Cell cycle regulation is largely based on protein phospho-dephosphorylation events, catalyzed by cyclin dependent kinases (Cdks) and phosphatases (PPases), respectively. During many years the Cdks were considered the main component of the cell cycle control system. Recently, the importance of the counteracting PPases has emerged. Research on yeast has provided many insights into such contribution. Here we present an overview of the protein phosphatase 2A family's roles during mitosis.
- PP2A phosphatase
- cell cycle
- mitosis
- chromosome segregation
- Mitotic exit network
- Septation initiation network
1. Introduction
At the metaphase to anaphase transition, the APC/CCdc20 (anaphase promoting complex) promotes the proteasomal destruction of cyclin B driving the inactivation of the mitotic Cdk1[1] and the separase (Esp1) inhibitor, securin[2][3][4] (Pds1 in budding yeast). Active separase promotes sister chromatids segregation by cleaving the Scc1 subunit of the cohesin complex and triggers mitotic exit through Cdc14 activation[5][6]. PP2ACdc55 prevents the untimely activation of the mitotic exit in different ways: by the adaptation to the spindle assembly checkpoint, regulating the cohesin cleavage and by inhibiting Cdc14 release from the nucleolus (Figure 1). Later on, during late anaphase PP2A regulate the mitotic exit network (MEN in budding yeast or SIN in fission yeast).
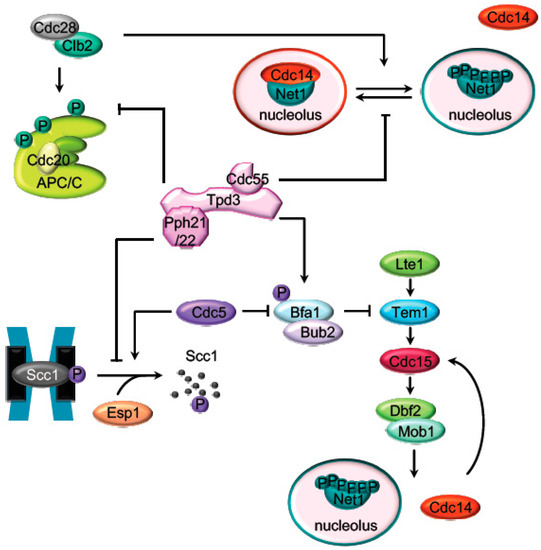
Figure 1.
Main PP2ACdc55
targets during mitosis. Representation of the major mitotic PP2ACdc55
substrates described inS. cerevisiae
. Before anaphase onset, PP2ACdc55
counteracts the Cdk1 phosphorylation of the APC/C subunits and the Cdc14 inhibitor, Net1. Scc1 dephosphorylation by PP2ACdc55
also prevent premature sister chromatids segregation before anaphase. PP2ACdc55 contributes to keep MEN inactive by counteracting Bfa1 phosphorylation in metaphase.
contributes to keep MEN inactive by counteracting Bfa1 phosphorylation in metaphase.2. The APC Dephosphorylation by PP2A
(1) The APC Dephosphorylation by PP2A
Cdc55
Cdc28–Clb2 phosphorylates the APC subunits Cdc16, Cdc23, and Cdc27 upon spindle damage conditions to activate APC
Cdc28–Clb2 phosphorylates the APC subunits Cdc16, Cdc23, and Cdc27 upon spindle damage conditions to activate APC
. The phospho-null mutants for these proteins
[9]
and the inactivation of Cdc28
[10]
impaired APC/C
Cdc20
activity. Conversely, PP2A
Cdc55
counteracts the Cdk1 phosphorylation of the APC/C subunit Cdc16
, keeping the spindle checkpoint assembly (SAC) active until the cell is prepared for anaphase. Tight balance between Cdc28–Clbs and PP2A
Cdc55
activities is important for the adaptation to the spindle checkpoint
[10]
.
