Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Beatrix Zheng and Version 6 by Beatrix Zheng.
For targeted nanoDDS, imaging serves as a “pilot” evaluation of where a targeted Nanoparticles (NPs) localizes, shedding light on “on-target efficiency”. The image-guided treatment regime can also facilitate identifying patients who lack the common target and will not respond to treatment, which is critical for treatment planning. Designing nanotheranostic particles with high efficiency and translational potential demands careful choice of the composition of NPs, imaging labels to be added to the NPs, in addition to their target of choice and cargo to be delivered.
- targeted drug delivery
- antibody–drug conjugates
- nanotheranostics
- image-guided therapy
- nanoparticles
- drug carriers
- radiolabelling
- anticancer therapy
1. Radiolabels
Nuclear imaging modalities, including PET and SPECT are to date two of the most-used imaging modalities for nanoDDS owing to their capabilities for whole-body systemic assessment and the ability to quantify their signal. More importantly, in nuclear imaging nanoDDS can be detected in the microdose range (<1% of the therapeutic dose), which facilitates clinical translation [1]. The most commonly used positron-emitting radionuclides in clinical studies are β- and γ- emitters due to their manageable energy levels and long ranges [2]. Given the average circulating time of nanoDDS, radionuclides used for labeling NPs are usually those with long half-lives. Technetium-99 m is the most frequently utilized radionuclide because of its wide availability, low cost, and its long half-life (6 h), which permits an imaging window of up to 24 h. Isotopes of iodine and copper are also often used [3].
While various methods can be used to radiolabel NPs, one important consideration is that the synthesis of NPs has to be a lot shorter than the decay of radioisotopes to preserve their radiotracing functionality. Coordination chemistry is used to covalently label NPs with radioisotopes by forming a stable chelator-isotope bond in a short period. Chelators for metallic radioisotopes include esadentate acyclic chelators (e.g., ethylenediaminetetraacetic acid EDTA or DTPA), tetradentate acyclic chelators (e.g., PTMS), and macrocyclic chelators such as 1,4,7-triazacyclononane-N,N’,N’’-triacetic acid (NOTA) and 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) [4]. Notably, careful selection of chelators is critical as certain radioisotopes can also be effectively bound using specific chelators [5]. For example, macrocyclic chelators are generally considered to bond more strongly to metallic radioisotopes [6]. It has also been found that NOTA is more suitable for 64Cu labeling than DOTA [7][8][9].
NPs can be labeled by either attaching the chelators on the surface or by adding them to the NP payload. Encapsulation of radiolabels within liposomes can be achieved passively by a process of extrusion [10]. However, this approach requires fresh liposome preparation before imaging, which is labor-intensive, and suffers from a low loading efficiency <10%. Another mechanism for liposome radiolabeling is to use a lipophilic chelator to incorporate radioisotopes into the lipid layer [11]. A combined approach of “remote loading” has been devised to allow radioisotopes to diffuse through lipid layer of liposome encapsulating hydrophilic chelators, forming chelates “remotely” inside liposomes [12][13][14]. This approach has become increasingly popular due to its efficiency and has been adopted in clinical trials [15]. Other chelator-free radiolabeling approaches have been developed and applied in preclinical studies [16][17][18]. For example, a new approach has been derived to label nanographene with 64Cu based on transition metal–π electron interactions [17]. Rapid 64Cu and 69Ga labeling of quantum dots was also achieved through a cation exchange approach [19].
One prominent clinical application of radiolabeled nanotheranostics is of radiolabeled liposomes. In the clinical trial of a formulation of PEGylated liposomal doxorubicin targeted to human epidermal growth factor receptor 2 (HER2) (NCT01304797) named MM-302 [15], 19 patients with metastatic breast cancer were selected for imaging restudyearch using Cu-64 labeled MM-302, [64Cu]MM-302. This radiolabeled liposome was surface-functionalized with an anti-HER2 scFV-PEG-DSPE, which inserts into liposome bilayer [20]. 64Cu chelated by a novel chelator 4-DEAP-ATSC was loaded by gradient into liposomes [21] (Figure 1). Directed at testing whether HER2-targeting increases the amount of drug accumulating at the metastases, and further correlating with the efficacy of trastuzumab treatment (a clinically approved anti-HER2 monoclonal antibody), [64Cu]MM-302 at a target dose of 400 MBq per patient was given and PET imaging at this 24 h showed that [64Cu]MM-302 remained in the circulation for over 24 h, with the liver and spleen being the major organs of NP uptake. Importantly, it found that high 64Cu-MM-302 deposition in tumors was associated with more favorable treatment outcomes. This research exemplifies the use of imaging probes for patient stratification and outcome prediction.
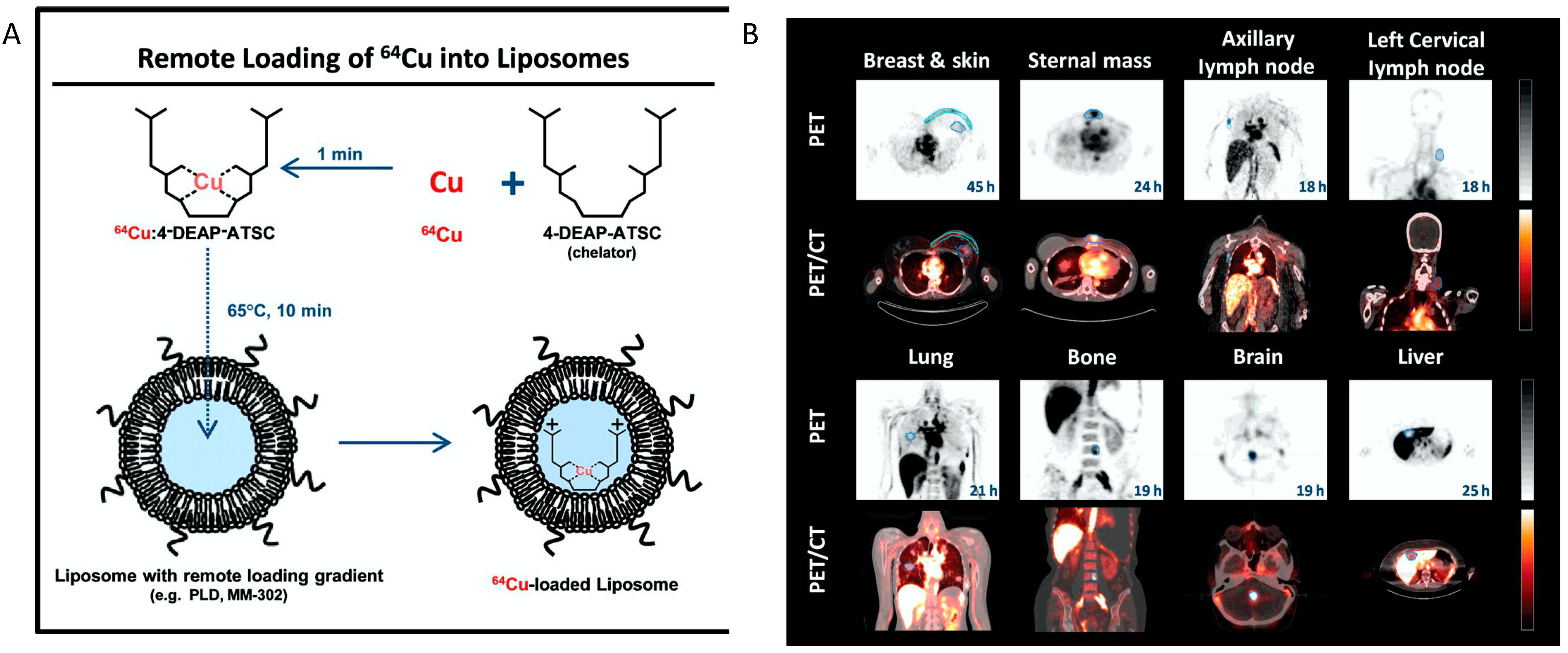
Figure 1. The construction64Cu-MM-302 and its application in lesion detection in the phase I clinical trial (NCT01304797). (A) Schematic depicting remote loading of 64Cu into liposomes using the novel gradient-loadable chelator 4-DEAP-ATSC. Heating liposomes above the lipid bilayer phase transition temperature facilitates transmembrane transport of unprotonated 4-DEAP-ATSC, which becomes protonated within the liposome and remains entrapped. (B) Representative PET and fused PET/CT images of 64Cu-MM-302 in lesions at different anatomic locations. Intensity scale bars represent deposition from 0 to 10%ID/kg (derived from SUVmedian). The regions of interest used to measure tumor deposition of 64Cu-MM-302 are shown in blue or turquoise outlines. 64Cu-MM-302 uptake was detected at above muscle background level in lesions of various anatomic locations that are common for HER2-positive metastatic diseases. Figures are adapted based on Refs. [15][21] with permissions. Copyright 2017 American Association for Cancer Research.
Because of their high energy levels and ionizing potential, α-emitters are usually included as the payload of an NP rather than attached to the particle surface. Their β- and γ-emitter counterparts can help define the dose and rate at which the radionuclides are delivered to tumor versus normal tissues before α particle therapy due to similar pharmacokinetics. One such example is the use of SPECT/PET imaging with 123I/124I-labeled agents before 131I-based radionuclide therapy [22][23][24]. These identical diagnostic/therapeutic pairs enable a theranostic regime for reliable delineation of biodistribution, target site accumulation, and prediction of responsive tumors.
