the surface more similar to the in vivo conditions, 3D culture systems containing ECM proteins have been proposed.
While hydrogels such as the collagen sandwich or Matrigel consist of almost exclusive ECM proteins, they are often integrated directly into the scaffold matrix in scaffold-based 3D cultivation systems, or the scaffold is subsequently coated with them. In addition to the liver-specific ECM proteins, fibronectin, collagen type I and gelatin are often used [11][12]. Since gelatin is a byproduct of collagen hydrolysis, it contains the same RGD peptides. Therefore, it is also often used as a component of the scaffold matrix [13].
Pre-incubation of the scaffold in serum-containing medium, may enhance cell adherence. In order to achieve optimal cell adherence, a relatively long pre-incubation period of up to 10 days, is sometimes required [14].
In general, scaffold-based 3D cultivation systems which are commonly used for the cultivation of liver cells, can be divided into two groups. The first group includes porous scaffold materials such as Cryogels®, porous natural products, or scaffolds made using electrospinning or a 3D printer [15][16][17]. In the second group, the live cells are completely enclosed by the scaffold matrix [16]. Among the 3D culture methods, many systems use hepatocytes on natural sponges or other natural products like silk fibroin protein [14][16][18][19].
2.2. Elasticity, Porosity, and Other Physical Properties of Scaffolds in Liver TE
The optimum stiffness and elasticity of a healthy human liver have been estimated between 400 and 600 Pa [20][21]. The liver lobules have no basal membrane and relatively little amount of extracellular matrix. Together with the numerous fenestrations and gaps within sinusoidal endothelial cells, this structure allows rapid bidirectional exchange of macromolecules between plasma and hepatocytes [22]. For a bio-artificial scaffold, at least a porosity of 95% is required to allow the exchange of nutrients and wastes products. Additionally, a large surface/volume ratio is necessary to promote hepatocyte attachment and maintenance [12]. The optimal pore size is required to maintain the polarity of the cells. It seems that cell-cell interactions are also required, which suggests rather larger pores. In a study carried out on rat hepatocytes, it has been shown that the pore sizes of 10 µm or 80 µm lead to an improved hepatic function. Moreover, an increase in metabolic function with the 80 µm pores was observed especially at a high cell concentration, which indicates that an interaction among the cells [23]. In order to provide a sufficient supply of nutrients and to allow facilitated exchange among the cells, the scaffold material should be highly permeable. Since there is no blood supply in vitro, it is necessary to reduce the number of cells particularly in static culture condition compared to the in vivo conditions. However, it should be considered that a reduction in the cell concentration also reduces the possibility of cell-cell interactions, which is accompanied by a reduced function of the cultivation system. In order to ensure that the cells can efficiently migrate through the scaffold, it is also necessary that the pores should be interconnected. The stiffness of the scaffold also has an influence on the metabolic activity of the cells. In a recent study, polydimethylsiloxane (PDMS) was used to generate different levels of rigidity, resulting in cells with superior metabolic activity when cultured on a substrate with approximate 2 kPa stiffness compared to 50 kPa on polystyrene substrate, where the stiffness can be approximately 3 GPa [24].
Synthetic hydrogels can be degradable or non-degradable. Compared to natural ones, the advantages of synthetic hydrogels increased the potential application of synthetic hydrogels in TE approaches. These hydrogels are reproducible, less immunogenic, mechanically tougher. But these hydrogels are not popular for liver tissue engineering in clinical application [25].
Table 1 shows some common 3D models which employ scaffold or hydrogel type and their advantages and disadvantages considering the culture method.