3. The Regulation of the Cohesin Cleavage by PP2A
(2) The Regulation of the Cohesin Cleavage by PP2A
Cdc55
PP2ACdc55 also regulates anaphase onset by counteracting the phosphorylation of the Scc1 subunit of the cohesin complex[12]. Scc1 is phosphorylated by the polo-like kinase Cdc5, promoting the Scc1 cleavage by separase[13][14][15][16]. Before anaphase, the dephosphorylation of the Scc1 by PP2ACdc55 prevents its recognition by separase, avoiding premature sister chromatids segregation[12]. In early anaphase, upon separase downregulation of PP2ACdc55, Scc1 dephosphorylation is inhibited, promoting cohesin cleavage.
4. The FEAR-Cdc14 Release by PP2A
(3) The FEAR-Cdc14 Release by PP2A
Cdc55
The first described mitotic function of PP2ACdc55 was its role in the activation of the phosphatase Cdc14 during anaphase. Cdc14 is kept sequestered in the nucleolus by its binding to the nucleolar protein Net1 during most of the cell cycle. At anaphase, Net1 is phosphorylated by the mitotic kinases Cdk1–Clb2 and polo-like Cdc5[17][18][19]. Phosphorylated-Net1 has low affinity toward Cdc14, and the phosphatase is translocated, first to the nucleus during early anaphase and to the cytoplasm in late anaphase. Early anaphase Cdc14 release is regulated by separase in conjunction with a series of proteins (Slk19, Spo12, Fob1, Cdc5, Cdk1–Clb2, Cdc55, and Hit1)[20][17][21][22][23][24][25][26] commonly known as the FEAR pathway (the cdcfourteen early anaphase release (FEAR)).
During most of the cell cycle, Net1 phosphorylation is counteracted by PP2ACdc55[20][8], and, as a consequence, Cdc14 is sequestered at the nucleolus. In cdc55Δ mutant cells, Net1 is phosphorylated already at metaphase, and Cdc14 is prematurely released from the nucleolus. In addition, it was shown that PP2ACdc55 has phosphatase activity against Net1 in vitro[27][20] and both proteins co-immunoprecipitate in vivo[27], suggesting that Net1 is a substrate of PP2ACdc55. During early anaphase, downregulation of the PP2ACdc55 phosphatase activity allows the accumulation of the Cdk1–Clb2-dependent Net1 phosphorylation and promotes the Cdc14 release from the nucleolus[20]. Remarkably, the anaphase-specific inhibition of the PP2ACdc55 phosphatase activity is due to phosphorylation of the regulatory subunit Cdc55 by Cdk1–Clb2 and depends on active separase and Zds1 proteins[27][20][21][23].
PP2ACdc55 is also required for the proper temporal initiation of meiotic events[28]. Similar to mitosis, PP2ACdc55 also regulates the FEAR pathway during meiosis[29][30]. PP2ACdc55 dephosphorylates Net1 and promotes Cdc14 release from the nucleolus, preventing precocious exit from meiosis I. In addition, PP2ACdc55 is required for reductional chromosome segregation in the absence of recombination independently of its role in the FEAR pathway[31].
5. MEN (SIN) Regulation by PP2A
(4) MEN (SIN) Regulation by PP2A
Cdc14 activation and release during anaphase is mediated by two parallel pathways: the FEAR and the mitotic exit network (MEN)[32]. MEN (also known as the Hippo pathway in higher eukaryotes) is a GTPase-driven signaling cascade (spindle pole body, SPB in yeast) that regulates mitotic exit, enables the control of the spindle orientation, and promotes cytokinesis in budding yeast.
The core of the MEN cascade consists of two serine/threonine kinases, Cdc15 (PAK kinase in higher eukaryotes) and Dbf2-Mob1 (LATS kinase in higher eukaryotes). They are activated in mid–late anaphase to maintain Cdc14 released from the nucleolus and promote its full activation[33][34][35]. During an unperturbed cell cycle, the inhibitor complex Bub2/Bfa1 keeps MEN inactive. PP2ACdc55 contributes to keep Bub2/Bfa1 active by dephosphorylating Bfa1 in metaphase[36]. When cells reach anaphase with a correct aligned mitotic spindle, Cdc5 phosphorylates Bfa1 and inactivates the Bub2/Bfa1 GAP activity[37][38]. The anaphase-specific inactivation of PP2ACdc55 also contributes to increase the Cdc5-dependent Bfa1 phosphorylation and promotes the activation of MEN. Therefore, PP2ACdc55 not only facilitates the FEAR-dependent Cdc14 release in early anaphase but also contributes to alleviation of the MEN inhibitory signal imposed by Bfa1/Bub2.
