Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Jacob Eide.
Inverted papillomas (IP) are the most common sinonasal tumor with a tendency for recurrence, potential attachment to the orbit and skull base, and risk of malignant degeneration into squamous cell carcinoma (SCC). While the overall rate of recurrence has decreased with the widespread adoption of high-definition endoscopic optics and advanced surgical tools, there remain challenges in managing tumors that are multiply recurrent or involve vital neurovascular structures. The World Health Organization has defined three subtypes of sinonasal Schneiderian papillomas: inverted, exophytic, and oncocytic lesions.
- tumor
- recurrence
- endoscopic
1. Preoperative Evaluation
Initial evaluation of patients suspected to have an intranasal mass begins with a comprehensive history and physical examination, including a nasal endoscopy. History should specifically address previous history of nasal mass or polyp removals, tobacco use or exposure, and symptoms related to mass effect (nasolacrimal obstruction, vision changes, headaches). Unilateral symptoms, negative allergic workup, and failure to respond to medical therapy for inflammatory processes should raise suspicion for sinonasal mass but are not specific to IP. Complete head and neck examination should be performed with attention to any visual deficits, numbness in the trigeminal nerve distribution, extraocular movement deficits, middle ear effusion, and presence of any cervical lymphadenopathy.
Classically, IPs are lobulated, firm tumors [1]. Symptoms are typically non-specific, including nasal obstruction, epistaxis, rhinorrhea, and facial pressure, and accordingly, IPs are commonly diagnosed in a delayed fashion [17][2]. Interestingly, in some series, as many as 23% of patients were asymptomatic from their mass, which was found incidentally [18][3]. Tissue confirmation is recommended prior to definitive resection and can frequently be accomplished in the clinic setting. However, IPs