Table 1. Common types materials used in 3D cultures, and their advantages and disadvantages.
| Type of 3D Culture | Cultivation Technique/Coating Material | Production Technique | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Hydrogel based Scaffold | Collagen Sandwich, Collagen Gel/Isolated from rat tails | Gel formation by crosslinking of the water-soaked collagen–fibers | a) Containing collagen type I b) Maintenance of hepatocytes polarity including transporter activity |
a) Reduced exchange of nutrients and waste products between cells and medium b) Dead cells were not removed within the matrix C) Disruption of living cells by proteases released from dead cells |
[26] |
| Matrigel/ECM proteins extracted from mice Englebreth-Holm-Swarm tumors | Cold Matrigel is mixed with medium and plated between 2 and 6 °C as fluid solution. Temperatures ≥ 10 °C results in a solid gel formation | a) Cell polarity preserved b) Containing various ECM proteins and growth factors c) Promotion of cell differentiation |
a) The same disadvantages as described for collagen b) The components of the Matrigel are not well defined |
[27][28] | |
| Scaffold | Decellularized Human Liver as a Natural Scaffold | Tissue was decellularized, remaining ECM was used as scaffold for culture | a) Perfectly represents the structural features as well as the biochemical components of the human liver matrix | a) Elaborate production b) Limited availability of donor tissue |
[29] |
| Cryogel/PHEMA, Bis-Acrylamide, Alginate, Gelatin, Collagen | Monomers are frozen in aqueous solution with crosslinking agents. Ice crystals form, which remains after polymerization and thawing as pores in the scaffold matrix | a) Simple preparation b) Create various pore sizes and stiffness |
a) Difficult standardization of the manufacturing process b) Variation in scaffold parameters possible only in certain range |
[11][13] | |
| Electrospinning/Natural or synthetic polymer solutions | electrostatic fiber formation which utilizes electrical forces to produce polymer fibers | a) Relatively high standardizable b) Using different materials c) Using different fiber strengths and degrees of intertwining adjustable |
a) Generating solid tissue structure during electrospinning intertwined fibers | [12][30] | |
| 3D printing/Natural products like gelatin and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxy succinimide (NHS) for crosslinking | Scaffold was printed by using a 3D printer | a) Uniform and reproducible b) Reduction of user error c) Precisely adjustable scaffold pore size d) interconnectivity and controlled geometry |
a) Requires elaborated equipment b) High standardization results in lacking of representation of the biological variability c) Generating pores with many different sizes is difficult |
[31] |
ECM: extracellular matrix. PHEMA: Poly 2-hydroxyethyl methacrylate.
3. Different Approaches in Liver TE
3.1. Decellularization/Recellularization Approach
Extracellular matrix plays a key role in cell adherence, polarity, proliferation, differentiation [32][33] and can promote liver functions such as cytochromes P450 (CYPs) activity in organoids [34].
Due to polymorphic differences that exist between human and other species, the ideal biomaterials for liver tissue engineering should be human derived. Decellularized organ is a suitable scaffold with a proper and specific microstructure for the implanted cells of the original organ.
By using the ECM of an acellularized liver, which can be integrated into the scaffold matrix, the highest similarity with the in vivo conditions can be achieved [35]. During decellularization, cells and other immunogenic factors are removed and only the natural scaffold of tissue remains. This approach could provide an alternative source of implantable organs in OLT [36]. Compared to other techniques, in this method, the original template of the vascular network and biliary system are maintained, and this is the greatest point that can be noted in liver tissue engineering [36]. In fact, three important parameters should be considered in recellularization technique including; i) selection of suitable cell types, ii) route of cell administration, and iii) optimized cell seeding protocols. [4]. In 2015, the first whole organ decellularization protocol was introduced by Ott et al. [37].
This decellularized human livers were later repopulated using hepatic stellate cells (LX2), hepatocellular carcinoma (Sk-Hep-1) and hepatoblastoma cells (HepG2). Ex vivo preservation was prolonged for up to 21 days, with excellent cell viability, motility and proliferation and remodeling of the extracellular matrix [29]. Another study developed a humanized liver by using acellularized porcine liver and combinations of human fetal hepatocytes and stellate cells. This study demonstrated that the acellularized matrix could support and induce phenotypic maturation of engrafted human fetal hepatocytes in a continuously perfused system [38].