In addition, most of the MEN proteins are regulated by phosphorylation, making MEN activity restrained by Cdk1 and stimulated by the action of the opposing phosphatases, Cdc14 and PP2ACdc55 (Figure 3). Cdk1 restrains MEN activity through Cdc15 and Mob1 phosphorylation[39]. At anaphase, Cdc15 is dephosphorylated by the FEAR-released Cdc14 facilitating its activation[40][41][42][43][44]. Mob1 dephosphorylation at late anaphase is necessary for Dbf2-Mob1 activation. Abrupt Cdk1 inactivation and Cdc14 release from the nucleolus contribute to Mob1 dephosphorylation in late anaphase[39]. In addition, PP2ACdc55 also dephosphorylates Mob1 protein[36]. At anaphase onset, PP2ACdc55 downregulation facilitates Cdk1-dependent phosphorylation of Mob1, contributing to Dbf2–Mob1 inhibition. During exit from mitosis, PP2ACdc55 reactivation could promote Mob1 dephosphorylation supporting Dbf2–Mob1 activation.
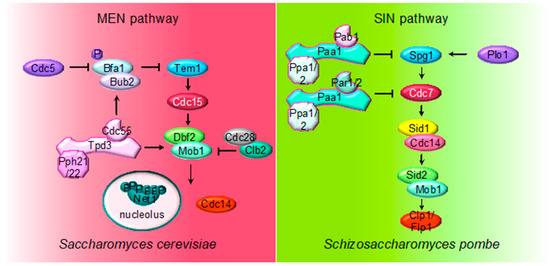
Figure 2. Multiple roles of PP2A regulating MEN and SIN pathways. The cartoon shows the different PP2A holoenzymes and their substrates regulating MEN during mitosis in S. cerevisiae and SIN in S. pombe during septation.
Although PP2ACdc55 is the main PP2A regulating mitotic exit, PP2ARts1 was also described to regulate MEN upon activation of the spindle position checkpoint (SPOC). The activation and functionality of SPOC depend on the ability of Bub2/Bfa1 to inhibit MEN. Phosphorylation of Bfa1 by Kin4 prevents the Cdc5-dependent phosphorylation of Bfa1, keeping MEN inactive[38][45][46][47]. PP2ARts1 phosphatase is a SPOC component acting upstream of Kin4. PP2ARts1 dephosphorylates Kin4, regulating the association of Kin4 to the SPBs, and thereby restraining MEN activity[48].
The MEN pathway is closely related to the septation initiation network (SIN) in Schizosaccharomyces pombe and the Hippo pathway in mammals. Their most conserved role is the regulation of cytokinesis. At the core of the SIN pathway, Sid1-Cdc14 is the PAK-like kinase (Cdc15) and Sid2-Mob1 is the LATS-kinase (Dbf2-Mob1). Two additional kinases, the polo-like Plo1 and the Ste20-family Cdc7 are also part of the SIN pathway. Mutations of the PP2A regulatory subunits (Pab1 and Par1) and the major catalytic subunit Ppa2 rescue conditional SIN mutants[49][50][51], suggesting that PP2A inhibits SIN signaling (Figure 2). PP2APab1 inhibits the SIN pathway[49] and, similar to S. cerevisiae, it has been proposed that the main candidate to be the target of PP2APab1 is the GAP Byr4-Cdc16 (Bub2/Bfa1 in S. cerevisiae)[49]. The other PP2A holoenzyme, PP2APar1/2, regulates the localization of the Cdc7 kinase inhibiting SIN, in order to avoid multiple rounds of septation[50][51][52].