Despite the fact that radiolabeled liposomes constitute an overwhelming majority of radiolabeled NPs in clinical studies, recent development of other types of radiolabeled NPs, including inorganic NPs, e.g., silicon NPs [25][26], and polymeric NPs including cellulose [27] and chitosan NPs [28], are gaining momentum. In studies performed by Cai et al., a novel type of ultrasmall porous silica nanoparticles (UPSN) (size ~15 nm) were labeled with isotopic pair yttrium-90/86 (90/86Y, with the high energy β-emitter 90Y being used for therapy and low energy emitter 86Y for imaging) through the DOTA chelators. The smaller size of these radiolabeled UPSNs led to enhanced in vivo pharmacokinetic behaviors, achieving an astonishingly high tumor accumulation (12% ID/g), long blood circulation, and greater evasion from the RES system. In mouse models of breast cancer, theranostic NPs enabled both sensitive detection of tumors (with 10.4 ± 0.8% ID/g uptake of 86Y-DOTA-UPSN in tumor sites), and efficient treatment monitoring and tumor retardation (~30% tumor regression) after injecting ~5.5 MBq 90Y-DOTA-UPSN [26].
2. Magnetic Resonance Imaging Labels
Most MRI labels generate contrast by indirectly affecting neighboring water molecules. The movement of these water molecules is detected and translated into an MRI image which is based on relative tissue water content. Paramagnetic labels, which generate movement through weak magnetic forces, include Manganese (Mn2+; Mn) as well as lanthanide metal ions such as gadolinium (Gd3+; Gd). These paramagnetic labels generate positive (brightening) signals in MRI images. Paramagnetic metal ions are used in chelated form since the accumulation of the naked ions in tissues typically induces toxicity [29]. Chelators, such as DTPA and DOTA, are also used in constructing metal ion MRI labels to confer thermodynamic and kinetic stability. The ability of MRI labels to generate image contrast is measured by its effects on shortening water T1 and T2 relaxation times, metrics termed r1 and r2 relaxivity, respectively. Current clinical Gd-based contrast agents have an r1 relaxivity of 3–4 s−1mM−1 at field strengths of 0.5 Tesla and 37 °C. Much scientific effort has been devoted to improving relaxivities of the paramagnetic agents to enhance detection sensitivity and lower contrast agent doses. While small-molecular targeted agents usually contain one or several paramagnetic ion chelates per molecule, demanding an abundant level of their molecular targets, targeted NPs that encompass hundreds or thousands of paramagnetic ion chelates per particle can enhance detection sensitivity by increasing chelate-to-target ratios.
One prominent type of paramagnetic NPs seen in clinical trials is AGuIX (Activation and Guidance of Irradiation by X-ray), which are sub-5 nm NPs composed of a polysiloxane matrix with gadolinium cyclic chelates covalently grafted on the inorganic matrix [30][31] (Figure 2). Gd retention by brain tumor cells following AGuIX injection means AGuIX NPs have high radiosensitizing properties [32] together with excellent positive MRI contrast (r1 = 8.9 mM−1s−1 per Gd at 3 Tesla) [31], making them powerful nanotheranostic agents. Phase-I clinical trials, regulated by the French Agence Nationale de Sécurité du Médicament et des produits de santé, have been conducted (NANO-RAD trial, NCT02820454 [33]). In this trial, patients with brain metastases were given intravenous injection of escalating doses of AGuIX (15, 30, 50, 75, or 100 mg/kg b.w.) on the day of initiation of whole-brain radiation therapy (30 Gy in 10 fractions). This research demonstrated no dose-limiting toxic effects up to AGuIX 100 mg/kg, with a mean half-life of AGuIX shown to be 1.3 h at all doses. Efficiency and persistence of AGuIX contrast enhancement were observed in brain metastases from patients with primary colon cancer, melanoma, lung, and breast cancers. More importantly, 13 of 14 evaluable patients had improved clinical outcomes evidenced by either stabilized or reduced tumor volume. A significant correlation was found between MRI contrast enhancement and tumor response, implicating a radiosensitizing effect. From the perspective of image-guided therapy, this research provides strong suggestion that imaging can serve as a non-invasive predictor of cancer treatment outcomes [34]. The phase II trial of AGuIX is underway to expand the protocol to multiple centers and 100 patients. It is worth noting that AGuIX mainly relies on EPR effect for tumor homing, and suffers from a low tumor residence time and strong off-target effect. A newer version of AGuIX, which includes porphyrin as an extra photosensitizer and is modified with peptides targeted to neuropilin-1 (NRP-1), a transmembrane receptor abundantly overexpressed in the tumor vascular system [35], is undergoing preclinical testing.
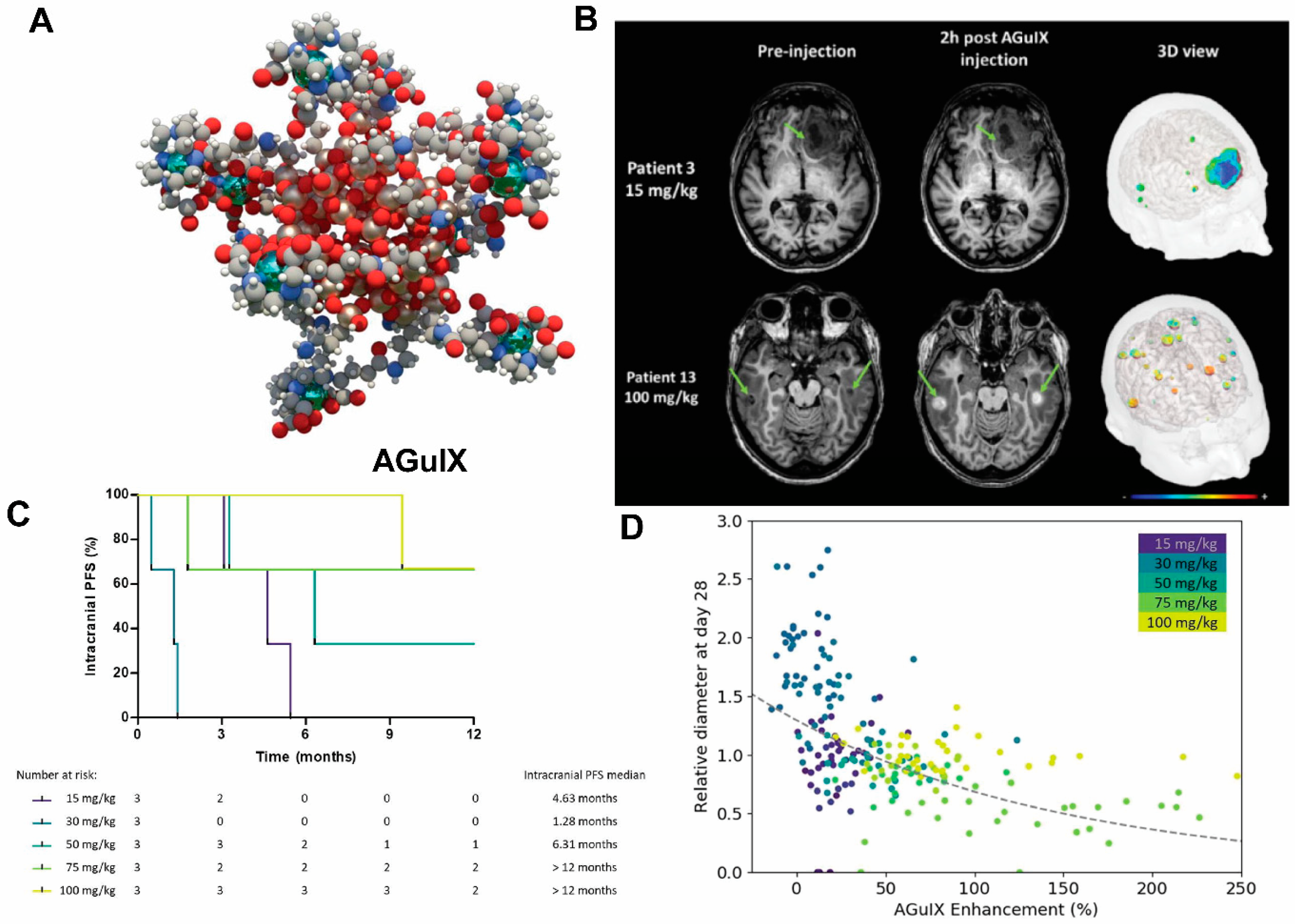
Figure 2. AGuIX as radiosynthesizer and MRI contrast-enhancing NPs in the phase I clinical trial (NANO-RAD trial). (A) Schematic representation of AGuIX. Gadolin interesting aium ions are chelated by 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid derivatives. Polysiloxane core (Si, metallic grey; O, red; H, white; C, grey; N, blue) is surrounded by covalently grafted chelates of gadolinium (Gd, metallic green). (B) AGuIX contrast-enhanced MRI at 2 h in brain metastases of 2 patients with lung cancer following intravenous AGuIX administration at 15 and 100 mg/kg, resplication of ectively. T1-weighted MRI images were obtained without injection of contrast agent before and at 2 h after a single AGuIX intravenous administration at the indicated concentration. Green arrows are pointing highlighted metastases. The 3-D vizualization of entire brain with specific contrast enhancement into metastases was obtained from T1-weighted MRI mapping. (C) Intracranial progression-free survival (PFS) of multiple patients with brain metastases treated with a combination of whole-brain radiotherapy (WBRT) and different dose levels of intravenous AGuIX. The color of survival curves corresponds to different AGuIX doses. (D) Correlation between change in size of brain metastases and AGuIX signal variation. Correlation of measured metastasis sizes for patients with brain metastases and treated with whole brain radiotherapy and different AGuIX doses. Points colored according to patient number and administrated dose with darker colors corresponding to lower AGuIX doses. Metastasis diameter at 28 days normalized to diameter at Day 0 (V28/V0) as a function of AGuIX enhancement (points) compared with predicted trend (dashed line), showing good agreement and dependence of metastasis evolution on AGuIX uptake. AGuIX, Activation and Guidance of Irradiation by X-Ray. Figures were adapted based on Refs. [31][34] with permissions.