An efficient and successful decellularization process, preserves the initial pattern of ECM and provides a proper niche for seeded cells. After cell homing, the neo-organ should be able to present some levels of functional maturation in the perfusion bioreactor and subsequently, the new organ could be transplanted without extreme immunosuppression. In 2010, Uygun et al. published the first study on recellularization of an acellularized liver that was transplanted in a rat model. The recellularized graft was maintained in a perfusion chamber for up to 2 weeks before implantation. This was the first report that supported functionality of the re-seeded hepatocytes which were cultured on a decellularized 3D ECM scaffold. However, this study highlighted some key questions; for instance, what is the proper flow rate for recellularization?
At slow flow rate, the reagents do not reach the depth of tissues and at fast flow rates, cell clamps are produced. Furthermore, during recellularization, intravascular liver thrombosis could be happening, and the vessels might be blocked [39].
To examine the best method for recellularization of an organ, a study from 2011 described as a multistep infusion is associated with the most favorable results. The described methods are as the following: i) direct parenchymal injection, ii) continuous perfusion and iii) multistep infusion. This study showed that multistep infusion is associated with the most favorable results [40].
In conclusion, different bio-scaffolds can be employed in transplantation and pharmaceutical and toxicological studies and may act as a reliable tool to study normal organ development as well as liver basic pathology [41].
3.2. Cell Encapsulation Techniques in Liver TE
Encapsulation is an advanced technology for immobilization of allogenic or xenogeneic cells in a semipermeable scaffold in order to escape immune system and deliver biological products to patients without any immunosuppression [42]. While many advanced technologies are under development, cell encapsulation is the only approach that currently meets all the essential prerequisites for a truly translational medicine [43]. An acceptable capsule should be biocompatible, and the microstructure should provide a suitable niche for cell, survival and proliferation as well as cell functionality. Furthermore, the implant of bio-materials is usually lodged in tissues where long time engraftment and lower immunogenicity are required [44].
Researchers showed that intraperitoneal transplantation of alginate-encapsulated “rat hepatocytes” could provide sufficient metabolic support to rescue an animal models with acute liver failure without immunosuppression up to 7 days [45]. More recently, human-induced pluripotent stem cell-derived hepatocyte-like cells (iPSC-HLC) were co-cultured with human stellate cells and encapsulated in alginate beads. This study showed improved differentiation efficiency of induced pluripotent stem cells (iPSCs) compared to the 2D monoculture conditions. Furthermore, the mentioned structure was implanted in immunocompetent mice for 24 days without any immune rejection [46].
In addition, acellularized ECM derived from liver could be used for cell encapsulation too. In fact, the rich content of growth factors in the ECM is important to provide proper interactions between the incorporated cells and surrounding ECM. Thus, using specific ECM can cause a remarkable effect in terms of better maintenance of encapsulated liver cells [47]. Despite recent promising results, this technology needs more validation for long-term in vitro maintenance and in vivo transplantation for clinical applications.
The major challenges in cell encapsulation technology are the risk of immunogenicity of the bio-materials and toxicity of particular components used for crosslinking. However, promotion of this field needs progress in several aspects, including more research in particular liver disease models, reduction and modification of fibrogenesis reaction during inflammation, and improvement of neovascularization through the model structure [32][48].
3.3. 3D Bio-Printing in Liver TE
3D bio-printing technology, as a multidisciplinary approach, benefits from chemistry, material science and biology [49]. Proper spatio-temporal status and polarity of cells as well as effective cell-cell and cell-ECM interactions could be provided using 3D bio-printers. 3D bio-printers can use different materials and structure them based on a computer-aided design (CAD) [50].
3D bio-printing technique aims to fabricate biomimetic self-assembling constructs and can use micro-tissues (or spheroids) as building blocks. However, solid organs, like the liver, are probably the most difficult ones to print because of their complex vascularization and innervation pattern [51].