Finally, PP2APab1 and PP2APar1 are also regulated by the PP1-like phosphatase Dis2. PP1 binds to and activates PP2APab1 through a conserved RVXF motif present in Pab1, the B55 subunit. Active PP2APab1 dephosphorylates Par1 and promotes PP1 recruitment, which in turn further activates PP2APar1 phosphatase. In this way, PP1-induced activation of both PP2AB55 and PP2AB56 coordinates mitotic progression and exit from mitosis[53].
References
- Matt Sullivan; David O. Morgan; Finishing mitosis, one step at a time. Nature Reviews Molecular Cell Biology 2007, 8, 894-903, 10.1038/nrm2276.
- O Cohen-Fix; J M Peters; M W Kirschner; D Koshland; Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p.. Genes & Development 1996, 10, 3081-3093, 10.1101/gad.10.24.3081.
- Zoe Hilioti; Yun Shin Chung; Yuko Mochizuki; Christopher F.J Hardy; Orna Cohen-Fix; The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Current Biology 2001, 11, 1643, 10.1016/s0960-9822(01)00505-x.
- Hong Hwa Lim; Phuay-Yee Goh; Uttam Surana; Cdc20 is essential for the cyclosome-mediated proteolysis of both Pds1 and Clb2 during M phase in budding yeast.. Current Biology 1998, 8, 231-237, 10.1016/s0960-9822(98)70088-0.
- Matt Sullivan; Frank Uhlmann; A non-proteolytic function of separase links the onset of anaphase to mitotic exit.. Nature 2003, 5, 249-54, 10.1038/ncb940.
- Frank Uhlmann; Minik Wernic; Marc-André Poupart; Eugene V Koonin; Kim Nasmyth; Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast.. Cell 2000, 103, 375-386, 10.1016/s0092-8674(00)00130-6.
- Valentina Rossio; Takeshi Michimoto; Takeshi Sasaki; Iwai Ohbayashi; Yoshiko Kikuchi; Satoshi Yoshida; Nuclear PP2A-Cdc55 prevents APC-Cdc20 activation during the spindle assembly checkpoint. Journal of Cell Science 2013, 126, 4396-4405, 10.1242/jcs.127365.
- Christopher M. Yellman; Daniel J. Burke; The Role of Cdc55 in the Spindle Checkpoint Is through Regulation of Mitotic Exit in Saccharomyces cerevisiae. Molecular Biology of the Cell 2006, 17, 658-666, 10.1091/mbc.E05-04-0336.
- Adam D. Rudner; Andrew W. Murray; Phosphorylation by Cdc28 Activates the Cdc20-Dependent Activity of the Anaphase-Promoting Complex. The Journal of Cell Biology 2000, 149, 1377-1390, 10.1083/jcb.149.7.1377.
- Claudio Vernieri; Elena Chiroli; Valentina Francia; Fridolin Gross; Andrea Ciliberto; Adaptation to the spindle checkpoint is regulated by the interplay between Cdc28/Clbs and PP2ACdc55. The Journal of Cell Biology 2013, 202, 765-778, 10.1083/jcb.201303033.
- Noel Lianga; Elizabeth C. Williams; Erin K. Kennedy; Carole Doré; Sophie Pilon; Stéphanie L. Girard; Jean-Sebastien Deneault; Adam D. Rudner; A Wee1 checkpoint inhibits anaphase onset. The Journal of Cell Biology 2013, 201, 843-862, 10.1083/jcb.201212038.
- Gilad Yaakov; Kurt Thorn; David O. Morgan; Separase biosensor reveals that cohesin cleavage timing depends on phosphatase PP2A(Cdc55) regulation.. Developmental Cell 2012, 23, 124-36, 10.1016/j.devcel.2012.06.007.
- Frank Uhlmann; Friedrich Lottspeich; Kim Nasmyth; Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 1999, 400, 37-42, 10.1038/21831.
- Sujiraporn Pakchuen; Mai Ishibashi; Emi Takakusagi; Katsuhiko Shirahige; Takashi Sutani; Physical Association of Saccharomyces cerevisiae Polo-like Kinase Cdc5 with Chromosomal Cohesin Facilitates DNA Damage Response.. Journal of Biological Chemistry 2016, 291, 17228-46, 10.1074/jbc.M116.727438.