can be found concurrently with inflammatory polyps and the diagnosis should still be suspected even in cases fofr negative biopsy, as there has been a reported 17% false negative rate [1]. There are also data suggesting a higher degree of radiographic contralateral sinus mucosal thickening in IP cases compared to controls (58.9% vs. 26.7%), which has caused some investigators to consider the role of chronic inflammation in IP development [3][4].
2. Imaging
Preoperative imaging is routinely obtained to evaluate the extent of the tumor and assist in surgical planning. Non-contrast computed tomography (CT) scans with thin cuts (<1 mm) are standard protocol to evaluate for areas of bony erosion, although findings typically demonstrate a non-specific soft tissue density with microcalcifications present in 20% of cases [19][5]. CT is particularly sensitive at detecting areas of hyperostosis, which has been used to predict the site of IP attachment with positive predictive values of 89–95% reported [20,21,22][6][7][8]. (Figure 21) This finding on imaging could be corroborated with nasal endoscopy, but the point of insertion is often obscured during exam.
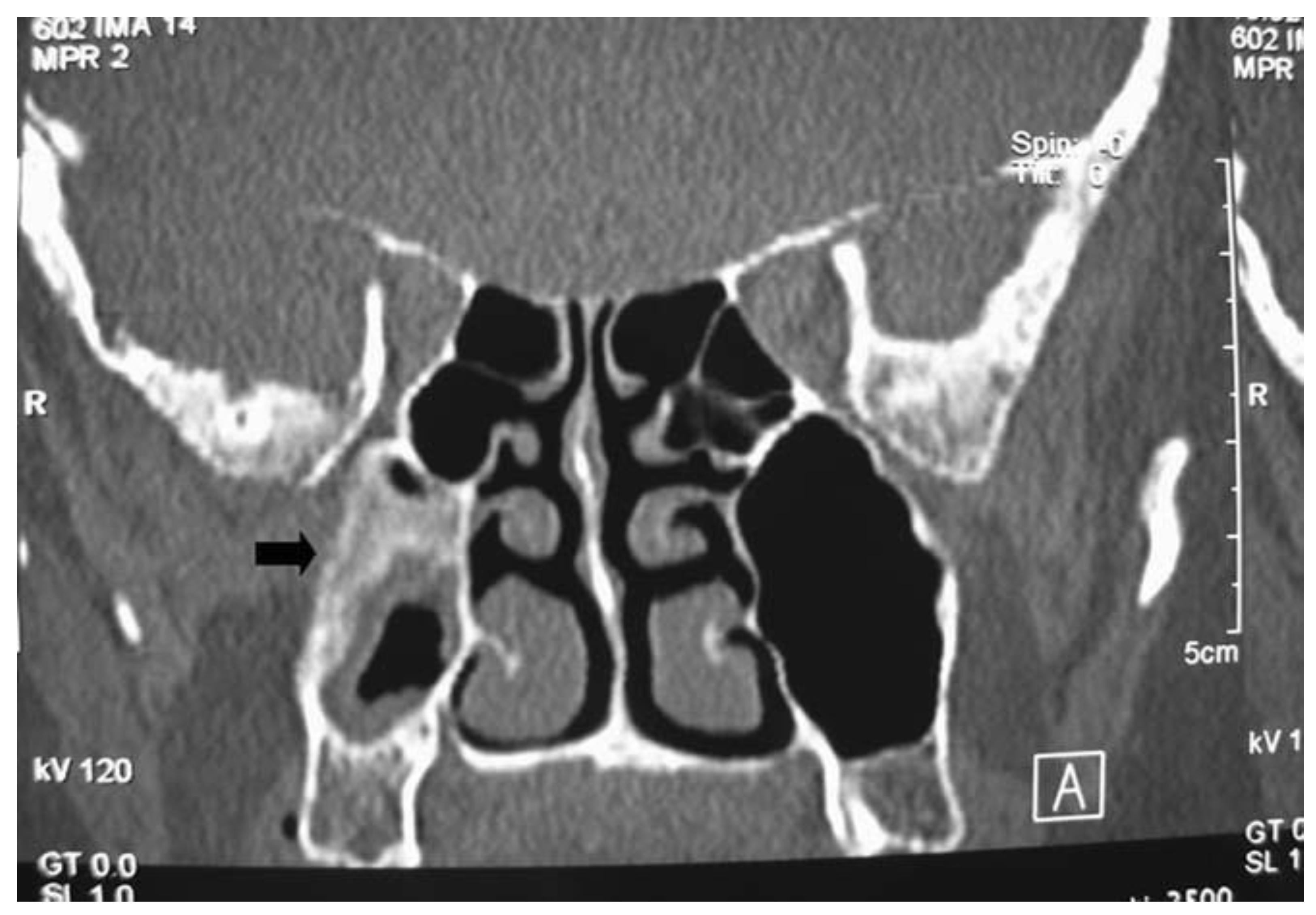
Figure 21. Coronal image of computed tomography showing a recurrent sinonasal inverted papilloma pedicled on the posterior maxillary sinus wall. There is significant hyperostosis at the origin of the lesion.
Magnetic resonance imaging (MRI) has increasingly become part of preoperative workup due to IP’s unique imaging characteristics. Typical findings are a hypodensity on T1, iso- or hypodensity on T2, contrast enhancement, and convoluted cerebriform pattern (CCP) [23,24][9][10]. (Figure 32) This imaging modality is superior to CT in delineating the tumor from inspissated secretions and in predicting malignant degeneration [25][11]. Specifically, benign IPs have been found to have a higher prevalence of CCP compared to IP-transformed SCC, and a significantly lower apparent diffusion coefficient (ADC) on