Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Divyam Girdhar and Version 3 by Jessie Wu.
Immune-compromised diseases lead to a majority of opportunistic conditions such as oral fungal infections, also known as oral mycosis. Often, the impairment of host resistance leads to the initiation of pathogenic conditions in the oral cavity, and progression through local colonization. The use of immunosuppressive drugs and immunodeficiency upon viral infection, especially in COVID-19 patients, has led to a significant increase in the frequency of oral mycosis globally.
- SARS-CoV-2
- delta variant
- mucormycosis
- COVID-19
1. Introduction
So far, the diagnostic measures for oral mycotic conditions have been dealt with using clinical and cytological/histopathological tests of the oral tissues [1][3], although advanced diagnostic and treatment methods are being developed to achieve new landmarks.
Mucormycosis and zygomycosis imply a group of distinctive mycoses, caused by two phyla of the fungal kingdom: Entomophthorales and Zygomycota, respectively [2][4]. The highly transmitted infectious disease COVID-19 caused by SARS-CoV-2 is responsible for causing respiratory infection [3][4][5][5,6,7], diffuse alveolar damage, and severe inflammation following immunosuppression as represented by a decrease in numbers of CD4-T and CD8-T cell counts [6][8].
Treatment protocols for COVID-19 patients usually involve higher doses of corticosteroids which reduce the immune response and increase glucose levels in the blood due to the steroid administration itself or even pre-existing diabetes mellitus which further leads to opportunistic diseases such as mucormycosis [7][9]. The main symptoms of COVID-19, including hypoxia, hyperglycemia, and hyperferritinemia, go hand-in-hand with predisposing conditions like diabetes mellitus (DM), diabetic ketoacidosis (DKA), and hyperglycemia (Figure 1). Moreover, the presence of high free iron levels in the serum of patients undergoing SARS-CoV-2 therapy may facilitate the attachment of fungal spore coat protein CotH3 to glucose-regulated protein 78 (GRP78) on the surface of endothelial cells. This phenomenon leads to aberrant signaling causing damage to epithelial cells, immune cells, cytokine storm, HIF-1α upregulation, and eventually thrombosis or tissue necrosis [8][10].
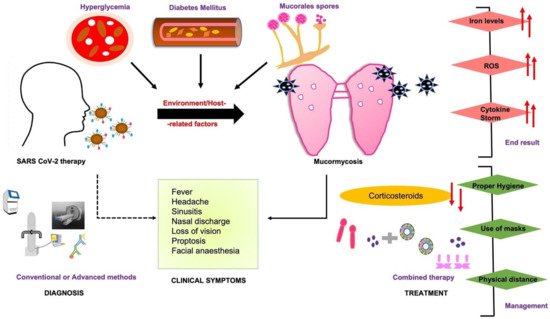
Figure 1. COVID-19-associated mucormycosis (CAM) prognosis depends upon early diagnosis by clinical symptoms, and treatment based on a combined strategy of conventional and advanced diagnostic assays. The early diagnosis may affect the end result and timely management of the disease. The dashed line represents some of the clinical outcomes linked to COVID-19-associated therapy or directly to mucormycosis (bold line).
2. General Mycology
Fungi belonging to the order Mucorales are majorly divided into six different families wherein Mucoraceae is the most frequent cause of mucormycosis [9][22]. The most important species in order of frequency are Rhizopus arrhizus (Oryzae), Rhizopus microsporus var. rhizopodiformis, Rhizomucor pusillus, Cunninghmella bertholletiae, Apophysomyces elegans, and Saksenaea vasiformis [9][10][11][12][22,23,24,25]. The newly emerging species include Rhizopus homothallicus, Thamnostylum lucknowense, Mucor irregularis, and Saksenaea erythrospora [13][26] (Table 1). Mucoraceae may be divided into sporangium producers, sporangiolum producers, and merosporangium producers based on morphology. For instance, lactophenol cotton blue-stained elements such as rhizoids, stolons, and columella or hyphae are able to differentiate between the fungal species [10][11][23,24].