- Gabriela Alexandru; Frank Uhlmann; Karl Mechtler; Marc-André Poupart; Kim Nasmyth; Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast.. Cell 2001, 105, 459-472, 10.1016/s0092-8674(01)00362-2.
- Nadine C D Hornig; Frank Uhlmann; Preferential cleavage of chromatin-bound cohesin after targeted phosphorylation by Polo-like kinase. The EMBO Journal 2004, 23, 3144-3153, 10.1038/sj.emboj.7600303.
- R. Azzam; Susan L. Chen; Wenying Shou; Angie S. Mah; Gabriela Alexandru; Kim Nasmyth; Roland S. Annan; Steven A. Carr; Raymond J. Deshaies; Phosphorylation by Cyclin B-Cdk Underlies Release of Mitotic Exit Activator Cdc14 from the Nucleolus. Science 2004, 305, 516-519, 10.1126/science.1099402.
- Frederick R. Cross; Wenying Shou; Ramzi Azzam; Susan L Chen; Michael J Huddleston; Christopher Baskerville; Harry Charbonneau; Roland S Annan; Steve A Carr; Raymond J Deshaies; Faculty of 1000 evaluation for Cdc5 influences phosphorylation of Net1 and disassembly of the RENT complex.. F1000 - Post-publication peer review of the biomedical literature 2002, 3, 1-14, 10.1186/1471-2199-3-3.
- Jose-Antonio Rodriguez-Rodriguez; Yolanda Moyano; Soraya Játiva; Ethel Queralt; Mitotic Exit Function of Polo-like Kinase Cdc5 Is Dependent on Sequential Activation by Cdk1. Cell Reports 2016, 15, 2050-2062, 10.1016/j.celrep.2016.04.079.
- Ethel Queralt; Chris Lehane; Bela Novak; Frank Uhlmann; Downregulation of PP2ACdc55 Phosphatase by Separase Initiates Mitotic Exit in Budding Yeast. Cell 2006, 125, 719-732, 10.1016/j.cell.2006.03.038.
- Ines Calabria; Barbara Baro; Jose-Antonio Rodriguez-Rodriguez; Nuria Russiñol; Ethel Queralt; Zds1 regulates PP2ACdc55 activity and Cdc14 activation during mitotic exit through its Zds_C motif. Journal of Cell Science 2012, 125, 2875-2884, 10.1242/jcs.097865.
- Ana Isabel De Los Santos-Velázquez; Inés G. De Oya; Javier Manzano-López; Fernando Monje-Casas; Late rDNA Condensation Ensures Timely Cdc14 Release and Coordination of Mitotic Exit Signaling with Nucleolar Segregation. Current Biology 2017, 27, 3248-3263.e5, 10.1016/j.cub.2017.09.028.
- Ethel Queralt; Frank Uhlmann; Separase cooperates with Zds1 and Zds2 to activate Cdc14 phosphatase in early anaphase. The Journal of Cell Biology 2008, 182, 873-883, 10.1083/jcb.200801054.
- Frank Stegmeier; Julie Huang; Rami Rahal; Jessica Zmolik; Danesh Moazed; Angelika Amon; The Replication Fork Block Protein Fob1 Functions as a Negative Regulator of the FEAR Network. Current Biology 2004, 14, 467-480, 10.1016/j.cub.2004.03.009.
- Frank Stegmeier; Rosella Visintin; Angelika Amon; Separase, Polo Kinase, the Kinetochore Protein Slk19, and Spo12 Function in a Network that Controls Cdc14 Localization during Early Anaphase. Cell 2002, 108, 207-220, 10.1016/s0092-8674(02)00618-9.
- Brett N. Tomson; Rami Rahal; Vladimír Reiser; Fernando Monje-Casas; Karim Mekhail; Danesh Moazed; Angelika Amon; Regulation of Spo12 phosphorylation and its essential role in the FEAR network.. Current Biology 2009, 19, 449-60, 10.1016/j.cub.2009.02.024.