MRI diffusion-weighted imaging (DWI) [25][11]. OneFor study with limited sample size suggested that MRI was better at predicting involvement of the frontal sinus than CT alone, with other studiess were suggesting that MRI is accurate at predicting the final pathologic stage of the IP in the vast majority of cases [26,27][12][13]. Frank bone erosion and aberration of the classic cerebriform appearance on MRI have been associated with malignant transformation, and some authors have suggested more advanced dynamic contrast MRI techniques as adjuncts [28][14].
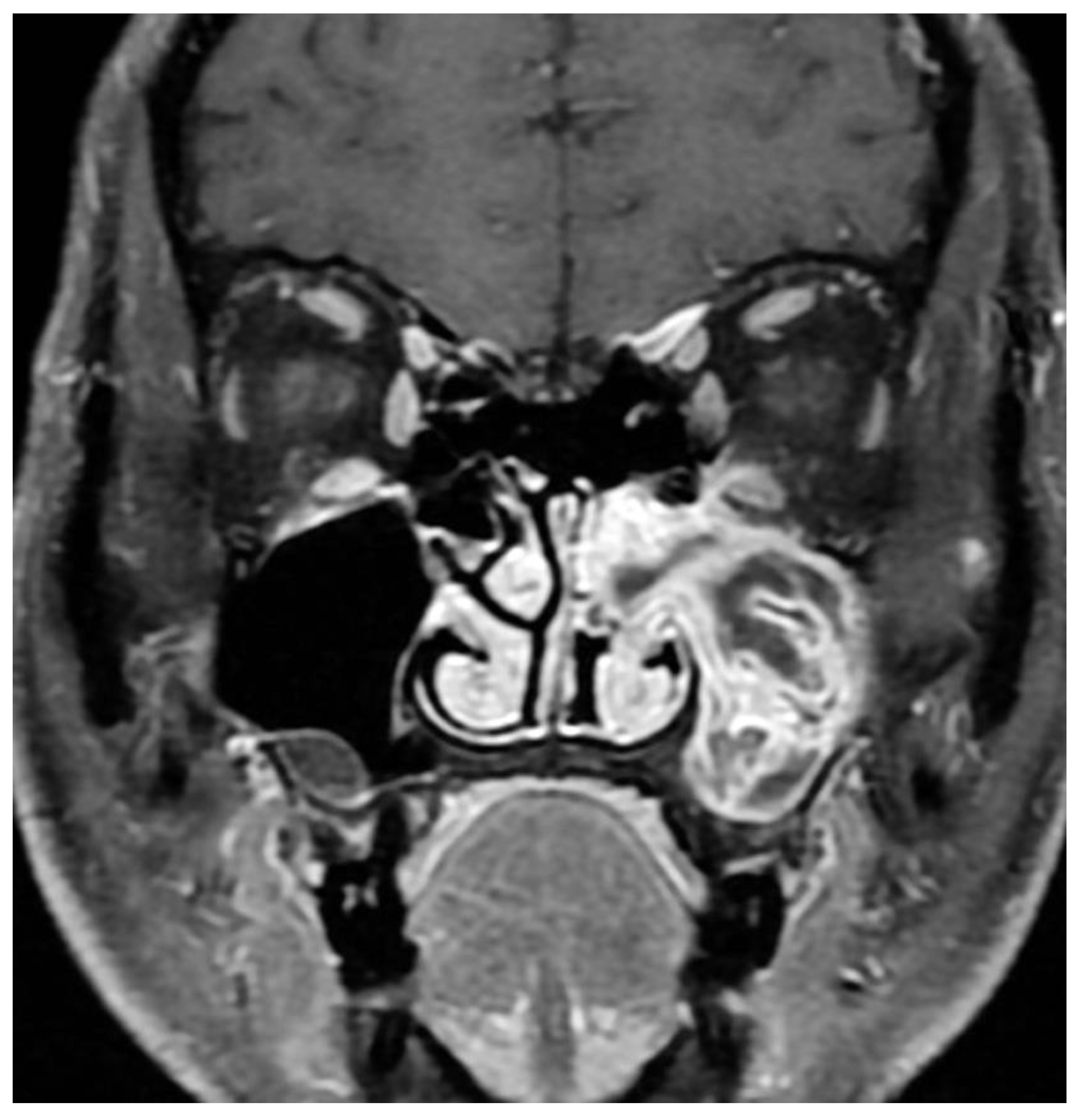
Figure 32. Alternating lines of high and low signal intensity (convoluted cerebriform pattern) seen in an inverted papilloma of the maxillary sinus.
3. Surgical Planning
Locally advanced IPs or IP-degenerated SCC require further workup and a collaborative approach. While most IPs can be managed by an experienced surgeon, rare tumors with significant extra-sinus extension or skull base involvement should prompt neurosurgical evaluation, and management should be undertaken by dedicated skull base teams for optimal results. Further workup to delineate involvement of key neurovascular structures may be required. Figure 43 shows images of a patient who was initially evaluated for headaches and confusion and was found to have a lesion of the sphenoid sinus with erosion of the sella and dehiscence of the carotid artery and optic nerve. Biopsy revealed the pathology as IP without dysplasia, and resection was performed with careful dissection off the neurovascular structures.
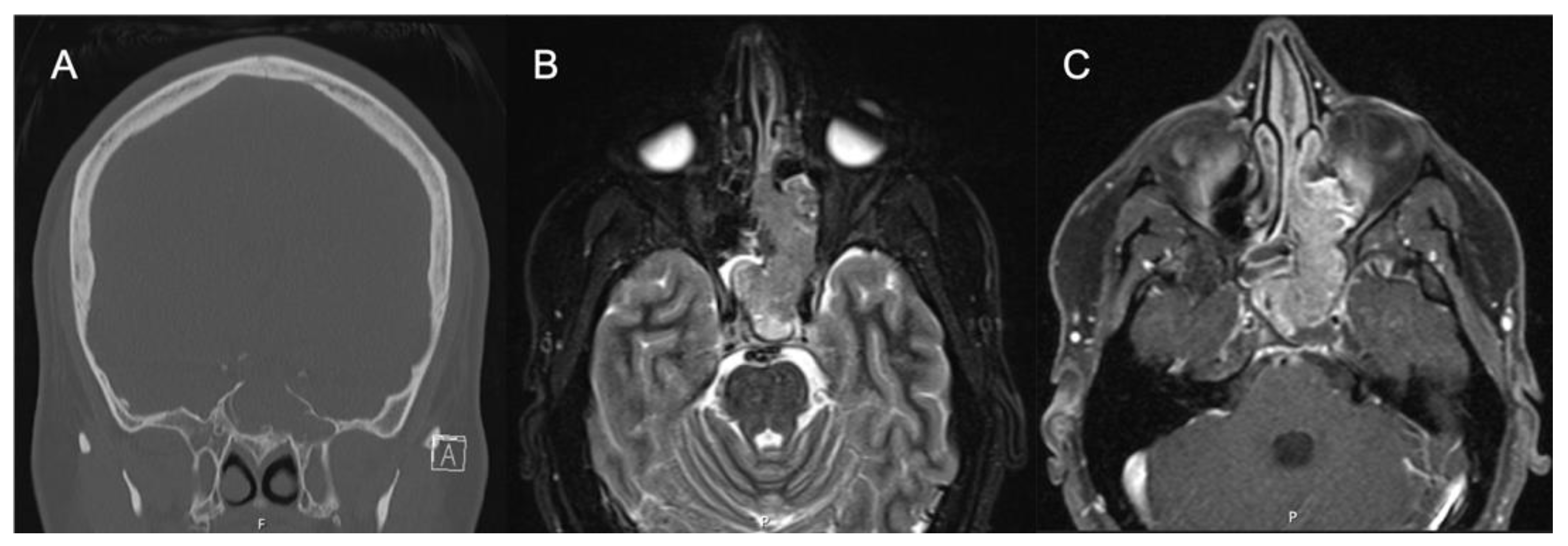
Figure 43. IP-degenerated squamous cell carcinoma. (A) Non-contrasted CT sinus demonstrating a mass of the left sphenoid with erosion of the skull base at the sella. (B) STIR sequence contrasted MRI demonstrating an expansile mass originating from the left sphenoid lateral wall. (C) T1 sequence contrasted MRI with fat suppression demonstrating a mass of the left sphenoid and ethmoid cavity.