During bio-printing process, live cells are suspended in a hydrogel solution, namely bio-ink. The bio-ink could be cross-linked during or immediately after the bio-printing process, to shape the final architecture of the designed construct. The hydrogel-based bio-inks may be made from natural or synthetic biomaterials, or a combination of both as hybrid materials. Natural biomaterial-based bio-inks include: alginate, gelatin, collagen, fibrin, fibronectin, gellan gum, hyaluronic acid, agarose, chitosan, silk, acellularized extracellular matrix, cellulose, etc. The synthetic bio-inks may include: polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), pluronic polymers [52][53], etc. The ideal bi-oink should have the proper physiochemical properties, such as suitable mechanical, rheological, chemical and biological ones [54]. A practical biomaterial for 3D bio-printing is usually a biocompatible substance, which should be easily manipulated and it could maintain or even enhance cell viability and functions [55]. Different types of 3D bio-printing technologies have been introduced so far, including ink-jet-based bio-printing [56], laser-assisted bio-printing [57], extrusion-based bio-printing [58], stereo-lithography-based bio-printing [59] and microvalve-based bio-printing [60] and many other novel emerging technologies [61]. Among these technologies, probably extrusion-based bio-printing has been the most widely used one to construct living 3D tissues and organs [62]. The first report using bio-printer was launched by Klebe in 1988, in which biomaterials such as collagen and fibronectin were printed while the hydrogel contained fibroblasts [63].
Later, Chang et al. used alginate as a bio-ink and designed a microchip model for drug metabolism studies. In this study, they used multi-head deposition system (MHDS) that carried out a layer-by-layer deposition of HepG2 cells and alginate simultaneously, then, integrated the 3D bio-printed construct into a microfluidic system [64]. In 2015, gelatin-alginate-fibrinogen-based hydrogel was used as a representative matrix model for natural liver ECM. The parenchymal and non-parenchymal cells were successfully embedded in this hydrogel. The results showed increased hepatocyte viability in 3D co-culture and enhanced drug metabolism [65].
Recently, a novel co-culture system using bio-printed tissue constructs seeded with primary hepatocyte, hepatic stellate and endothelial cells, has been described that successfully mimics liver fibrosis condition [66]. In 2016, a 3D microscale hexagonal architecture was printed using hydrogel in which, hiPSCs-hepatic progenitor cells (HPCs), human umbilical vein endothelial cells and adipose-derived stromal cells were embedded. This 3D model showed a practical phenotype and increased the physiologic function of cells over the weeks [59]. In 2017, a study demonstrated a method for fabricating scalable liver-like tissue by fusing hundreds of liver bud-like spheroids. Such fabricated liver-like tissue exhibited self-organization ex vivo and was successfully engrafted in rat liver. This was a new method for transplantation of ex vivo generated organoids [67]. In fact, bio-printing technology facilitates automated and high-throughput fabrication of sophisticated and controlled 3D structures. Thus, combining them with bioreactors may lead to the realization of next-generation organ-on-a-chip platforms [68].
In conclusion, 3D bio-printing is a promising technology in the field of bio-artificial organ generation, which may overcome various limitations encountered in different models [51] and improve maturation of hepatocyte like cells (HLCs) [60]. Furthermore, this technology could preserve ex vivo hepatocyte function and maintenance [56]. Also, thanks to the multi-nozzle 3D bio-printers and novel biocompatible polymers, the artificial organs could be more similar to the original tissue compartments [62].
3.4. Microfluidic Systems in Liver TE
Organ-on-a-chip technologies are microfluidic systems that can recapitulate in vivo structures. These are systems, with or without perfusion, in which lobular or spheroid-based structures mimic a minimized environment in order to build functional units [69]. This promising point has attracted attention of many pharmaceutical companies. Up to now, at least 28 organ-on-a-chip companies have been registered in less than 7 years [70]. Mimicking hepatic structure and complexity is one of the reliable approaches in this field. Liver-on-a-chip systems have been shown to be able to predict possible toxicity and improve the sensitivity of certain drugs which are comparable with in vivo data [71]. One study developed an in vitro liver sinusoid chip by integrating four types of primary murine hepatic cells, including parenchymal and non-parenchymal liver cells, into two adjacent fluid channels separated by a porous permeable membrane. This microfluidic chip replicated liver physiological cell composition, microscopic architecture and mechanical microenvironment [72]. Spheroid-based microfluidic model is another approach that overcomes many problems of static cell culture systems. In 2016, a model was developed using bio-printing hepatic spheroids encapsulated in a hydrogel scaffold in a microfluidic device for drug-induced toxicity [73]. In another study, a spheroid-based model was established using co-cultivation of rat hepatocytes and hepatic stellate cells to prolong hepatic functions under chip culture condition [74].