Table 1. The table lists different fungal species associated with CAM versus emerging species.
| CAM-Associated Species | Prevalence | References |
|---|---|---|
| Rhizopus spp. | Mexico, Iran, India 40–50% |
[9][10][11][12][13][14][22,23,24,25,26,27] |
| Cunninghamella spp. | Global 40–60% |
|
| Lichtheimia spp. | Europe (France) Up to 80% |
|
| Rhizomucor spp. | Australia, France, Italy, India 90–100% |
|
| R arrhizus | Worldwide 60% |
|
| Mucor spp. | USA, Mexico, Iran, Greece 70–90% |
|
| Apophysomyces | Asia (India) 90% |
|
| Emerging Species | ||
| Rhizopus homothallicus | Asia 7.6% |
|
| Thamnostylum lucknowense | Asia | |
| Mucor irregularis | China, India | |
| Saksenaea spp. | France, Australia 60–70% |
|
| ToR microspores | India 11% |
The identity of the fungal species does not infer the relevant therapy as diseases caused by these species is phenotypically identical. The optimal temperature for the growth of aerobic Mucorales is 28–30 °C for an incubation period of 2–5 days. The clinically relevant Mucorales initiate and facilitate the decay of organic material so that exposure to spores of these fungi is inevitable. Despite their ubiquitous existence, the infection caused by the Mucorales is not common owing to its low virulence but is an indication of a serious underlying predisposition [2][4].
3. Immune System Abnormalities
The role of immune modulation in mucormycosis has been evident as natural killer (NK) cells produce cytokines and chemokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor (TNF-α), and interferon-gamma (IFN-γ), which influence the regulation of other immune cells and have both direct and indirect cytotoxic effects on the fungal growth. Necrotic areas have been evident in tissues with no obvious fungal development suggesting that thrombotic ischemia may occur due to the systemic platelet activation. Moreover, phagocytes release pro-inflammatory cytokines such as TNF-α, interleukin-1 beta (IL-1β), and IFN-γ which activate other immune cells. In this regard, for neutropenic patients with mucormycosis, the use of GM-CSF and granulocyte colony-stimulating factors (G-CSF) is highly recommended [15][28]. Nevertheless, the phagocytes of normal hosts kill Mucorales by the generation of oxidative metabolites and the cationic defensins [16][29] and therefore remain the major host defense mechanism against mucormycosis. However, evidence of the high prevalence of autoantibodies against immunomodulatory proteins (including cytokines, chemokines, complement components, and cell-surface proteins) in COVID-19 patients further supports the exacerbations associated with mucormycosis [17][30].
4. Diagnosis
Since some of the symptoms are shared between different sources of fungal infection, the specific diagnosis of mucormycosis can be evaluated through major pathologic manifestations such as areas of vasculitis with thrombosis, hemorrhage, and infarction. The identification of morphological differences between Mucorales and Aspergillus is also a commonly used laboratory practice to diagnose this fungal condition.4.1. Microscopic Examination
Phenotypic observation and visualization of characteristic hyphae are one of the most definitive ways to characterize the differences between mucormycosis and aspergillosis owing to the former’s irregularly shaped and right-angled broad hyphae (10–20 µm in diameter) structure. Both can be differentiated by culture and pathological examination of biopsy specimens. In addition, certain Gram-negative bacilli such as Pseudomonas aeruginosa can mimic vasculitic lesions in skin or viscera as produced by Mucorales for which hyphae can be easily visualized in routine hematoxylin-eosin-stained sections or the periodic acid–Schiff reaction and Grocott–Gomori methenamine-silver nitrate stains [18][51]. A rapid diagnosis of mucormycosis could be made through direct histopathological examination of the tissue through fluorescence microscopy using brighteners such as Blankophor and Calcofluor White, which bind to chitin and cellulose in the fungal cell wall and fluoresce under ultraviolet light [8][10].4.2. Imaging Methods
CT scans and magnetic resonance imaging (MRI) scans of the head typically reveal only evidence of sinus involvement associated with the opacification of sinuses and thickening of optic muscles, especially the medial rectus muscle as demonstrated by lucencies on a CT scan [18][19][20][21][22][51,52,53,54,55]. Proptosis may also be evident in some cases. The results of an MRI scan may also reveal abnormalities in involved structures such as the ocular muscles and sinuses as a byproduct of prominent mucosal thickening and secretions. Another commonly reported abnormality is cavernous sinus thrombosis of vessels due to ischemic changes in the area of distribution by failure in the enhancement of the affected vessels [22][55]. In the absence of comparative studies, the benefits of CT vs. MRI are not known; however, MRI scanning could be a preferred measure for a diabetic patient for whom intravenous contrast agents may be contraindicated. In addition to imaging methods, functional and metabolic imaging using PET/CT coupled with [18F]-fluorodeoxyglucose (FDG) has been considered a valuable tool in the diagnosis and management of mucormycosis due to its sensitivity and accuracy in picking up anatomic abnormalities. Advanced biosensor-based diagnosis and detection of CAM are discussed in detail in [8][10].4.3. Species Identification and Antifungal Susceptibility Testing