Involvement of the orbit should likewise prompt referral to ophthalmology evaluation for baseline exam and joint management. Figure 54 contains images of a patient who presented to ourthe office with recurrent epiphora after an external dacryocystorhinostomy (DCR) over a year ago. The patient also noted chronic unilateral nasal obstruction and a history of minor procedures for polyp removal spanning two decades outside of the United States. An endoscopic exam revealed an intranasal mass which was biopsy positive for IP-degenerated SCC. The coronal CT cut shows the bony dehiscence that was likely a result of her prior DCR, and the T1-weighted contrast enhanced MRI image shows extension of the tumor into the orbit and infiltration into the periorbita. A PET/CT demonstrated local FDG avidity without regional or distant uptake. This patient subsequently underwent an endoscopic-assisted resection with a modified Denker’s procedure and lateral rhinotomy, resection of the periorbita, and reconstruction with canthoplasty.
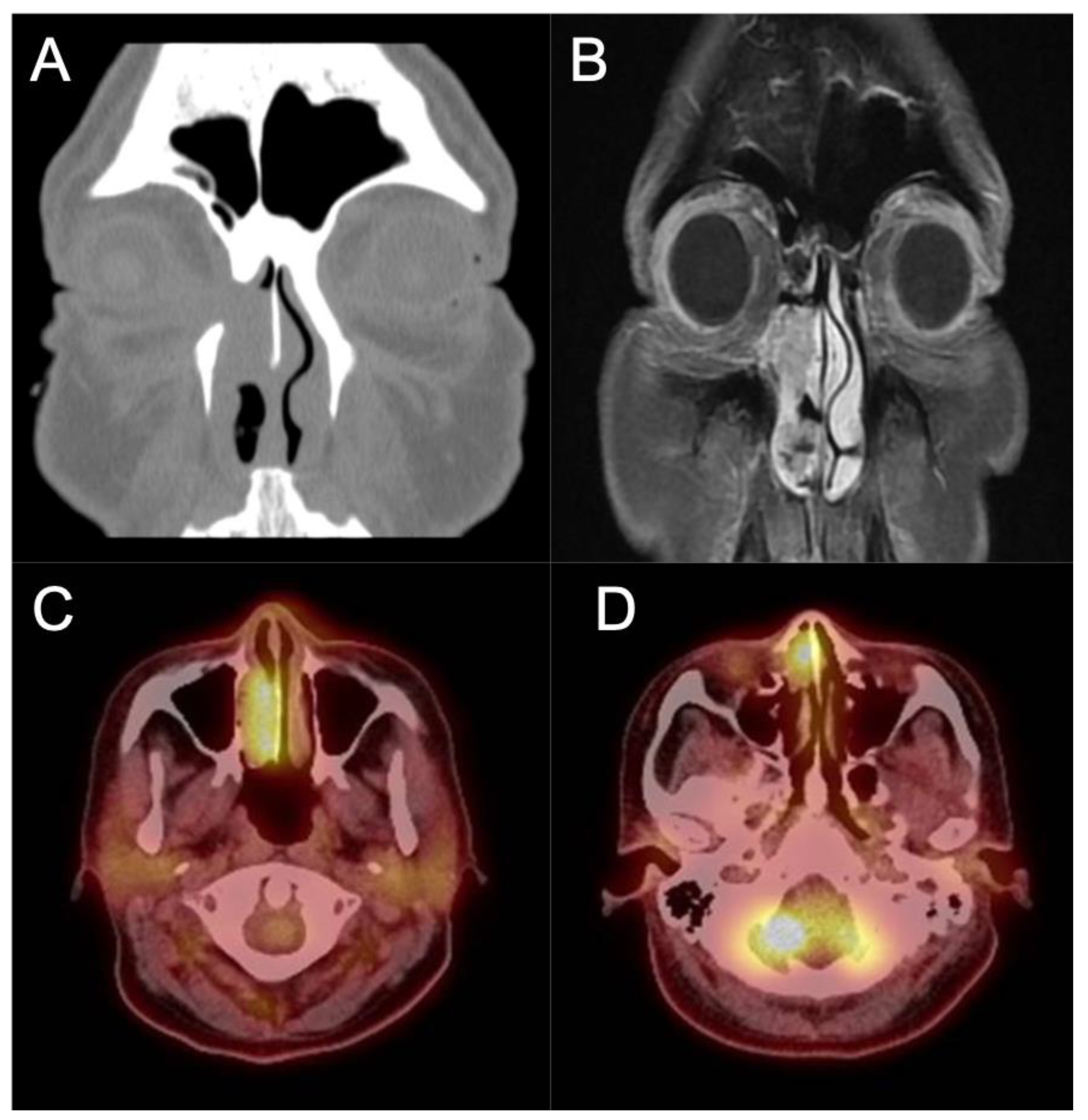
Figure 54. IP-degenerated squamous cell carcinoma. (A) Non-contrasted CT scan demonstrating an erosive mass in the right nasal cavity with invasion into the orbit. (B) T1 sequence contrasted MRI scan demonstrating the invasion of the mass into the medial orbit. (C,D) PET CT scan demonstrating avidity of the right nasal mass.