An interesting application of Gd-based NPs is in photodynamic therapy (PDT). Since Gd has a high 1O2 quantum yield upon light irradiation and several studies show the high-relaxivity, Gd-encapsulating NPs such as Gd-graphene carbon [36] and gadofullerenes can serve as photosensitizing agents. Upon activation under light of a specific wavelength, these particles trigger a cascade of tumor-damaging photochemical and photobiologic reactions, such as generating reactive oxygen species (ROS). Guan et al. prepared a β-alanine(Ala)-modified gadofullerene (Gd@C82-Ala, diameter = 130 nm) that shortens the light interval between Gd-Alanine under light irradiation and induces malignant tumor cell and vascular disruption. This research showed that following Gd@C82-Ala administration, localized treatment with white light irradiation for 30 min led to significant retardation of tumor growth accompanied by increased blood vessel porosity and immune cell recruitment [37]. Gadofullerene has also been used to treat melanoma [38]. These studies, together with the capability of Gd-encapsulatig NPs as sensitive MRI probes with a high transmetallation stability [39], indicate great potential of Gd-encapsulatig NPs as nanotheranostics for cancer.
Beside paramagnetic ion chelates, superparamagnetic NPs, majorly iron oxide (Fe3O4) NPs or magnetite, are frequently used by themselves or after being incorporated into another NP matrix. In fact, iron oxide NPs constitute a large portion of clinically approved NPs, e.g., Feraheme, a dextran-coated iron oxide particle for treating anemia, and therefore has been the focus of NP research. The size of iron oxide particles may range from several nanometers, i.e., super-small iron oxide nanoparticles (SPIONs), to micron-sized nanoparticles (IONs), with intrinsic r1 and r2 relaxivities scaling with the size and coating composition. Larger IONs (diameter >10 nm) predominantly generate T2/T2* contrasts, which manifest as “darkening” contrasts in images. Ultrasmall IONs (USPION, diameter <10 nm) can also generate T1 contrasts, and the composition of USPIONs can be tuned, e.g., by adding gadolinium [40], to exhibit both T1 and T2 contrasts (also dubbed as dual-contrast agents). Overall, SPIONs are favored as a cargo in nanoDDS due to their small size, unless IONs themselves serve as the drug carriers.
IONs may also enhance therapeutic efficiencies of nanoDDS. The thermal effects of iron oxide NPs under an alternating magnetic field (AMF) can be used for cancer therapy [41][42]. By targeting iron oxide NPs to cancer cells, magnetic hyperthermia treatment (MHT) can induce specific cancer cell death as the tumor environment temperatures increase to >41 °C. The specific absorption rate (the rate of energy absorbed per unit mass under radio frequency) [41] increases with particle size, and therefore most studies use IONs of 20–50 nm [41]. In the study by Ishimura et al. [43], folic acid-conjugated PEG-coated SPION clusters were constructed as targeted nanotheranostics for MRI and MHT. The clustering of SPIONs not only prolonged blood circulation, but also enhanced relaxivity and SAR. It was shown that after intravenous injection, the clusters showed significant MRI contrast enhancement in breast cancer tissues and exerted high magnetic hyperthermia effect (f = 230 kHz, H = 8 kA/m). Additionally, the magnetism of magnetite could also be exploited to create another driving force for targeted delivery. For example, magnetically labeled nanoDDS can be navigated to cancerous regions under an external magnetic field–a technique termed magnetic targeting, which has been shown to improve efficacy in preclinical models [44][45][46]. Active targeting of NPs can also be combined with magnetic targeting to enhance chemotherapy drug delivery.
It would be ideal to combine detection properties of both the NPs and the drug without additional labeling. Recent development of Chemical Exchange Saturation Transfer (CEST) MRI gives a glimpse of this possibility. This imaging modality offers the potential to detect diamagnetic compounds, i.e., compounds which do not possess metallic labels, which encompasses most drugs and organic NP matrices. In a recent study by Yuan et al., a self-assembly enzyme-responsive NP was constructed for image-guided cancer therapy (Figure 3). The building blocks of the NPs are an anticancer agent olsalazine (Olsa) conjugated to the cell-penetrating peptide RVRR. Under enzymic reaction by furin, these NPs self-assemble into large intracellular NPs [47]. Both the NPs and their constituent peptide components are readily detected with CEST MRI by virtue of exchangeable Olsa hydroxyl protons. In vivo studies showed that the NPs result in generation of a 6.5- fold increase in tumor CEST contrasts and 5.2-fold increase in anti-tumor therapeutic effect in colon cancer, compared to Olsa treatment alone. Besides Olsa, this effect is thought to apply to some other chemotherapy drugs including gemcitabine [48][49] and melphalan [50]. Readers are referred to reviews on CEST-detectable nanoDDS for more details on the topic [51][52].
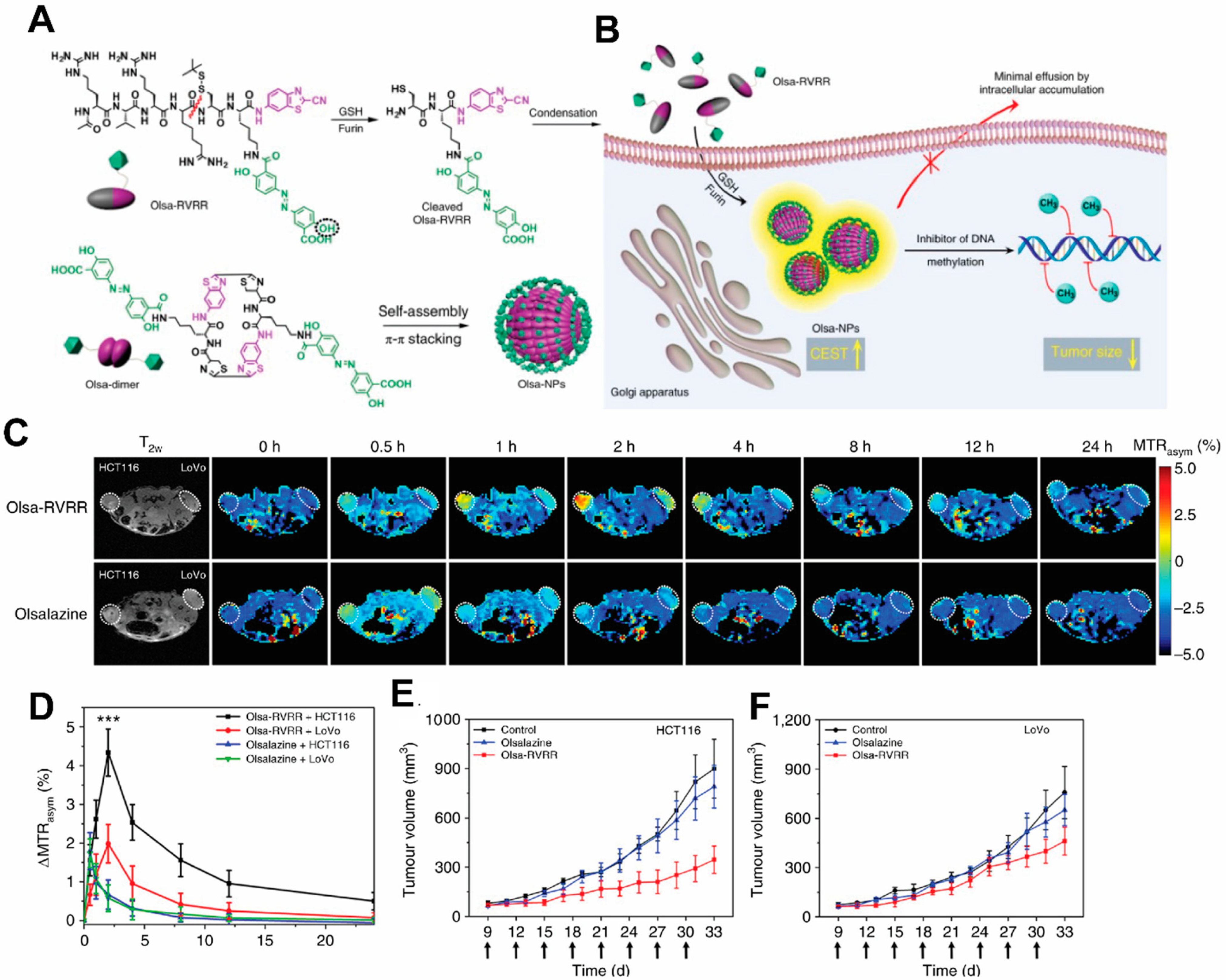
Figure 3. Schematic illustration for the formation of Olsa-NPs by furin-mediated intracellular reduction and condensation of Olsa-RVRR, resulting in enhanced CEST signal and tumor treatment efficacy. (A) Self-assembly of Olsa-RVRR into Olsa-NPs through a series of steps. Red line indicates the site of furin cleavage, and the circled hydroxyl group indicates the exchangeable hydroxyl proton that provides OlsaCEST signal at 9.8 ppm from the water frequency. (B) After Olsa-RVRR enters the cytoplasm of high furin-expressing cells (the HCT116 colon cancer cells in this research), it undergoes reduction by GSH and cleavage of the peptide by furin near the Golgi complex where cleaved Olsa-RVRR is generated. Amphiphilic oligomers (mostly dimers) are then formed from the click reaction between two cleaved Olsa-RVRR molecules, followed by self-assembly into Olsa-NPs as a result of intermolecular π-π stacking. The intracellular accumulation of Olsa-NPs then serves as a reservoir of Olsa molecule-enhancing CEST contrast and inhibiting DNA methylation for tumor therapy. (C,D) Dynamic T2-weighted (T2w) and OlsaCEST serial MRI of tumor-bearing mice after intravenous injection of 0.2 mmol kg−1 Olsa-RVRR or Olsa (left, HCT116; right, LoVo colon cancer cells). Time course MTRasym maps (C) and MTRasym OlsaCEST signal (D) for tumors after background correction by the subtraction of the MTRasym value at 0 h. Data are shown as mean ± s.d. for n = 4 mice; one-way ANOVA, followed by Dunnett’s post hoc test; ***: p < 0.001 versus all other groups. (E,F) Anti-tumor effects of Olsa and Olsa-RVRR for HCT116 (E) and LoVo (F) tumors. Arrows indicate time points of repeated drug administration (every 3 d × 8) after tumor cell injection. Data are shown as mean ± s.d. (n = 4 mice). The figure is adapted with permission based on Ref. [47]. Copyright 2019 Springer Nature.