Species identification using commercially available kits has aided a better epidemiological understanding of mucormycosis and is indeed valuable for quick and reliable outbreak investigations. For instance, the ID32C kit (BioMerieux, Marcy l’E’ toile, France) has been used successfully for the identification of species such as Lichtheimia corymbifera, Lichtheimia ramose, and Rhizomucor pusillus; and API 50CH (BioMerieux) has been used for Mucor species [23][24][56,57]. Reproducible techniques for antifungal susceptibility testing are important methods to evaluate uniformity in reporting and to facilitate interlaboratory comparisons. Many factors influence in vitro susceptibility testing results, such as endpoint definition, inoculum size, incubation time, incubation temperature, and the medium used for testing. A range of traditional methods such as broth microdilution, disk diffusion, and agar screening, as well as commercial methods such as YeastOne and VITEK2, that are easy to set up and perform, are useful for this application [18][51]. Finally, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) (Bruker Biotyper, Germany, and Vitek MS France), mass spectrometry, and T2MR (T2 biosystems) are FDA-approved platforms involving the complex between particles coated with target-specific agents with respect to the altered microenvironment for the rapid detection of mucormycosis [8][25][10,58].4.4. Molecular Assays
Molecular-biology-based assays, including conventional polymerase chain reaction (PCR) [26][59], restriction fragment length polymorphism analyses (RFLP) [27][60], DNA sequencing [28][61] of defined gene regions, and melt curve analysis of PCR products [29][62], can be used either for the detection of Mucorales based on internal transcribed spacer or the 18S rRNA genes [30][63]. Nonetheless, the low number of patients in culture-based studies is a limiting factor that results in varied sensitivity and specificity, never approaching 100%. Moreover, studies performed with formalin-fixed, paraffin-embedded, or fresh tissue samples [31][64] lead to variable results as well. Considering the lack of evaluation and other limiting factors, none of the culture or phenotypical approaches can be recommended as a standalone approach in clinical routine diagnostics [31][64]. In lieu of this, molecular-based diagnosis [32][33][65,66] has been gaining attention to yield promising results and confirm the culture-proven cases. Presently, molecular-based diagnostic assays from blood and serum can be recommended as valuable add-on tools that complement conventional diagnostic procedures.4.4.1. Serology Assays
Serological techniques such as immunohistochemistry (IHC), enzyme-linked immunosorbent assays (ELISA), immunoblots, and immunodiffusion tests have been reported to highlight variable success as ELISA was observed to be more sensitive than double immunodiffusion (DID) tests. SDS-PAGE-based immunoblots responded positively to R. arrhizus antigens but recognized only a few of the 20–30 gel-separated bands. Mucorales-specific T cells versus control were successfully detected by an enzyme-linked immunospot (ELISpot) assay using heat-killed germinated conidia from patients with invasive mucormycosis [34][67]. Additionally, serum tests involving the use of a 1,3-beta-D-glucan assay yield promising results for the Mucorales group based on the presence of glucan in their spore cell walls [8][10].4.4.2. Differential Diagnosis Methods
CAM is mostly characterized by histopathology or culture-based assays as broad, irregular, pauci septate hyphae whereas cryptococcosis and endemic mycoses such as fungal infections are identified by encapsulated yeast cells and budding spherules, respectively. However, unlike aspergillosis and candidiasis, CAM can be identified from both blood and BAL samples. Molecular assays such as Genus-NAAT (Nucleic Acid Amplification Test) and pan fungal PCR, other than mass spectrometry, are recommended for the identification of CAM (Figure 2. However, neither enzyme immunoassays nor antigen detection assays are very efficient, as for other non-CAM fungal species [35][68].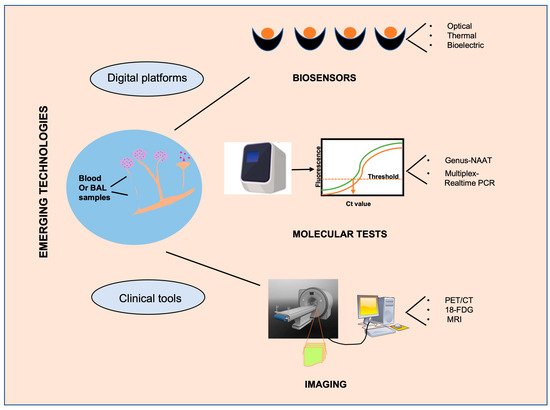
Figure 2.