4. Staging
There have been several proposed staging systems for IP, but perhaps the most commonly used is the Krouse staging system [29][15]. It is important to note that this system was developed in the pre-endoscopic era and based on prior surgical experience and other staging systems for sinonasal malignancies. It also prioritized ease of use, and at the time was not stratified based on patient outcomes. Kim et al. performed a meta-analysis investigating recurrence based on surgical approach and completed a sub-group analysis of 4 papers that included the Krouse classification. They noted a higher risk ratio for recurrence in higher Krouse classifications but their data did not achieve significance [30][16]. Lisan et al. performed a subsequent meta-analysis of 13 studies, specifically designed to investigate the association with the Krouse system with recurrence. Interestingly, they found that there was no significant difference in recurrence between T1 and T2 lesions or between T3 and T4 lesions. There was, however, a 51% increase in recurrence between T2 and T3 lesions [31][17]. These findings highlight the fact that while the Krouse classification allows for standardization of reporting these tumors, there is a significant amount of heterogeneity within groups, particularly within the T4 group that included all carcinomas. A number of other staging systems have since been proposed, including the Oikawa, Han, Cannady, and Meng systems, but there is limited evidence that these systems correlate with tumor recurrence [32,33][18][19].
5. Pathology and Molecular Changes Associated with Malignant Degeneration
Histologically, IPs are characterized by a thickened epithelium enclosed by the basement membrane on hematoxylin and eosin stain, thus appearing “inverted” compared to other Schneiderian papillomas [34][20]. Varying degrees of dysplasia can be observed in the non-keratinizing epithelium, ranging from none, mild, and moderate to severe dysplasia. The degree of dysplasia should be noted by the examining pathologist with attention to possible SCC or other concurrent malignancies [34][20]. Although HPV has long been implicated in the pathogenesis of IP, detection rate has not been consistent across techniques (PCR, DNA/mRNA in situ hybridization) and are not routinely performed. It has been reported, however, that high-risk HPV in IP is correlated with an increase in epidermal growth factor receptor (EGFR) expression, which leads to dysplasia and invasion [35][21]. There is also a growing interest in understanding the molecular pathways that lead to carcinogenesis. One area of interest is epigenetic changes that modify gene expression. In a comparative studyone between 15 patients with IP and 12 with SCC ex-IP, three genes were identified (OPA3, MIR661, and PLEC) at six different genetic sites that were hypermethylated compared to controls. MIR661 encodes a microRNA that can either promote or suppress tumor aggressiveness depending on p53 expression, and miR-661 mRNA was significantly upregulated in SCC ex-IP [36,37][22][23]. The OPA3 protein is a key portion of the mitochondrial outer membrane and is found to be decreased in SCC ex-IP [36,38][22][24]. Plectin, the protein encoded by PLEC, regulates the intermediate filament structure of cells and was noted to be upregulated in SCC ex-IP, similar to reports in ovarian and pancreatic tumors [36,39,40][22][25][26].
MicroRNAs are small, non-coding RNA segments that regulate gene expression via messenger RNA cleavage and inhibition of translation to cell proteins [41][27]. They represent another level of transcriptional regulation that, when aberrant, can lead to cancer progression. Analysis of microRNA expression in SCC ex-IP compared to IP demonstrated a significantly higher level of miR-296-3p [42][28]. This microRNA subsequently downregulates PTEN, a known tumor suppressor via inhibition of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway [42,43][28][29]. PTEN depletion has been reported to be associated with worse outcomes in non-sinonasal SCC and may be an important prognostic factor for SCC ex-IP in the future [44][30].