A worthy liver-on-chip platform was reported by Lee et al. and it mimics sinusoidal and hepatic cord-like structures [75]. Some studies used hepatocytes 2D culture in liver-on-chip hepatocytes cultured in a 2D monolayer on top of a porous membrane sandwiched between two micro-channels. These systems allow hepatocytes to associate with other cells like endothelial cells [75][76].
In a recent study, researchers designed a very large-scale liver-lobule (VLSLL) on-a-chip device that provided a micro-physiological niche for hepatocytes [77].
Even though liver-on-a-chip is still in its early phases of development, recent progresses in the prediction of drug toxicity are highly promising.
References
- Vosough, M.; Omidinia, E.; Kadivar, M.; Shokrgozar, M.-A.; Pournasr, B.; Aghdami, N.; Baharvand, H.J. Generation of functional hepatocyte-like cells from human pluripotent stem cells in a scalable suspension culture. Stem Cells Dev. 2013, 22, 2693–2705.
- Farzaneh, Z.; Pakzad, M.; Vosough, M.; Pournasr, B.; Baharvand, H.J. Differentiation of human embryonic stem cells to hepatocyte-like cells on a new developed xeno-free extracellular matrix. Histochem. Cell Boil. 2014, 142, 217–226.
- Lee, S.-W.; Wang, X.; Chowdhury, N.R.; Roy-Chowdhury, J. Hepatocyte transplantation: State of the art and strategies for overcoming existing hurdles. Ann. Hepatol. 2004, 3, 48–53.
- Mazza, G.; Al-Akkad, W.; Rombouts, K.; Pinzani, M. Liver tissue engineering: From implantable tissue to whole organ engineering. Hepatol. Commun. 2018, 2, 131–141.
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16.
- Rahman, S.; Nagrath, M.; Ponnusamy, S.; Arany, P. Nanoscale and macroscale scaffolds with controlled-release polymeric systems for dental craniomaxillofacial tissue engineering. Materials 2018, 11, 1478.
- Meyer, U.; Meyer, T.; Handschel, J.; Wiesmann, H.P. Fundamentals of Tissue Engineering and Regenerative Medicine; Springer: New York, NY, USA, 2009.
- Li, Z.; Xie, M.-B.; Li, Y.; Ma, Y.; Li, J.-S.; Dai, F.-Y. Recent progress in tissue engineering and regenerative medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766.
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262.
- Yang, Y.; Ritchie, A.C.; Everitt, N.M. Comparison of glutaraldehyde and procyanidin cross-linked scaffolds for soft tissue engineering. Mater. Sci. Eng. C 2017, 80, 263–273.
- Ruoss, M.; Haussling, V.; Schugner, F.; Olde Damink, L.H.H.; Lee, S.M.L.; Ge, L.; Ehnert, S.; Nussler, A.K. A Standardized Collagen-Based Scaffold Improves Human Hepatocyte Shipment and Allows Metabolic Studies over 10 Days. Bioengineering 2018, 5, 86.
- Rajendran, D.; Hussain, A.; Yip, D.; Parekh, A.; Shrirao, A.; Cho, C.H. Long-term liver-specific functions of hepatocytes in electrospun chitosan nanofiber scaffolds coated with fibronectin. J. Biomed. Mater. Res. A 2017, 105, 2119–2128.