- Soraya Játiva; Ines Calabria; Yolanda Moyano-Rodriguez; Patricia Garcia; Ethel Queralt; Cdc14 activation requires coordinated Cdk1-dependent phosphorylation of Net1 and PP2A-Cdc55 at anaphase onset.. Cellular and Molecular Life Sciences 2019, 76, 3601-3620, 10.1007/s00018-019-03086-5.
- Nolt, J.K.; Rice, L.M.; Gallo-Ebert, C.; Bisher, M.E.; Nickels, J.T. PP2ACdc55 is required for multiple events during meiosis I. Cell Cycle 2011.
- Kerr, G.W.; Sarkar, S.; Tibbles, K.L.; Petronczki, M.; Millar, J.B.A.; Arumugam, P. Meiotic nuclear divisions in budding yeast require PP2A Cdc55-mediated antagonism of Net1 phosphorylation by Cdk. J. Cell Biol. 2011.
- Farid Bizzari; Adele L. Marston; Cdc55 coordinates spindle assembly and chromosome disjunction during meiosis. The Journal of Cell Biology 2011, 193, 1213-1228, 10.1083/jcb.201103076.
- Gary W. Kerr; Jin Huei Wong; Prakash Arumugam; PP2ACdc55’s role in reductional chromosome segregation during achiasmate meiosis in budding yeast is independent of its FEAR function. Scientific Reports 2016, 6, 30397, 10.1038/srep30397.
- Bàrbara Baro; Ethel Queralt; Fernando Monje-Casas; Regulation of Mitotic Exit in Saccharomyces cerevisiae. Advanced Structural Safety Studies 2016, 1505, 3-17, 10.1007/978-1-4939-6502-1_1.
- Angie S. Mah; Joanne Jang; Raymond J. Deshaies; Protein kinase Cdc15 activates the Dbf2-Mob1 kinase complex. Proceedings of the National Academy of Sciences 2001, 98, 7325-7330, 10.1073/pnas.141098998.
- Rosella Visintin; Angelika Amon; Regulation of the Mitotic Exit Protein Kinases Cdc15 and Dbf2. Molecular Biology of the Cell 2001, 12, 2961-2974, 10.1091/mbc.12.10.2961.
- Satoshi Yoshida; Akio Toh-E; Regulation of the localization of Dbf2 and mob1 during cell division of saccharomyces cerevisiae.. Genes & Genetic Systems 2001, 76, 141-147, 10.1266/ggs.76.141.
- Barbara Baro; Jose-Antonio Rodriguez-Rodriguez; Ines Calabria; María Luisa Hernáez; Concha Gil; Ethel Queralt; Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase. PLOS Genetics 2013, 9, e1003966, 10.1371/journal.pgen.1003966.
- Marco Geymonat; Ad Spanos; Philip A. Walker; Steven G. Sedgwick; Leland H. Johnston; In Vitro Regulation of Budding Yeast Bfa1/Bub2 GAP Activity by Cdc5*. Journal of Biological Chemistry 2003, 278, 14591-14594, 10.1074/jbc.c300059200.
- Fenghua Hu; Yanchang Wang; U Liu; Yumei Li; Jun Qin; Stephen J Elledge; Regulation of the Bub2/Bfa1 GAP Complex by Cdc5 and Cell Cycle Checkpoints. Cell 2001, 107, 655-665, 10.1016/s0092-8674(01)00580-3.
- Cornelia König; Hiromi Maekawa; Elmar Schiebel; Mutual regulation of cyclin-dependent kinase and the mitotic exit network. The Journal of Cell Biology 2010, 188, 351-368, 10.1083/jcb.200911128.
- Ulrike Gruneberg; Kirsteen Campbell; Clare Simpson; Joan Grindlay; Elmar Schiebel; Nud1p links astral microtubule organization and the control of exit from mitosis. The EMBO Journal 2000, 19, 6475-6488, 10.1093/emboj/19.23.6475.