3. Ultrasound Labels
Ultrasound (US) is one of the earliest-employed diagnostic imaging tools. Its application in cancer offers unique benefits of both portability and real-time depiction of tumors [53]. US has also been employed as a remarkable therapeutic tool by locally inducing drug release from carriers [54][55][56] to perform thermal ablation therapies, i.e., high-intensity focused ultrasound (HIFU) [57], among other applications [58]. A major class of ultrasound contrast agents are gas-filled nano-/micro-bubbles and liposomes with high echogenicity [59], i.e., the ability to reflect the ultrasound waves, thus generating enhanced sonogram. Under focused US, which induces oscillation of the gas bubbles in a fluid (a mechanical phenomenon termed inertial cavitation) [60], gas bubbles grow unstable and subsequently collapse during compression under the inertia of the surrounding fluid. Hence, US can be used to enhance delivery efficiency of therapeutic agents to the tumor beyond the intrinsic targeting of NPs [61]. In HIFU, gas-containing NPs intensify the thermal response in target sites to enhance specific thermal ablation and decrease damage to normal tissues. The commercial organic microbubbles or liposomes in use for US imaging are lipid-coated perfluoropropane (phase transition temperature of 56 °C) microbubbles [62], namely Levovist, Sonovue, and Optison, which undergo an instant phase transition into echogenic gas bubbles. Their micrometer size and limited longevity due to premature rupture make them undesirable as drug delivery systems and HIFU agents, and they have only been used clinically as US contrast agents thus far. Efforts have been devoted to developing particles encapsulating other phase-transition materials including perfluorohexane (PFH, phase transition temperature of 56 °C) [63] and perfluoropetane (PFP, phase transition temperature of 29 °C) [64], for a controllable phase transition, and the use of NPs for a higher targeting to tumors. Another interesting study also encapsulated calcium carbonate using poly(d,l-lactide-co-glycolide)(PLG) to construct a NP for treating neuroblastoma (Figure 4). In this research, the gas-generating NPs (GNPs) were modified with a rabies virus glycoprotein (RVG) peptide targeted to the nicotinic acetylcholine receptor (nAChR) abundantly expressed in neuroblastoma. At the tumor’s low-pH microenvironment, these NP are triggered by the pH change and generate carbon dioxide bubbles that imposes physical shock to cancer cells, simultaneously enhancing US contrasts [65]. This allows for the verification of the accumulation of NPs within tumors by US imaging. Despite not carrying additional chemotherapy drugs, thereby obviating side effects associated with conventional chemotherapy, necrotic cell death induced by the GNPs led to markedly retarded tumor growth.
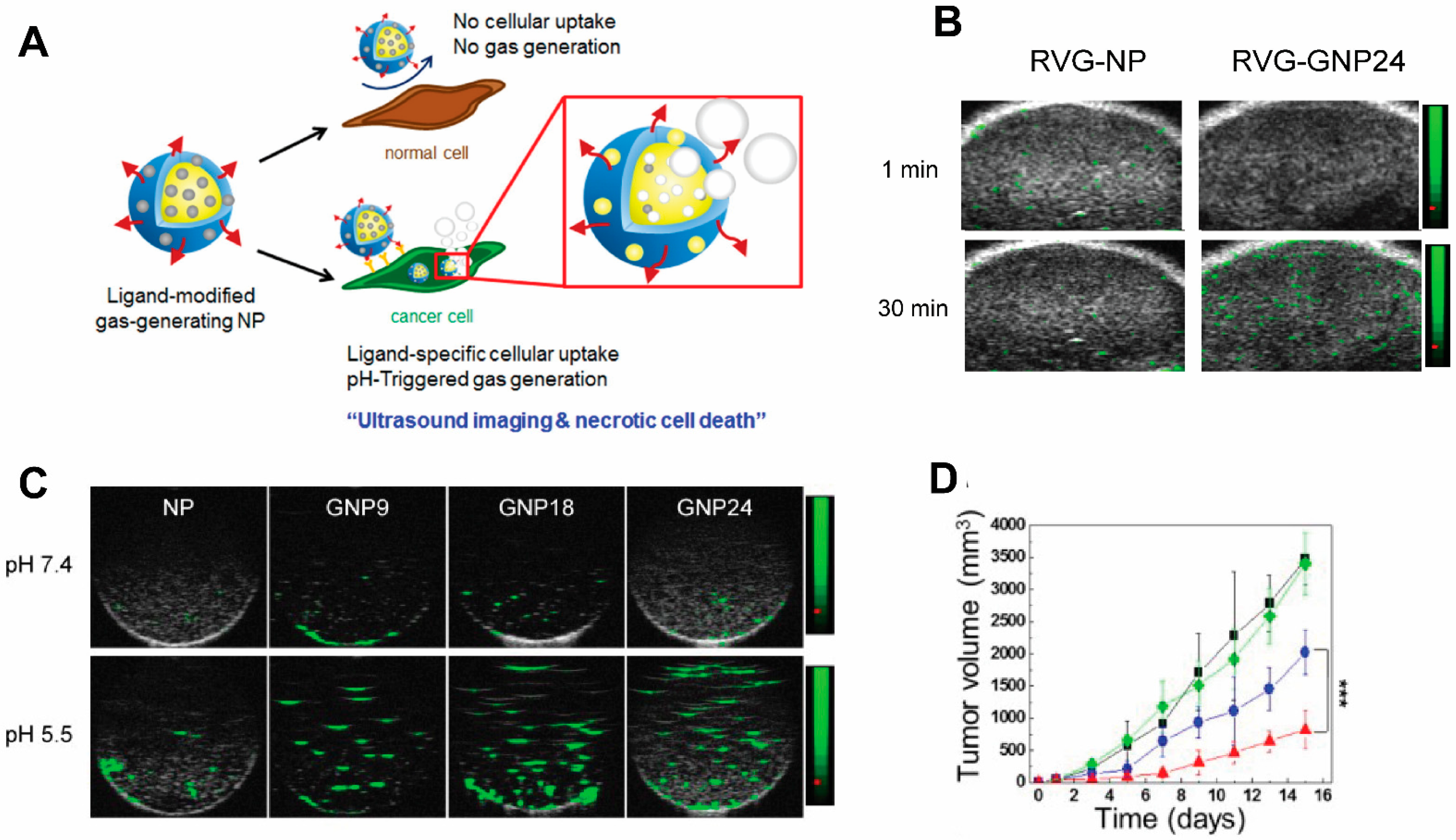
Figure 4. A targeted gas-generating nanotheranostic particle for ultrasound-guided treatment of neuroblastoma. (A) Schematic illustration of ligand-modified gas-generating nanoparticles for cancer-specific cellular uptake and pH-triggered gas generation. PLG nanoparticles loaded with fine-grained calcium carbonate provide theranostic functionality for cancer detection and treatment. pH change triggered carbon dioxide gas generation and these bubbles enabled simultaneous US imaging and necrosis of cancer without using conventional contrast or anti-cancer agents. (B) In vitro ultrasound signals of non-gas-generating NP and gas-generating nanoparticles (GNP9, GNP18, and GNP24) under neutral and acidic conditions ([GNP] = 10 mg/mL). (C) In vivo ultrasound imaging of tumors after intravenous injection of RVG-NP and RVG-GNP24 into a tumor-bearing mouse model (25 mg/kg, polymer/mouse at 1 and 30 min). (D) Changes in tumor volume of mice treated with saline (black diamond), RVG-NP (green square), RVG-GNP9 (blue circle), and RVG-GNP24 (red triangle) (10 mg/kg polymer/mouse and 20 mg/kg docetaxel/mouse; five daily intravenous injections; *** p < 0.001). Figures are adapted based on Ref. [65] with permission. Copyright 2016 Elsevier.
4. Optoacoustic Labels
OAI, also known as photoacoustic imaging, is an emerging modality based on the “light-in sound-out” principle, which has garnered increasing attention. In OAI, NPs can be loaded with small-molecule organic dyes with high photothermic conversion efficiency, such as IR780 or ICG, to become imageable. Several near-infrared light (NIR)-absorbing NPs, such as gold nanoparticles, iron oxide particles, semiconductor NPs, can also be used in OAI to illustrate the biodistribution of injected NPs [66][67]. However, not all OAI agents are created equal, and the conversion efficiency of optical energy into pressure waves is dependent on several factors. Controlling the geometry, composition, coatings, and solvents around plasmonic nanostructures can each help to generate the optimum OA signal [68].
Organic NIR dyes are common OAI contrast agents for NP labeling, as NIR dyes have a high extinction coefficient and low quantum yield, with an ideal spectral window (NIR-I: 650–950 nm or NIR-II: 1000–1700 nm) that overlaps negligibly with the biological background. The absorption wavelengths in this range also allows the excitation light to penetrate as deep as a few centimeters into the tissue. Common OAI dyes include squaraine, semicyanine, pentamethine cyanine, heptamethine cyanine, porphyrin, perylene-diimide, aza-BODIPY, and benzobisthiadiazole [69][70].