There have been multiple studiones looking at gene expression and mutation in SCC ex-IP. A recent systematic review one has summarized the results of various genomic sequencing of IP and SCC ex-IP, finding progressively increasing numbers of mutated genes as the samples became more dysplastic [45][31]. They specifically found that KRAS, APC, and STK11 genes were mutated at a higher rate in SCC arising from IP [45][31]. Interestingly, oither author was also noted somatic EGFR mutations in IP degenerated SCC and only found KRAS mutations in malignancies related to oncocytic papilloma, albeit in a limited number of patient samples [46][32]. Genomic sequencing of IP associated SCC has also noted mutations in lysine methyltransferase 2A (KMT2D), cyclin-dependent kinase inhibitor 2A (CDKN2A), tumor protein 53 (TP53), phosphodiesterase 4D interacting protein (PDE4DIP), and neurofibromin 1 (NF1) [47][33]. Parallel work has identified FoxM1 as a proliferation transcription factor that has been associated with a variety of malignancies and has been found to be significantly elevated in inverted papilloma and SCC ex-IP [48][34]. Importantly, expression of FoxM1 was found to correlate with Krouse stage and histological grade, suggesting that it plays a role in the transition from normal epithelium to IP and malignancy [48][34]. Finally, EGFR mutations have been identified in 88% of IPs and 77% of IP-SCC but not identified in de novo SCC. EGFR inhibitors have been shown to be effective in vitro and may be a viable treatment for IPs and IP-SCC in the future [8,49][35][36]. While the exact etiology of carcinogenesis remains unclear, recent genomic studiones implicating specific molecular pathways appear to be promising.
6. Adjuvant Therapy
The most common form of adjuvant therapy is external beam radiation therapy (XRT), which is typically reserved for SCC ex-IP following complete resection. Additionally, XRT should be considered for inoperable tumors (poor patient candidate, involvement of neurovascular structures) [1,13][1][37]. Patients should be counselled that for SCC ex-IP, complete surgical resection followed by XRT has a superior 5-year survival (84%) compared to 41% for XRT alone [66,67][38][39]. There has also been limited evidence of response to carboplatin/paclitaxel for inoperable IP with CIS that significantly debulked the tumor and allowed for surgical resection [68][40]. Finally, topical 5-fluorouracial has had positive preliminary results in recurrent challenging csituasestions, although further investigation is needed [69][41].
7. Treatment Algorithm
Figure 95 summarizes the overall workup, management, and surveillance strategy for IPs and SCC ex-IP. The algorithm highlights the need for a multi-disciplinary approach for locally advanced tumors, proper patient counseling, and shared decision making in treatment modality and surveillance.
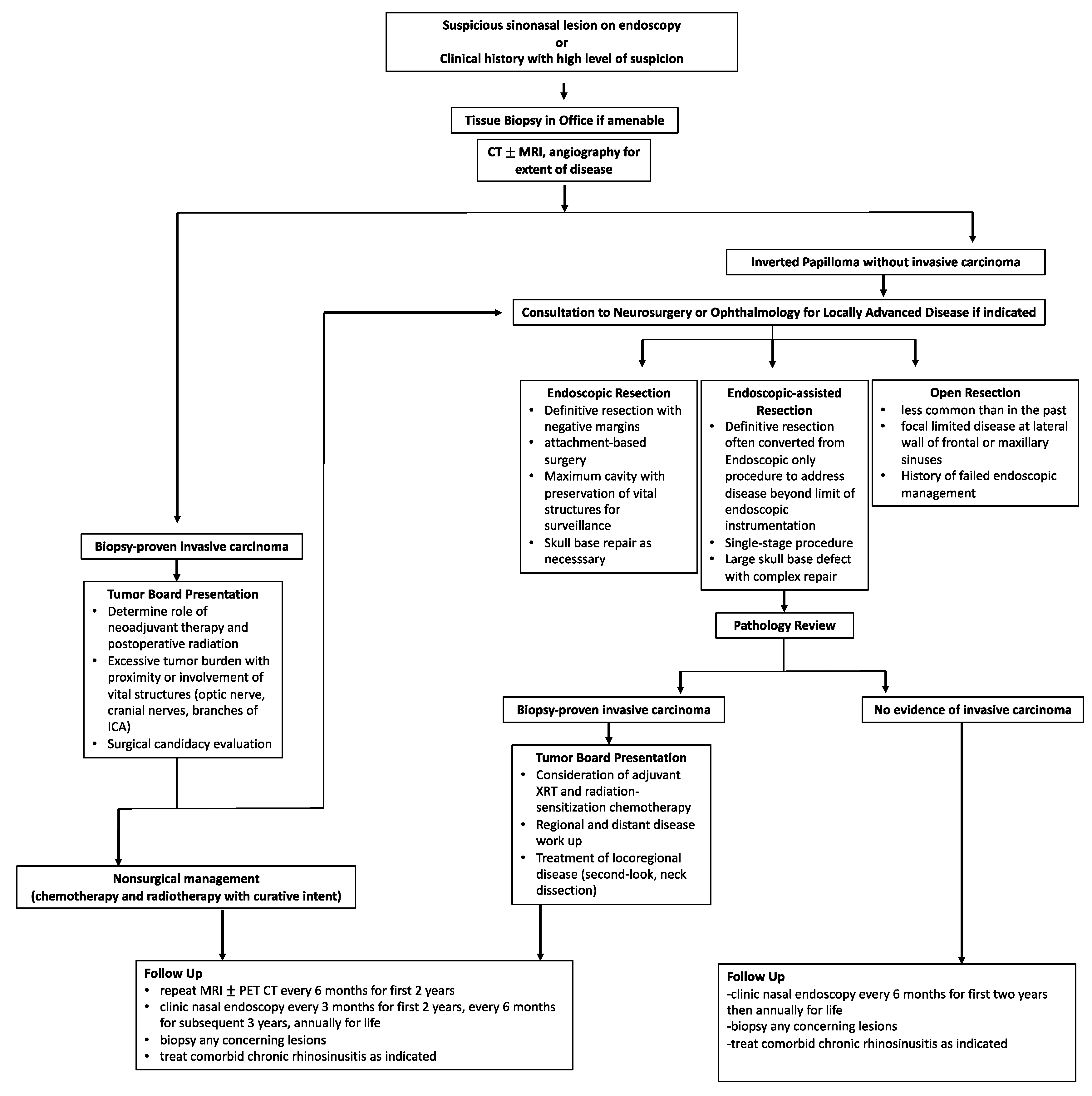
Figure 95.