The figure highlights emerging diagnostic technologies specifically for CAM that can eventually lead to a rapid diagnosis, favorable prognosis, and advanced treatment.
5. Link to SARS-CoV-2 Variant
The exclusive presence and surge of mucormycosis cases during the second wave led to speculations of its direct association with the COVID-19 delta variant. Since the COVID-19 delta variant is more contagious and resistant to vaccines than the wild-type strain, it carries a higher risk of hospitalization with a predisposition for rhinocerebral mucormycosis [36][80]. It is likely that other than the environmental, geographic, and genetic factors, the onset of mucormycosis is caused by the COVID-19 delta variant due to its ability to affect the pancreas, eventually contributing to intense hyperglycemia [37][81]. This, in turn, ultimately leads to the same predisposing factors as mentioned above for CAM patients causing endothelial injury and immune dysfunction.
According to a recent study by Alshahawey et al. [38][21], India reported an increase in mucormycosis prevalence from 12.9 cases/year during 1990–1999 to 35.6 cases/year during 2000–2004, then to 50 cases/year during 2006–2007, and eventually 89 cases/year during 2013–2015. The annual prevalence of global mucormycosis may be ~910,000 cases worldwide with 900,000 cases reported from India only. As of 20 July 2021, India counted 45,432 confirmed cases and 4252 deaths from black fungal infections as per the reports of the Ministry of Health and Family Welfare, which is far higher than the global rate. Of these, 80–94% of the cases had a history of diabetes, approximately 14.9% were suffering from DKA, and almost 86% of the patients were on corticosteroid treatment post-COVID-19 disease with predominance in male patients [8][38][10,21]. The neighboring countries, such as Pakistan and Bangladesh, have also shown an increasing trend in the number of black fungus cases during the wave of the COVID-19 delta variant [39][82].
In the south Asian countries with weak public health infrastructure, factors such as low vaccination rates and large immunocompromised populations may represent an epicenter for producing new variants. However, cases have also been reported in countries such as Brazil, Chile, Mexico, Paraguay, the United States, Italy, and the United Kingdom. Similarly, with the new omicron variant circulating, the possibility of CAM returning is convincing, as indicated by 500 new registered cases in the northern state, Haryana, in India. In Indian states, Gujarat and Maharashtra being the most affected during the COVID-19 delta variant infections, the number of CAM cases has again started to appear in the Mumbai city of Maharashtra [40][83]. Furthermore, 300 new cases were reported from middle east countries, which makes it crucial to consider precautionary factors as wearing proper masks, timely identification, maintaining hand hygiene, physical distance, avoiding mass gathering, and sufficient mass vaccination [40][83] to combat the collateral effects of COVID-19, especially in developing countries (Figure 3).