A large class of OAI nanotheranostics are NPs that encapsulate NIR dyes. MSNs are a class of NPs extensively studied for OAI-guided drug delivery. In the work by the researchers' group and others, MSNs with various sizes and pore structures have been developed. In work by MacCuaig et al., MSNs with wormhole pores were used to load IR780 OAI dye and chemotherapy drug paclitaxel. The asymmetric morphology of wormhole pores was to provide a higher surface area for increased loading capacity and slower cargo release. The particle also has a chitosan coating as the gate keeper, as chitosan shrink at physiological pH to entrap the cargo but expands at low pH for cargo release. To endow targeting capabilities, a pH-low-insertion peptide (pHLP) V7 was conjugated to the NP surface so that the NPs home to low-pH tumor microenvironment, where V7 peptide facilitate cellular uptake of the NPs. The resultant NP, named V7-TROS, was demonstrated to efficiently translocate into the cytoplasmic compartment for the release of the IR780 dye and paclitaxel, leading to enhanced tumor contrast and anti-neoplastic efficacy on ovarian cancer [71]. The low-pH targeted nanotheranostics were also found to enhance tumor detection using OAI and cargo uptake in orthotopic pancreatic cancer [33] and triple-negative breast cancers [72]. With the guidance of multiple spectral optoacoustic tomography (MSOT), the research also showed that active targeting outperforms NP size in facilitating tumor-specific uptake (Figure 5).
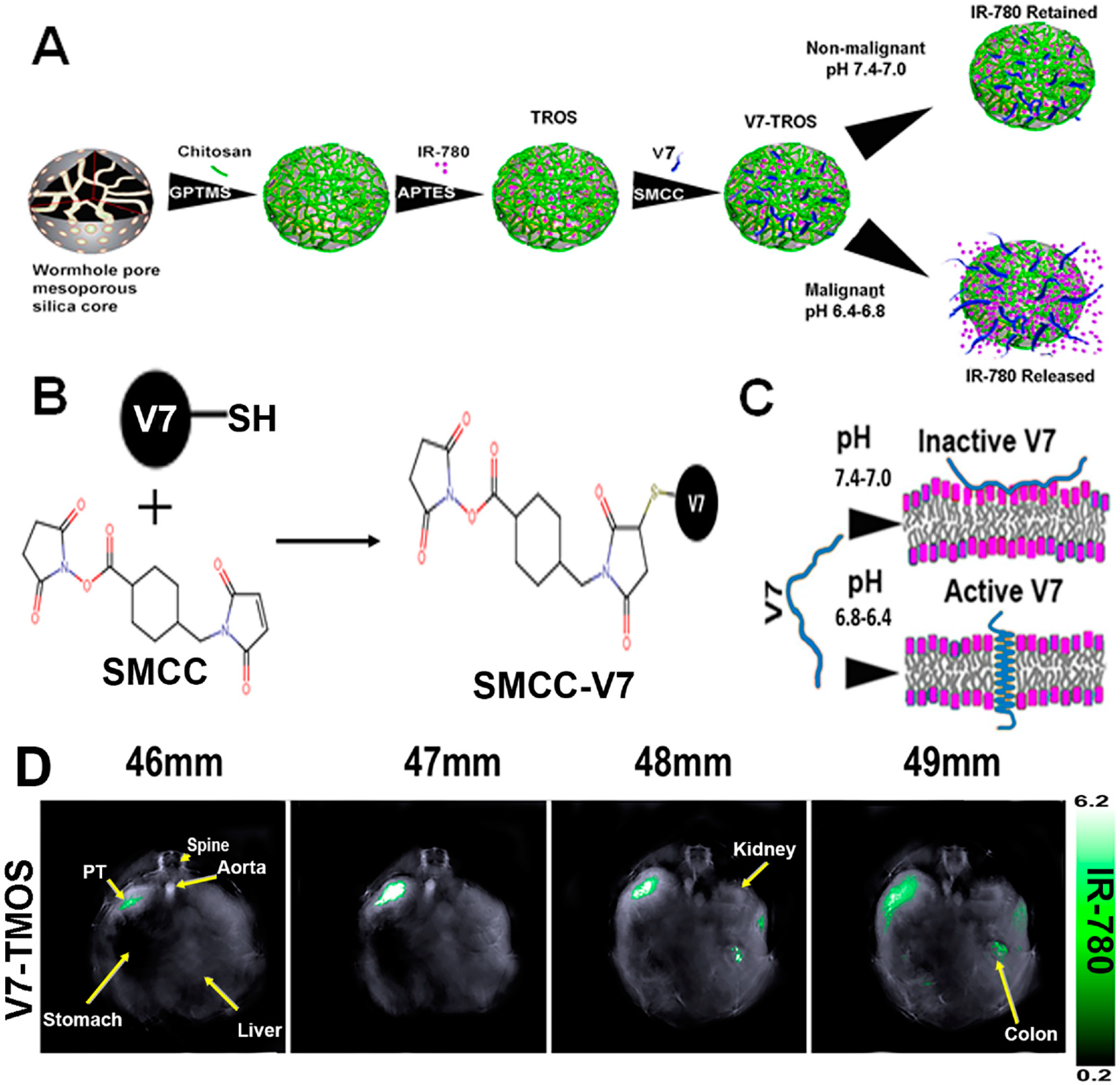
Figure 5. Construction of the low-pH-targeted MSN NP for photoacoustic imaging of pancreatic ductal adenocarcinoma. (A) Schematic Illustration of the Components and Formation of V7-TROS NPs, including V7-TMOS, V7-TEOS and V7-TPOS. (B) Conjugation Chemistry of SMCC to the Cysteine Residue on the V7 Peptide, and (C) the Activation Mechanism of the V7 Peptide in Acidic Environments. (D) Biodistribution of V7-TMOS in axial slices showing accumulation within the tumor, kidney, liver, and spleen Figures were adapted based Refs. [71][73] with permissions. Copyright 2021 American Chemical Society.
5. Computed Tomography Labels
Currently CT stands as the leading radiologic method for biomedical imaging. The contrast agents for CT are X-ray attenuating agents, including iodine and high atomic metallic nanoparticles such as gold [7374][7475] and bismuch NPs [7576][7677]. As a large dose is required to generating CT contrasts, nanotheranostics designed for CT are relatively rarer compared to those designed for other imaging modalities. One prominent example of CT nanotheranostics is NBTXR3, which are 50 nm Hafnium oxide (HfO2) crystalline NPs functionalized with anionic phosphate coating (Figure 6A,B). NBTXR3 NPs act as radioenhancers to increase energy deposition in tumor during radiotherapy and their CT contrasts allows visualization of their accumulation in tumors. There are several clinical trials that investigate the efficacy of NBTXR3 in an array of cancer types (Table 1). In trials on pancreatic ductal adenocarcinoma (Figure 6C) [7778] and locally advanced squamous cell carcinoma (Figure 6D) [7879], the accumulation and retention of NBTXR3 in tumor were visualized by CT, which is valuable in evaluating the biodistribution of injected NPs and confirming persistence of NBTXR3 during the entire duration of radiotherapy.
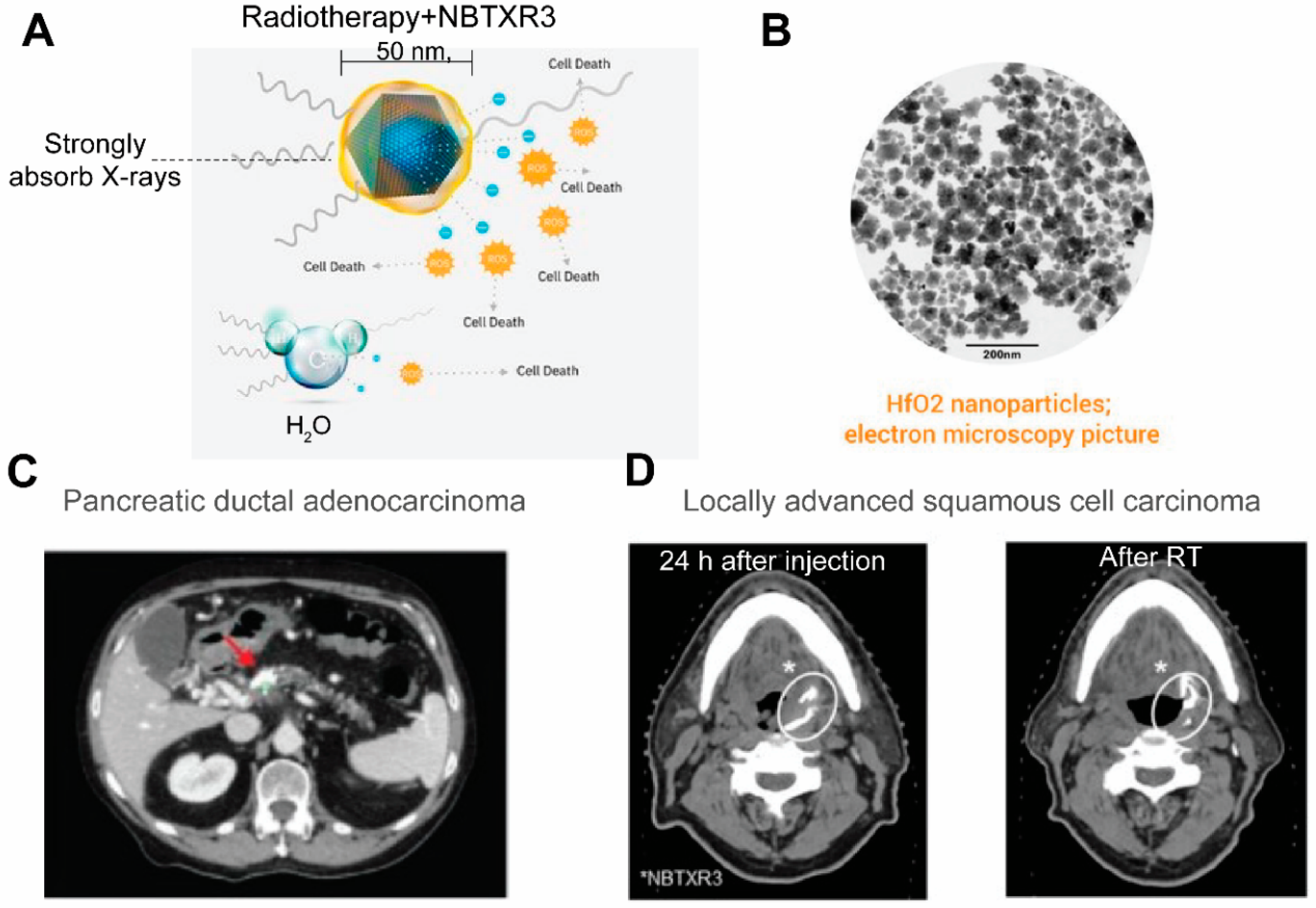
Figure 6. NBTXR3 able s radioenhancers that are trackable by CT. (A) An illustration of the composition of NBTXR3 and its radioenhancing function. NBTXR3 consists of the HfO2 crystalline core (blue) and phosphate coating (yellow). Upon ionizing radiation, HfO2 induces the generation of a substantial amount of electrons that create more energy deposition in tumor than water molecules, hence promoting cancer cell death. Source: twitter@Nanobiotix. (B) Electron microscopy image of NBTXR3. Source: https://www.sharepitch.com/healthcare/archives/05-2018, accessed on 22 April 2022. (C) Radiopaque NBTXR3 visualized on simulation CT image of a patient with pancreatic ductal adenocarcinoma (red arrow: tumor). Adapted based on Ref. [78]. (D) CT scans showing intratumoral localisation of NBTXR3 at 24 h after injection and after radiotherapy (RT) in a patient with locally advanced squamous cell carcinoma. *: position of NBTXR3 accumulation.Adapted based on Ref. [79].