Treatment algorithm for the diagnosis, management, and surveillance of both IP and malignant SCC ex-IP.
References
- Lisan, Q.; Laccourreye, O.; Bonfils, P. Sinonasal inverted papilloma: From diagnosis to treatment. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 337–341.
- Klimek, T.; Atai, E.; Schubert, M.; Glanz, H. Inverted papilloma of the nasal cavity and paranasal sinuses: Clinical data, surgical strategy and recurrence rates. Acta Oto-Laryngol. 2000, 120, 267–272.
- Minovi, A.; Kollert, M.; Draf, W.; Bockmühl, U. Inverted papilloma: Feasibility of endonasal surgery and long-term results of 87 cases. Rhinology 2006, 44, 205–210.
- Papagiannopoulos, P.; Tong, C.L.; Kuan, E.C.; Tajudeen, B.A.; Yver, C.M.; Kohanski, M.A.; Cohen, N.A.; Kennedy, D.W.; Palmer, J.N.; Adappa, N.D. Inverted papilloma is associated with greater radiographic inflammatory disease than other sinonasal malignancy. Int. Forum Allergy Rhinol. 2020, 10, 278–281.
- Momeni, A.K.; Roberts, C.C.; Chew, F.S. Imaging of chronic and exotic sinonasal disease: Review. AJR Am. J. Roentgenol. 2007, 189, S35–S45.
- Yousuf, K.; Wright, E.D. Site of attachment of inverted papilloma predicted by CT findings of osteitis. Am. J. Rhinol. 2007, 21, 32–36.
- Lee, D.K.; Chung, S.K.; Dhong, H.J.; Kim, H.Y.; Kim, H.J.; Bok, K.H. Focal hyperostosis on CT of sinonasal inverted papilloma as a predictor of tumor origin. AJNR Am. J. Neuroradiol. 2007, 28, 618–621.
- Bhalla, R.K.; Wright, E.D. Predicting the site of attachment of sinonasal inverted papilloma. Rhinology 2009, 47, 345–348.
- Savy, L.; Lloyd, G.; Lund, V.J.; Howard, D. Optimum imaging for inverted papilloma. J. Laryngol. Otol. 2000, 114, 891–893.
- Jeon, T.Y.; Kim, H.J.; Chung, S.K.; Dhong, H.J.; Kim, H.Y.; Yim, Y.J.; Kim, S.T.; Jeon, P.; Kim, K.H. Sinonasal inverted papilloma: Value of convoluted cerebriform pattern on MR imaging. AJNR Am. J. Neuroradiol. 2008, 29, 1556–1560.
- Yan, C.H.; Tong, C.C.L.; Penta, M.; Patel, V.S.; Palmer, J.N.; Adappa, N.D.; Nayak, J.V.; Hwang, P.H.; Patel, Z.M. Imaging predictors for malignant transformation of inverted papilloma. Laryngoscope 2019, 129, 777–782.
- Oikawa, K.; Furuta, Y.; Oridate, N.; Nagahashi, T.; Homma, A.; Ryu, T.; Fukuda, S. Preoperative staging of sinonasal inverted papilloma by magnetic resonance imaging. Laryngoscope 2003, 113, 1983–1987.
- Kasbekar, A.V.; Swords, C.; Attlmayr, B.; Kulkarni, T.; Swift, A.C. Sinonasal papilloma: What influences the decision to request a magnetic resonance imaging scan? J. Laryngol. Otol. 2018, 132, 584–590.
- Zhang, L.; Fang, G.; Yu, W.; Yang, B.; Wang, C.; Zhang, L. Prediction of malignant sinonasal inverted papilloma transformation by preoperative computed tomography and magnetic resonance imaging. Rhinology 2020, 58, 248–256.
- Krouse, J.H. Development of a staging system for inverted papilloma. Laryngoscope 2000, 110, 965–968.