- R. Cenamor; Javier Jiménez; Victor Jimenez Cid; C. Nombela; M. Sánchez; The Budding Yeast Cdc15 Localizes to the Spindle Pole Body in a Cell-Cycle-Dependent Manner. Molecular Cell Biology Research Communications 1999, 2, 178-184, 10.1006/mcbr.1999.0173.
- Sue L. Jaspersen; David O. Morgan; Cdc14 activates cdc15 to promote mitotic exit in budding yeast.. Current Biology 2000, 10, 615-618, 10.1016/s0960-9822(00)00491-7.
- Shuichan Xu; Han-Kuei Huang; Peter Kaiser; Martin Latterich; Tony Hunter; Phosphorylation and spindle pole body localization of the Cdc15p mitotic regulatory protein kinase in budding yeast.. Current Biology 2000, 10, 329-332, 10.1016/s0960-9822(00)00382-1.
- Ruth Menssen; Albert Neutzner; Wolfgang Seufert; Asymmetric spindle pole localization of yeast Cdc15 kinase links mitotic exit and cytokinesis. Current Biology 2001, 11, 345-350, 10.1016/s0960-9822(01)00095-1.
- Katharine E. D’Aquino; Fernando Monje-Casas; Jennifer Paulson; Vladimír Reiser; Georgette M. Charles; Leslie Lai; Kevan M. Shokat; Angelika Amon; The Protein Kinase Kin4 Inhibits Exit from Mitosis in Response to Spindle Position Defects. Molecular Cell 2005, 19, 223-234, 10.1016/j.molcel.2005.06.005.
- Hiromi Maekawa; Claire Priest; Johannes Lechner; Gislene Pereira; Elmar Schiebel; The yeast centrosome translates the positional information of the anaphase spindle into a cell cycle signal. The Journal of Cell Biology 2007, 179, 423-436, 10.1083/jcb.200705197.
- Gislene Pereira; Elmar Schiebel; Kin4 Kinase Delays Mitotic Exit in Response to Spindle Alignment Defects. Molecular Cell 2005, 19, 209-221, 10.1016/j.molcel.2005.05.030.
- Leon Y. Chan; Angelika Amon; The protein phosphatase 2A functions in the spindle position checkpoint by regulating the checkpoint kinase Kin4. Genes & Development 2009, 23, 1639-1649, 10.1101/gad.1804609.
- Aurelia Lahoz; María Alcaide-Gavilán; Rafael R. Daga; Juan Jimenez; Antagonistic Roles of PP2A-Pab1 and Etd1 in the Control of Cytokinesis in Fission Yeast. Genetics 2010, 186, 1261-1270, 10.1534/genetics.110.121368.
- Xavier Le Goff; Stéphanie Buvelot; Ekaterina Salimova; Frédéric Guerry; Susanne Schmidt; Nathalie Cueille; Elena Cano; Viesturs Simanis; The protein phosphatase 2A B'-regulatory subunit par1p is implicated in regulation of the S. pombe septation initiation network.. FEBS Letters 2001, 508, 136-142, 10.1016/s0014-5793(01)03047-2.
- W Jiang; R L Hallberg; Correct regulation of the septation initiation network in Schizosaccharomyces pombe requires the activities of par1 and par2.. Genetics 2001, 158, 1413-1429, null.
- N. Sadananda Singh; Nan Shao; Janel R. McLean; Mayalagu Sevugan; Liping Ren; Ting Gang Chew; Andrea Bimbó; Reetu Sharma; Xie Tang; Kathleen L. Gould; Mohan K. Balasubramanian; SIN-inhibitory phosphatase complex promotes Cdc11p dephosphorylation and propagates SIN asymmetry in fission yeast.. Current Biology 2011, 21, 1968-78, 10.1016/j.cub.2011.10.051.
- Agnes Grallert; Elvan Boke; Anja Hagting; Ben Hodgson; Yvonne Connolly; John. R. Griffiths; Duncan L. Smith; Jonathon Pines; Iain M. Hagan; A PP1–PP2A phosphatase relay controls mitotic progression. Nature 2014, 517, 94-98, 10.1038/nature14019.