Table 1. Cancer nanotheranostics in clinical trials.
| Drug Name | Composition | Imaging Label (Modality) | Therapeutic Agent (Mechanism) | NCT | Phase(s) | Cancer Type |
|---|---|---|---|---|---|---|
| [64Cu]MM-302 | HER2-targeted 64Cu-labeled liposome containing doxorubicin | 64Cu (PET) | Doxorubicin (chemotherapy) | NCT01304797 | I | Breast cancer |
| NCT02213744 | II | |||||
| [89Zr]-Df-CriPec® | 89Zr labeled micellar docetaxel conjugate | 89Zr (PET) | Docetaxel (chemotherapy) | NCT03712423 | I | Solid Tumor |
| AGuIX® | Polysiloxane matrix nanoparticles with Gd chelates | Gd (MRI) | Gd (radiosensitizer) | NCT02820454 | I | Multiple brain metastases |
| NCT03818386 | II | |||||
| NCT04899908 | II | |||||
| NCT03308604 | I | Locally advanced cervical cancer | ||||
| NCT04881032 | I/II | Newly Diagnosed Glioblastoma | ||||
| NBTXR3 | Hafnium oxide nanoparticles | Hafnium oxide (CT) | Hafnium oxide (radioenhancer) | NCT01433068 | I | Soft tissue sarcoma |
| NCT02379845 | II/III | |||||
| NCT02805894 | I/II | Prostate adenocarcinoma | ||||
| NCT04505267 | I | Non-small cell lung cancer | ||||
| NCT04834349 | II | Head and neck squamous cell cancer (inoperable or recurrent) | ||||
| NCT04484909 | I | Pancreatic cancer | ||||
| NCT04615013 | I | Esophageal adenocarcinoma | ||||
| NCT04862455 | II | Head and neck squamous cancer (recurrent or metastatic) | ||||
| NCT05039632 | II | |||||
| NCT04892173 | III | Locally advanced squamous cell carcinoma |
References
- Man, F.; Lammers, T.; De Rosales, R.T.M. Imaging Nanomedicine-Based Drug Delivery: A Review of Clinical Studies. Mol. Imaging Biol. 2018, 20, 683–695.
- Cuaron, J.; Hirsch, J.; Medich, D.; Rosenstein, B.; Martel, C.; Hirsch, A. A Proposed Methodology to Select Radioisotopes for Use in Radionuclide Therapy. Am. J. Neuroradiol. 2009, 30, 1824–1829.
- Lipowska, M.; Klenc, J.; Taylor, A.T.; Marzilli, L.G. fac-99mTc/Re-tricarbonyl complexes with tridentate aminocarboxyphosphonate ligands: Suitability of the phosphonate group in chelate ligand design of new imaging agents. Inorg. Chim. Acta 2018, 486, 529–537.
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902.
- Ni, D.; Jiang, D.; Ehlerding, E.B.; Huang, P.; Cai, W. Radiolabeling Silica-Based Nanoparticles via Coordination Chemistry: Basic Principles, Strategies, and Applications. Acc. Chem. Res. 2018, 51, 778–788.
- Good, S.; Walter, M.A.; Waser, B.; Wang, X.; Müller-Brand, J.; Béhé, M.P.; Reubi, J.-C.; Maecke, H.R. Macrocyclic chelator-coupled gastrin-based radiopharmaceuticals for targeting of gastrin receptor-expressing tumours. Eur. J. Pediatr. 2008, 35, 1868–1877.
- Chang, A.J.; Sohn, R.; Lu, Z.H.; Arbeit, J.M.; Lapi, S.E. Detection of Rapalog-Mediated Therapeutic Response in Renal Cancer Xenografts Using 64Cu-bevacizumab ImmunoPET. PLoS ONE 2013, 8, e58949.
- Shokeen, M.; Anderson, C.J. Molecular Imaging of Cancer with Copper-64 Radiopharmaceuticals and Positron Emission Tomography (PET). Acc. Chem. Res. 2009, 42, 832–841.
- Zhang, Y.; Hong, H.; Engle, J.W.; Bean, J.; Yang, Y.; Leigh, B.R.; Barnhart, T.E.; Cai, W. Positron Emission Tomography Imaging of CD105 Expression with a 64Cu-Labeled Monoclonal Antibody: NOTA Is Superior to DOTA. PLoS ONE 2011, 6, e28005.
- Rauscher, A.; Frindel, M.; Rajerison, H.; Gouard, S.; Maurel, C.; Barbet, J.; Faivre-Chauvet, A.; Mougin-Degraef, M. Improvement of the Targeting of Radiolabeled and Functionalized Liposomes with a Two-Step System Using a Bispecific Monoclonal Antibody (Anti-CEA x Anti-DTPA-In). Front. Med. 2015, 2, 83.
- Borràs, J.; Mesa, V.; Suades, J.; Barnadas-Rodriguez, R. Direct Synthesis of Rhenium and Technetium-99m Metallosurfactants by a Transmetallation Reaction of Lipophilic Groups: Potential Applications in the Radiolabeling of Liposomes. Langmuir 2020, 36, 1993–2002.
- Aranda-Lara, L.; Morales-Avila, E.; Luna-Gutiérrez, M.A.; Olivé-Alvarez, E.; Isaac-Olivé, K. Radiolabeled liposomes and lipoproteins as lipidic nanoparticles for imaging and therapy. Chem. Phys. Lipids 2020, 230, 104934.
- Petersen, A.L.; Binderup, T.; Rasmussen, P.; Henriksen, J.R.; Elema, D.R.; Kjaer, A.; Andresen, T.L. 64Cu loaded liposomes as positron emission tomography imaging agents. Biomaterials 2011, 32, 2334–2341.
- Engudar, G.; Schaarup-Jensen, H.; Fliedner, F.P.; Hansen, A.E.; Kempen, P.; Jølck, R.I.; Kjaer, A.; Andresen, T.L.; Clausen, M.H.; Jensen, A.; et al. Remote loading of liposomes with a 124I-radioiodinated compound and their in vivo evaluation by PET/CT in a murine tumor model. Theranostics 2018, 8, 5828–5841.
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 4190–4202.
- Pratt, E.C.; Shaffer, T.M.; Grimm, J. Nanoparticles and radiotracers: Advances toward radionanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 872–890.
- Shi, S.; Xu, C.; Yang, K.; Goel, S.; Valdovinos, H.; Luo, H.; Ehlerding, E.B.; England, C.G.; Cheng, L.; Chen, F.; et al. Chelator-Free Radiolabeling of Nanographene: Breaking the Stereotype of Chelation. Angew. Chem. Int. Ed. 2017, 56, 2889–2892.
- Wall, M.A.; Shaffer, T.M.; Harmsen, S.; Tschaharganeh, D.-F.; Huang, C.-H.; Lowe, S.W.; Drain, C.M.; Kircher, M.F. Chelator-Free Radiolabeling of SERRS Nanoparticles for Whole-Body PET and Intraoperative Raman Imaging. Theranostics 2017, 7, 3068–3077.
- Tang, T.; Wei, Y.; Yang, Q.; Yang, Y.; Sailor, M.J.; Pang, H.-B. Rapid chelator-free radiolabeling of quantum dots for in vivo imaging. Nanoscale 2019, 11, 22248–22254.
- Miller, K.; Cortes, J.; Hurvitz, S.A.; Krop, I.E.; Tripathy, D.; Verma, S.; Riahi, K.; Reynolds, J.G.; Wickham, T.J.; Molnar, I.; et al. HERMIONE: A randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naive, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer 2016, 16, 352.