- Kumari, J.; Karande, A.A.; Kumar, A. Combined Effect of Cryogel Matrix and Temperature-Reversible Soluble–Insoluble Polymer for the Development of in Vitro Human Liver Tissue. ACS Appl. Mater. Interfaces 2016, 8, 264–277.
- Amirikia, M.; Shariatzadeh, S.M.A.; Jorsaraei, S.G.A.; Soleimani Mehranjani, M. Impact of pre-incubation time of silk fibroin scaffolds in culture medium on cell proliferation and attachment. Tissue Cell 2017, 49, 657–663.
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745.
- Burkhardt, B.; Martinez-Sanchez, J.J.; Bachmann, A.; Ladurner, R.; Nüssler, A.K. Long-term culture of primary hepatocytes: New matrices and microfluidic devices. Hepatol. Int. 2014, 8, 14–22.
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22.
- Godoy, P.; Hewitt, N.J.; Albrecht, U.; Andersen, M.E.; Ansari, N.; Bhattacharya, S.; Bode, J.G.; Bolleyn, J.; Borner, C.; Boettger, J. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530.
- Chen, J.P.; Lin, T.C. Loofa sponge as a scaffold for culture of rat hepatocytes. Biotechnol. Prog. 2005, 21, 315–319.
- Mueller, S.; Sandrin, L. Liver stiffness: A novel parameter for the diagnosis of liver disease. Hepatic Med. Evid. Res. 2010, 2, 49.
- Desai, S.S.; Tung, J.C.; Zhou, V.X.; Grenert, J.P.; Malato, Y.; Rezvani, M.; Español-Suñer, R.; Willenbring, H.; Weaver, V.M.; Chang, T.T. Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology 2016, 64, 261–275.
- Martinez-Hernandez, A.; Amenta, P.S. The hepatic extracellular matrix. Virchows Archiv A 1993, 423, 77–84.
- Ranucci, C.S.; Kumar, A.; Batra, S.P.; Moghe, P.V. Control of hepatocyte function on collagen foams: Sizing matrix pores toward selective induction of 2-D and 3-D cellular morphogenesis. Biomaterials 2000, 21, 783–793.
- Natarajan, V.; Berglund, E.J.; Chen, D.X.; Kidambi, S. Substrate stiffness regulates primary hepatocyte functions. RSC Adv. 2015, 5, 80956–80966.
- Ye, S.; Boeter, J.W.; Penning, L.C.; Spee, B.; Schneeberger, K.J.B. Hydrogels for liver tissue engineering. Bioengineering 2019, 6, 59.
- Schyschka, L.; Sánchez, J.M.; Wang, Z.; Burkhardt, B.; Müller-Vieira, U.; Zeilinger, K.; Bachmann, A.; Nadalin, S.; Damm, G.; Nussler, A. Hepatic 3D cultures but not 2D cultures preserve specific transporter activity for acetaminophen-induced hepatotoxicity. Arch. Toxicol. 2013, 87, 1581–1593.
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890.
- Swift, B.; Pfeifer, N.D.; Brouwer, K.L.R. Sandwich-cultured hepatocytes: An in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab. Rev. 2010, 42, 446–471.
- Mazza, G.; Rombouts, K.; Rennie Hall, A.; Urbani, L.; Vinh Luong, T.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 13079.
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347.
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater. 2018, 69, 63–70.
- Moya, M.L.; Garfinkel, M.R.; Liu, X.; Lucas, S.; Opara, E.C.; Greisler, H.P.; Brey, E.M. Fibroblast growth factor-1 (FGF-1) loaded microbeads enhance local capillary neovascularization. J. Surg. Res. 2010, 160, 208–212.
- Ghodsizadeh, A.; Hosseinkhani, H.; Piryaei, A.; Pournasr, B.; Najarasl, M.; Hiraoka, Y.; Baharvand, H.J. Galactosylated collagen matrix enhanced in vitro maturation of human embryonic stem cell-derived hepatocyte-like cells. Biotechnol. Lett. 2014, 36, 1095–1106.