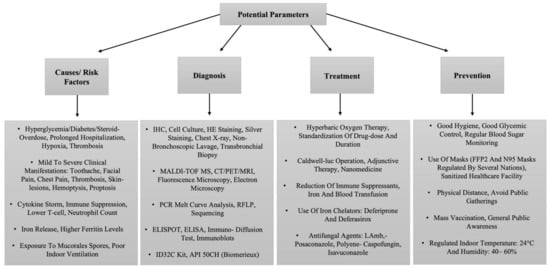

Figure 3.
The tabular representation of a list of potential parameters related to the management of CAM.
SARS-CoV-2 and Mucormycosis: Underlying Factors
Diabetes Mellitus is a predominant risk factor for increased severity of COVID-19 infection and higher mortality. Increased ACE2 receptor and GRP78 expression, dysregulated immune response, alveolar and endothelial dysfunction, an acidic environment (DKA) causing hyperglycemia that favors SARS-CoV-2 replication, and a hyperferritinemic state due to increased availability of iron, eventually makes DM the most common risk factor for mucormycosis [8][10].
In severe cases of COVID-19, lymphocytopenia has been observed as the pathological mechanism of SARS-CoV-2, majorly affecting T cells (CD4+ and CD8+) [14][27]. However, T cells play an important role in controlling invasive Mucorales infection through various cytokines (IL-4, IL-10, IL-17, and interferon-gamma) that damage the fungal hyphae, indicating that severe COVID-19 infection on its own is a risk factor for mucormycosis [14][41][27,47].
Nevertheless, chronic antibiotic use by itself is one of the prime risk factors for opportunistic fungal infections including mucormycosis which in some cases can even lead to the emergence of antimicrobial resistance [14][27].
Not all COVID-19 patients present co-morbidities such as DM and DKA; therefore, in India, which is known as the diabetes capital of the world, the highest rates of mucormycosis cases were found after the COVID-19 surge that accounted for more than 70% of cases [17][42][30,31]. Moreover, associations with poor hygiene and ventilator-associated infections lead to the high prevalence of the same in developing countries, with India leading the charts owing to its high population density. Moreover, in COVID-19 patients, >88% of the mucormycosis cases are localized as sinus and cerebral as compared with the pulmonary (>24%) and cutaneous prevalence (>19%) in non-CAM patients [14][27] (Table 2).
Table 2.
Prevalence of COVID-19-associated mucormycosis cases in India and other countries by February 2022.
| Country | No. of Cases | Affected Organ | References |
|---|---|---|---|
| India | 2849 | Rhino Orbital Cerebral | [43][44][85,86] |
| USA | 5 | Rhino Orbital Cerebral | [45][46][47][48][87,88,89,90] |
| Italy | 1 | Rhino Orbital Cerebral | [49][91] |
| Iran | 18 | Rhino Orbital Cerebral | [50][51][52][92,93,94] |
| Turkey | 1 | Rhino Orbital Cerebral | [53][95] |
| Mexico | 1 | Rhino Orbital Cerebral | [54][96] |
| India | 1 | Pulmonary | [55][97] |
| UK | 1 | Pulmonary | [56][98] |
| USA | 2 | Pulmonary | [57][58][99,100] |
| Australia | 1 | Pulmonary | [59][101] |
| India | 2 | Gastro-Intestinal | [60][61][33,102] |
| Brazil | 1 | Gastro-Intestinal | [62][103] |
| USA | 1 | Cutaneous | [63][104] |
Different intervention factors, particularly the hospitalization infrastructure have crucial roles to play in the outbreak of CAM in developing countries, including crowded hospitals, unavailability of healthcare resources, overburdened healthcare workers, and poor diagnostic quality [40][83]. On the other hand, developed countries’ hospitals ensure quality control of oxygen supply, proper sanitization of oxygen cylinders, disposable oxygen humidifiers, and even the use of clean distilled water in oxygen humidifiers and concentrators. Of note, during the extreme pandemic periods (March 2020 to May 2021) in India, the high-standard hospitals observed 0 CAM cases among >5000 hospitalized COVID-19 patients [64][84]. Moreover, people in developing countries can often procure medications without a prescription, making zealous use of steroids, hence exacerbating the situation. Nonetheless, differential gender susceptibility with 78% of CAM cases being reported in male patients [14][27] is another aspect that needs to be investigated in future studies.