- Lee, H.; Zheng, J.; Gaddy, D.; Orcutt, K.D.; Leonard, S.; Geretti, E.; Hesterman, J.; Harwell, C.; Hoppin, J.; Jaffray, D.A.; et al. A gradient-loadable 64Cu-chelator for quantifying tumor deposition kinetics of nanoliposomal therapeutics by positron emission tomography. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 155–165.
- Jentzen, W.; Verschure, F.; van Zon, A.; van de Kolk, R.; Wierts, R.; Schmitz, J.; Bockisch, A.; Binse, I. 124I PET Assessment of Response of Bone Metastases to Initial Radioiodine Treatment of Differentiated Thyroid Cancer. J. Nucl. Med. 2016, 57, 1499–1504.
- Lopci, E.; Chiti, A.; Castellani, M.R.; Pepe, G.; Antunovic, L.; Fanti, S.; Bombardieri, E. Matched pairs dosimetry: 124I/131I metaiodobenzylguanidine and 124I/131I and 86Y/90Y antibodies. Eur. J. Pediatr. 2011, 38, 28–40.
- Marsh, I.R.; Grudzinski, J.J.; Baiu, D.C.; Besemer, A.; Hernandez, R.; Jeffery, J.J.; Weichert, J.P.; Otto, M.; Bednarz, B.P. Preclinical Pharmacokinetics and Dosimetry Studies of 124I/131I-CLR1404 for Treatment of Pediatric Solid Tumors in Murine Xenograft Models. J. Nucl. Med. 2019, 60, 1414–1420.
- Goel, S.; Ferreira, C.A.; Dogra, P.; Yu, B.; Kutyreff, C.J.; Siamof, C.M.; Engle, J.W.; Barnhart, T.E.; Cristini, V.; Wang, Z.; et al. Size-Optimized Ultrasmall Porous Silica Nanoparticles Depict Vasculature-Based Differential Targeting in Triple Negative Breast Cancer. Small 2019, 15, e1903747.
- Ferreira, C.A.; Goel, S.; Ehlerding, E.B.; Rosenkrans, Z.T.; Jiang, D.; Sun, T.; Aluicio-Sarduy, E.; Engle, J.W.; Ni, D.; Cai, W. Ultrasmall Porous Silica Nanoparticles with Enhanced Pharmacokinetics for Cancer Theranostics. Nano Lett. 2021, 21, 4692–4699.
- Imlimthan, S.; Khng, Y.C.; Keinänen, O.; Zhang, W.; Airaksinen, A.J.; Kostiainen, M.A.; Zeglis, B.M.; Santos, H.A.; Sarparanta, M. A Theranostic Cellulose Nanocrystal-Based Drug Delivery System with Enhanced Retention in Pulmonary Metastasis of Melanoma. Small 2021, 17, e2007705.
- Gaikwad, G.; Rohra, N.; Kumar, C.; Jadhav, S.; Sarma, H.D.; Borade, L.; Chakraborty, S.; Bhagwat, S.; Dandekar, P.; Jain, R.; et al. A facile strategy for synthesis of a broad palette of intrinsically radiolabeled chitosan nanoparticles for potential use in cancer theranostics. J. Drug Deliv. Sci. Technol. 2021, 63, 102485.
- Tweedle, M.F.; Wedeking, P.; Kumar, K. Biodistribution of Radiolabeled, Formulated Gadopentetate, Gadoteridol, Gadoterate, and Gadodiamide in Mice and Rats. Investig. Radiol. 1995, 30, 372–380.
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.-L.; Quesada, J.-L.; et al. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 2021, 160, 159–165.
- Lux, F.; Tran, V.L.; Thomas, E.; Dufort, S.; Rossetti, F.; Martini, M.; Truillet, C.; Doussineau, T.; Bort, G.; Denat, F.; et al. AGuIX((R)) from bench to bedside-Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2019, 92, 20180365.
- Sancey, L.; Lux, F.; Kotb, S.; Roux, S.; Dufort, S.; Bianchi, A.; Crémillieux, Y.; Fries, P.; Coll, J.-L.; Rodriguez-Lafrasse, C.; et al. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br. J. Radiol. 2014, 87, 20140134.
- Serri, C.; Quagliariello, V.; Iaffaioli, R.V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: A preliminary in vitro study. J. Cell. Physiol. 2018, 234, 4959–4969.
- Verry, C.; Dufort, S.; Lemasson, B.; Grand, S.; Pietras, J.; Troprès, I.; Crémillieux, Y.; Lux, F.; Mériaux, S.; Larrat, B.; et al. Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci. Adv. 2020, 6, eaay5279.
- Gries, M.; Thomas, N.; Daouk, J.; Rocchi, P.; Choulier, L.; Jubréaux, J.; Pierson, J.; Reinhard, A.; Jouan-Hureaux, V.; Chateau, A.; et al. Multiscale Selectivity and in vivo Biodistribution of NRP-1-Targeted Theranostic AGuIX Nanoparticles for PDT of Glioblastoma. Int. J. Nanomed. 2020, 15, 8739–8758.
- Chen, H.; Qiu, Y.; Ding, D.; Lin, H.; Sun, W.; Wang, G.D.; Huang, W.; Zhang, W.; Lee, D.; Liu, G.; et al. Gadolinium-Encapsulated Graphene Carbon Nanotheranostics for Imaging-Guided Photodynamic Therapy. Adv. Mater. 2018, 30, e1802748.
- Guan, M.; Zhou, Y.; Liu, S.; Chen, D.; Ge, J.; Deng, R.; Li, X.; Yu, T.; Xu, H.; Sun, D.; et al. Photo-triggered gadofullerene: Enhanced cancer therapy by combining tumor vascular disruption and stimulation of anti-tumor immune responses. Biomaterials 2019, 213, 119218.
- Lu, Z.; Jia, W.; Deng, R.; Zhou, Y.; Li, X.; Yu, T.; Zhen, M.; Wang, C. Light-assisted gadofullerene nanoparticles disrupt tumor vasculatures for potent melanoma treatment. J. Mater. Chem. B 2020, 8, 2508–2518.
- Han, Z.; Wu, X.; Roelle, S.; Chen, C.; Schiemann, W.P.; Lu, Z.-R. Targeted gadofullerene for sensitive magnetic resonance imaging and risk-stratification of breast cancer. Nat. Commun. 2017, 8, 692.
- Si, Y.; Zhang, G.; Wang, D.; Zhang, C.; Yang, C.; Bai, G.; Qian, J.; Chen, Q.; Zhang, Z.; Wu, Z.; et al. Nanostructure-enhanced water interaction to increase the dual-mode MR contrast performance of gadolinium-doped iron oxide nanoclusters. Chem. Eng. J. 2019, 360, 289–298.
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091.
- Lartigue, L.; Innocenti, C.; Kalaivani, T.; Awwad, A.; Sanchez Duque, M.D.M.; Guari, Y.; Larionova, J.; Guérin, C.; Montero, J.-L.G.; Barragan-Montero, V.; et al. Water-Dispersible Sugar-Coated Iron Oxide Nanoparticles. An Evaluation of their Relaxometric and Magnetic Hyperthermia Properties. J. Am. Chem. Soc. 2011, 133, 10459–10472.
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic Nanoparticle Clusters for Cancer Theranostics Combining Magnetic Resonance Imaging and Hyperthermia Treatment. Theranostics 2013, 3, 366–376.
- Al Faraj, A.; Shaik, A.S.; Al Sayed, B. Preferential magnetic targeting of carbon nanotubes to cancer sites: Noninvasive tracking using MRI in a murine breast cancer model. Nanomedicine 2015, 10, 931–948.
- Svenskaya, Y.; Garello, F.; Lengert, E.; Kozlova, A.; Verkhovskii, R.; Bitonto, V.; Ruggiero, M.R.; German, S.; Gorin, D.; Terreno, E. Biodegradable polyelectrolyte/magnetite capsules for MR imaging and magnetic targeting of tumors. Nanotheranostics 2021, 5, 362–377.
- Schleich, N.; Po, C.; Jacobs, D.; Ucakar, B.; Gallez, B.; Danhier, F.; Préat, V. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J. Control. Release 2014, 194, 82–91.
- Yuan, Y.; Zhang, J.; Qi, X.; Li, S.; Liu, G.; Siddhanta, S.; Barman, I.; Song, X.; McMahon, M.T.; Bulte, J.W.M. Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy. Nat. Mater. 2019, 18, 1376–1383.
- Li, Y.; Chen, H.; Xu, J.; Yadav, N.N.; Chan, K.W.; Luo, L.; McMahon, M.; Vogelstein, B.; Van Zijl, P.C.; Zhou, S.; et al. CEST theranostics: Label-free MR imaging of anticancer drugs. Oncotarget 2016, 7, 6369–6378.
- Han, Z.; Li, Y.; Zhang, J.; Liu, J.; Chen, C.; Van Zijl, P.C.M.; Liu, G. Molecular imaging of deoxycytidine kinase activity using deoxycytidine-enhanced CEST MRI. Cancer Res. 2019, 79, 2775–2783.
- Ngen, E.J.; Bar-Shir, A.; Jablonska, A.; Liu, G.; Song, X.; Ansari, R.; Bulte, J.W.M.; Janowski, M.; Pearl, M.; Walczak, P.; et al. Imaging the DNA Alkylator Melphalan by CEST MRI: An Advanced Approach to Theranostics. Mol. Pharm. 2016, 13, 3043–3053.
- Han, Z.; Liu, G. CEST MRI trackable nanoparticle drug delivery systems. Biomed. Mater. 2021, 16, 024103.
- Castelli, D.D.; Terreno, E.; Longo, D.; Aime, S. Nanoparticle-based chemical exchange saturation transfer (CEST) agents. NMR Biomed. 2013, 26, 839–849.