- Saheli, M.; Sepantafar, M.; Pournasr, B.; Farzaneh, Z.; Vosough, M.; Piryaei, A.; Baharvand, H. Three-dimensional liver-derived extracellular matrix hydrogel promotes liver organoids function. J. Cell. Biochem. 2018, 119, 4320–4333.
- Damania, A.; Kumar, A.; Teotia, A.K.; Kimura, H.; Kamihira, M.; Ijima, H.; Sarin, S.K.; Kumar, A. Decellularized Liver Matrix-Modified Cryogel Scaffolds as Potential Hepatocyte Carriers in Bioartificial Liver Support Systems and Implantable Liver Constructs. ACS Appl. Mater. Interfaces 2018, 10, 114–126.
- Vorotnikova, E.; McIntosh, D.; Dewilde, A.; Zhang, J.; Reing, J.E.; Zhang, L.; Cordero, K.; Bedelbaeva, K.; Gourevitch, D.; Heber-Katz, E. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010, 29, 690–700.
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221.
- Barakat, O.; Abbasi, S.; Rodriguez, G.; Rios, J.; Wood, R.P.; Ozaki, C.; Holley, L.S.; Gauthier, P.K. Use of decellularized porcine liver for engineering humanized liver organ. J. Surg. Res. 2012, 173, e11–e25.
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.-L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820.
- Soto-Gutierrez, A.; Zhang, L.; Medberry, C.; Fukumitsu, K.; Faulk, D.; Jiang, H.; Reing, J.; Gramignoli, R.; Komori, J.; Ross, M.; et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng. C Methods 2011, 17, 677–686.
- Baptista, P.M.; Siddiqui, M.M.; Lozier, G.; Rodriguez, S.R.; Atala, A.; Soker, S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011, 53, 604–617.
- Orive, G.; Santos, E.; Poncelet, D.; Hernández, R.M.; Pedraz, J.L.; Wahlberg, L.U.; De Vos, P.; Emerich, D. Cell encapsulation: Technical and clinical advances. Trends Pharmacol. Sci. 2015, 36, 537–546.
- Hernández, R.M.; Orive, G.; Murua, A.; Pedraz, J.L. Microcapsules and microcarriers for in situ cell delivery. Adv. Drug Deliv. Rev. 2010, 62, 711–730.
- Rokstad, A.M.A.; Lacik, I.; de Vos, P.; Strand, B.L. Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation. Adv. Drug Deliv. Rev. 2014, 67, 111–130.
- Jitraruch, S.; Dhawan, A.; Hughes, R.D.; Filippi, C.; Soong, D.; Philippeos, C.; Lehec, S.C.; Heaton, N.D.; Longhi, M.S.; Mitry, R.R. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS ONE 2014, 9, e113609.
- Song, W.; Lu, Y.-C.; Frankel, A.S.; An, D.; Schwartz, R.E.; Ma, M. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci. Rep. 2015, 5, 16884.
- Ghim, J.H.; Hussein, K.H.; Park, K.-M.; Woo, H.M. Hepatic cell encapsulation using a decellularized liver scaffold. Biomed. Eng. Lett. 2015, 5, 58–64.
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S. Size-and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651.
- Lužanin, O.; Movrin, D.; Plančak, M. Effect of layer thickness, deposition angle, and infill on maximum flexural force in FDM-built specimens. J. Technol. Plast. 2014, 39, 49–58.
- Arnold, C.B.; Serra, P.; Piqué, A. Laser direct-write techniques for printing of complex materials. MRS Bull. 2007, 32, 23–31.
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785.
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174.
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R.; Inci, I. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946.
- Lee, H.J.; Kim, Y.B.; Ahn, S.H.; Lee, J.S.; Jang, C.H.; Yoon, H.; Chun, W.; Kim, G.H. A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv. Heal. Mater. 2015, 4, 1359–1368.
- Skardal, A.; Zhang, J.; McCoard, L.; Oottamasathien, S.; Prestwich, G.D. Dynamically crosslinked gold nanoparticle–hyaluronan hydrogels. Adv. Mater. 2010, 22, 4736–4740.
- Arai, K.; Yoshida, T.; Okabe, M.; Goto, M.; Mir, T.A.; Soko, C.; Tsukamoto, Y.; Akaike, T.; Nikaido, T.; Zhou, K.; et al. Fabrication of 3D-culture platform with sandwich architecture for preserving liver-specific functions of hepatocytes using 3D bioprinter. J. Biomed. Mater. Res. A 2017, 105, 1583–1592.
- Antoshin, A.; Churbanov, S.; Minaev, N.; Deying, Z.; Yuanyuan, Z.; Shpichka, A.; Timashev, P.; Deying, Z.; Yuanyuan, Z. LIFT-bioprinting, is it worth it? Bioprinting 2019, 15, e00052.
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343.
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.-S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211.
- Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D.-J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102.
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D bioprinting using stem cells. Pediatric Res. 2018, 83, 223–231.
- Liu, F.; Liu, C.; Chen, Q.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Progress in organ 3D bioprinting. Int. J. Bioprinting 2018, 4, 4.
- Klebe, R.J. Cytoscribing: A method for micropositioning cells and the construction of two-and three-dimensional synthetic tissues. Exp. Cell Res. 1988, 179, 362–373.
- Chang, R.; Emami, K.; Wu, H.; Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2010, 2, 045004.
- Zhao, X.; Du, S.; Chai, L.; Zhou, X.; Liu, L.; Xu, Y.; Wang, J.; Zhang, W.; Liu, C.H.; Wang, X. Anti-cancer drug screening based on a adipose-derived stem cell/hepatocyte 3D printing technique. J. Stem Cell Res. Ther. 2015, 5.
- Norona, L.M.; Nguyen, D.G.; Gerber, D.A.; Presnell, S.C.; LeCluyse, E.L. Editor’s highlight: Modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol. Sci. 2016, 154, 354–367.
- Yanagi, Y.; Nakayama, K.; Taguchi, T.; Enosawa, S.; Tamura, T.; Yoshimaru, K.; Matsuura, T.; Hayashida, M.; Kohashi, K.; Oda, Y. In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci. Rep. 2017, 7, 14085.
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101.
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772.
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278.
- Khetani, S.R.; Berger, D.R.; Ballinger, K.R.; Davidson, M.D.; Lin, C.; Ware, B.R. Microengineered Liver Tissues for Drug Testing. J. Lab. Autom. 2015, 20, 216–250.
- Du, Y.; Li, N.; Yang, H.; Luo, C.; Gong, Y.; Tong, C.; Gao, Y.; Lü, S.; Long, M. Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab Chip 2017, 17, 782–794.
- Knowlton, S.; Tasoglu, S. A bioprinted liver-on-a-chip for drug screening applications. Trends Biotechnol. 2016, 34, 681–682.
- Lee, S.-A.; Kang, E.; Ju, J.; Kim, D.-S.; Lee, S.-H. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte–hepatic stellate cell interactions and flow effects. Lab Chip 2013, 13, 3529–3537.
- Lee, P.J.; Hung, P.J.; Lee, L.P. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol. Bioeng. 2007, 97, 1340–1346.
- Dash, A.; Simmers, M.B.; Deering, T.G.; Berry, D.J.; Feaver, R.E.; Hastings, N.E.; Pruett, T.L.; LeCluyse, E.L.; Blackman, B.R.; Wamhoff, B.R. Hemodynamic flow improves rat hepatocyte morphology, function, and metabolic activity in vitro. Am. J. Physiol. Cell Physiol. 2013, 304, C1053–C1063.
- Banaeiyan, A.A.; Theobald, J.; Paukštyte, J.; Wölfl, S.; Adiels, C.B.; Goksör, M. Design and fabrication of a scalable liver-lobule-on-a-chip microphysiological platform. Biofabrication 2017, 9, 015014.
More