- Zhou, L.-Q.; Li, P.; Cui, X.-W.; Dietrich, C.F. Ultrasound nanotheranostics in fighting cancer: Advances and prospects. Cancer Lett. 2019, 470, 204–219.
- Vallet-Regi, M.; Manzano, M.; Baeza, A. Controlled Release with Emphasis on Ultrasound-Induced Release. Enzymes 2018, 43, 101–122.
- Qin, H.; Teng, R.; Liu, Y.; Li, J.; Yu, M. Drug Release from Gelsolin-Targeted Phase-Transition Nanoparticles Triggered by Low-Intensity Focused Ultrasound. Int. J. Nanomed. 2022, 17, 61–71.
- Novoselova, M.V.; German, S.V.; Abakumova, T.O.; Perevoschikov, S.V.; Sergeeva, O.V.; Nesterchuk, M.V.; Efimova, O.I.; Petrov, K.S.; Chernyshev, V.S.; Zatsepin, T.S.; et al. Multifunctional nanostructured drug delivery carriers for cancer therapy: Multimodal imaging and ultrasound-induced drug release. Colloids Surf. B Biointerfaces 2021, 200, 111576.
- Yildirim, A.; Shi, D.; Roy, S.; Blum, N.T.; Chattaraj, R.; Cha, J.N.; Goodwin, A.P. Nanoparticle-Mediated Acoustic Cavitation Enables High Intensity Focused Ultrasound Ablation Without Tissue Heating. ACS Appl. Mater. Interfaces 2018, 10, 36786–36795.
- Sjöstrand, S.; Evertsson, M.; Jansson, T. Magnetomotive Ultrasound Imaging Systems: Basic Principles and First Applications. Ultrasound Med. Biol. 2020, 46, 2636–2650.
- Qin, S.; Caskey, C.F.; Ferrara, K.W. Ultrasound contrast microbubbles in imaging and therapy: Physical principles and engineering. Phys. Med. Biol. 2009, 54, R27–R57.
- Bawiec, C.R.; Rosnitskiy, P.B.; Peek, A.T.; Maxwell, A.D.; Kreider, W.; ter Haar, G.R.; Sapozhnikov, O.A.; Khokhlova, V.A.; Khokhlova, T.D. Inertial Cavitation Behaviors Induced by Nonlinear Focused Ultrasound Pulses. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2884–2895.
- Chowdhury, S.M.; Abou-Elkacem, L.; Lee, T.; Dahl, J.; Lutz, A.M. Ultrasound and microbubble mediated therapeutic delivery: Underlying mechanisms and future outlook. J. Control Release 2020, 326, 75–90.
- Hyvelin, J.-M.; Gaud, E.; Costa, M.; Helbert, A.; Bussat, P.; Bettinger, T.; Frinking, P. Characteristics and Echogenicity of Clinical Ultrasound Contrast Agents: An In Vitro and In Vivo Comparison Study. J. Ultrasound Med. 2017, 36, 941–953.
- Wang, X.; Chen, H.; Zheng, Y.; Ma, M.; Chen, Y.; Zhang, K.; Zeng, D.; Shi, J. Au-nanoparticle coated mesoporous silica nanocapsule-based multifunctional platform for ultrasound mediated imaging, cytoclasis and tumor ablation. Biomaterials 2012, 34, 2057–2068.
- Li, J.; Ji, H.; Jing, Y.; Wang, S. pH- and acoustic-responsive platforms based on perfluoropentane-loaded protein nanoparticles for ovarian tumor-targeted ultrasound imaging and therapy. Nanoscale Res. Lett. 2020, 15, 31.
- Lee, J.; Min, H.-S.; Gil You, D.; Kim, K.; Kwon, I.C.; Rhim, T.; Lee, K.Y. Theranostic gas-generating nanoparticles for targeted ultrasound imaging and treatment of neuroblastoma. J. Control Release 2016, 223, 197–206.
- McNally, L.R.; Mezera, M.; Morgan, D.E.; Frederick, P.J.; Yang, E.S.; Eltoum, I.E.; Grizzle, W.E. Current and emerging clinical applications of multispectral optoacoustic tomography (MSOT) in oncology. Clin. Cancer Res. 2016, 22, 3432–3439.
- MacCuaig, W.M.; Jones, M.A.; Abeyakoon, O.; McNally, L.R. Development of Multispectral Optoacoustic Tomography as a Clinically Translatable Modality for Cancer Imaging. Radiol. Imaging Cancer 2020, 2, e200066.
- Mantri, Y.; Jokerst, J.V. Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14, 9408–9422.
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjugate Chem. 2020, 31, 194–213.
- Laramie, M.D.; Fouts, B.L.; MacCuaig, W.M.; Buabeng, E.; Jones, M.A.; Mukherjee, P.; Behkam, B.; McNally, L.R.; Henary, M. Improved pentamethine cyanine nanosensors for optoacoustic imaging of pancreatic cancer. Sci. Rep. 2021, 11, 4366.
- Samykutty, A.; Grizzle, W.E.; Fouts, B.L.; McNally, M.W.; Chuong, P.; Thomas, A.; Chiba, A.; Otali, D.; Woloszynska, A.; Said, N.; et al. Optoacoustic imaging identifies ovarian cancer using a microenvironment targeted theranostic wormhole mesoporous silica nanoparticle. Biomaterials 2018, 182, 114–126.
- Thomas, A.; Chiba, A.; Samykutty, A.; McNally, M.W.; McNally, L.R. Tumor specific cargo release in ex vivo patient samples and murine models of triple negative breast cancer by a pH-targeted nanoparticle: V3-RUBY. Cancer Res. 2020, 80, P3-06-04.
- Rostami, A.; Sazgarnia, A. Gold nanoparticles as cancer theranostic agents. Nanomed. J. 2019, 6, 147–160. MacCuaig, W.M.; Fouts, B.L.; McNally, M.W.; Grizzle, W.E.; Chuong, P.; Samykutty, A.; Mukherjee, P.; Li, M.; Jasinski, J.B.; Behkam, B.; et al. Active Targeting Significantly Outperforms Nanoparticle Size in Facilitating Tumor-Specific Uptake in Orthotopic Pancreatic Cancer. ACS Appl. Mater. Interfaces 2021, 13, 49614–49630.
- Curry, T.; Kopelman, R.; Shilo, M.; Popovtzer, R. Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol. Imaging 2014, 9, 53–61. Rostami, A.; Sazgarnia, A. Gold nanoparticles as cancer theranostic agents. Nanomed. J. 2019, 6, 147–160.
- Yang, C.; Guo, C.; Guo, W.; Zhao, X.; Liu, S.; Han, X. Multifunctional Bismuth Nanoparticles as Theranostic Agent for PA/CT Imaging and NIR Laser-Driven Photothermal Therapy. ACS Appl. Nano Mater. 2018, 1, 820–830. Curry, T.; Kopelman, R.; Shilo, M.; Popovtzer, R. Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol. Imaging 2014, 9, 53–61.
- Wei, B.; Zhang, X.; Zhang, C.; Jiang, Y.; Fu, Y.-Y.; Yu, C.; Sun, S.-K.; Yan, X.-P. Facile Synthesis of Uniform-Sized Bismuth Nanoparticles for CT Visualization of Gastrointestinal Tract In Vivo. ACS Appl. Mater. Interfaces 2016, 8, 12720–12726. Yang, C.; Guo, C.; Guo, W.; Zhao, X.; Liu, S.; Han, X. Multifunctional Bismuth Nanoparticles as Theranostic Agent for PA/CT Imaging and NIR Laser-Driven Photothermal Therapy. ACS Appl. Nano Mater. 2018, 1, 820–830.
- Bagley, A.F.; Ludmir, E.B.; Maitra, A.; Minsky, B.D.; Smith, G.L.; Das, P.; Koong, A.C.; Holliday, E.B.; Taniguchi, C.M.; Katz, M.H.; et al. NBTXR3, a first-in-class radioenhancer for pancreatic ductal adenocarcinoma: Report of first patient experience. Clin. Transl. Radiat. Oncol. 2022, 33, 66–69. Wei, B.; Zhang, X.; Zhang, C.; Jiang, Y.; Fu, Y.-Y.; Yu, C.; Sun, S.-K.; Yan, X.-P. Facile Synthesis of Uniform-Sized Bismuth Nanoparticles for CT Visualization of Gastrointestinal Tract In Vivo. ACS Appl. Mater. Interfaces 2016, 8, 12720–12726.
- Bonvalot, S.; Le Pechoux, C.; De Baere, T.; Kantor, G.; Buy, X.; Stoeckle, E.; Terrier, P.; Sargos, P.; Coindre, J.M.; Lassau, N.; et al. First-in-Human Study Testing a New Radioenhancer Using Nanoparticles (NBTXR3) Activated by Radiation Therapy in Patients with Locally Advanced Soft Tissue Sarcomas. Clin. Cancer Res. 2016, 23, 908–917. Bagley, A.F.; Ludmir, E.B.; Maitra, A.; Minsky, B.D.; Smith, G.L.; Das, P.; Koong, A.C.; Holliday, E.B.; Taniguchi, C.M.; Katz, M.H.; et al. NBTXR3, a first-in-class radioenhancer for pancreatic ductal adenocarcinoma: Report of first patient experience. Clin. Transl. Radiat. Oncol. 2022, 33, 66–69.
- Bonvalot, S.; Le Pechoux, C.; De Baere, T.; Kantor, G.; Buy, X.; Stoeckle, E.; Terrier, P.; Sargos, P.; Coindre, J.M.; Lassau, N.; et al. First-in-Human Study Testing a New Radioenhancer Using Nanoparticles (NBTXR3) Activated by Radiation Therapy in Patients with Locally Advanced Soft Tissue Sarcomas. Clin. Cancer Res. 2016, 23, 908–917.
More