- Kim, J.S.; Kwon, S.H. Recurrence of sinonasal inverted papilloma following surgical approach: A meta-analysis. Laryngoscope 2017, 127, 52–58.
- Lisan, Q.; Moya-Plana, A.; Bonfils, P. Association of Krouse Classification for Sinonasal Inverted Papilloma With Recurrence: A Systematic Review and Meta-analysis. JAMA Otolaryngol.-Head Neck Surg. 2017, 143, 1104–1110.
- Nakayama, T.; Tsunemi, Y.; Kashiwagi, T.; Kuboki, A.; Yamakawa, S.; Konno, W.; Mori, A.; Iimura, J.; Tsukidate, T.; Tanaka, Y.; et al. Comparison of Current Staging Systems for Sinonasal Inverted Papilloma. Am. J. Rhinol. Allergy 2021, 35, 64–71.
- Mak, W.; Webb, D.; Al-Salihi, S.; Dadgostar, A.; Javer, A. Sinonasal inverted papilloma recurrence rates and evaluation of current staging systems. Rhinology 2018, 56, 407–414.
- Centre International de Recherche sur le Cancer. Pathology and Genetics of Head and Neck Tumours; WHO: Geneva, Switzerland, 2005.
- Re, M.; Gioacchini, F.M.; Bajraktari, A.; Tomasetti, M.; Kaleci, S.; Rubini, C.; Bertini, A.; Magliulo, G.; Pasquini, E. Malignant transformation of sinonasal inverted papilloma and related genetic alterations: A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2991–3000.
- Yang, Z.; Zhang, Y.; Wang, X.; Huang, J.; Guo, W.; Wei, P.; Li, G.; Wang, Z.; Huang, Z.; Zhang, L. Putative biomarkers of malignant transformation of sinonasal inverted papilloma into squamous cell carcinoma. J. Int. Med. Res. 2019, 47, 2371–2380.
- Hoffman, Y.; Bublik, D.R.; Pilpel, Y.; Oren, M. miR-661 downregulates both Mdm2 and Mdm4 to activate p53. Cell Death Differ. 2014, 21, 302–309.
- Ryu, S.W.; Jeong, H.J.; Choi, M.; Karbowski, M.; Choi, C. Optic atrophy 3 as a protein of the mitochondrial outer membrane induces mitochondrial fragmentation. Cell Mol. Life Sci. 2010, 67, 2839–2850.
- Bausch, D.; Thomas, S.; Mino-Kenudson, M.; Fernández-del, C.C.; Bauer, T.W.; Williams, M.; Warshaw, A.L.; Thayer, S.P.; Kelly, K.A. Plectin-1 as a novel biomarker for pancreatic cancer. Clin. Cancer Res. 2011, 17, 302–309.
- Puiffe, M.L.; Le Page, C.; Filali-Mouhim, A.; Zietarska, M.; Ouellet, V.; Tonin, P.N.; Chevrette, M.; Provencher, D.M.; Mes-Masson, A.M. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 2007, 9, 820–829.
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233.
- Kakizaki, T.; Hatakeyama, H.; Nakamaru, Y.; Takagi, D.; Mizumachi, T.; Sakashita, T.; Kano, S.; Homma, A.; Fukuda, S. Role of microRNA-296-3p in the malignant transformation of sinonasal inverted papilloma. Oncol. Lett. 2017, 14, 987–992.
- Stambolic, V.; MacPherson, D.; Sas, D.; Lin, Y.; Snow, B.; Jang, Y.; Benchimol, S.; Mak, T.W. Regulation of PTEN transcription by p53. Mol. Cell 2001, 8, 317–325.
- da Costa, A.A.; D’Almeida Costa, F.; Ribeiro, A.R.; Guimarães, A.P.; Chinen, L.T.; Lopes, C.A.; de Lima, V.C. Low PTEN expression is associated with worse overall survival in head and neck squamous cell carcinoma patients treated with chemotherapy and cetuximab. Int. J. Clin. Oncol. 2015, 20, 282–289.
- Yasukawa, S.; Kano, S.; Hatakeyama, H.; Nakamaru, Y.; Takagi, D.; Mizumachi, T.; Suzuki, M.; Suzuki, T.; Nakazono, A.; Tanaka, S.; et al. Genetic mutation analysis of the malignant transformation of sinonasal inverted papilloma by targeted amplicon sequencing. Int. J. Clin. Oncol. 2018, 23, 835–843.
- Maisch, S.; Mueller, S.K.; Traxdorf, M.; Weyerer, V.; Stoehr, R.; Iro, H.; Hartmann, A.; Agaimy, A. Sinonasal papillomas: A single centre experience on 137 cases with emphasis on malignant transformation and EGFR/KRAS status in “carcinoma ex papilloma”. Ann. Diagn. Pathol. 2020, 46, 151504.
- Uchi, R.; Jiromaru, R.; Yasumatsu, R.; Yamamoto, H.; Hongo, T.; Manako, T.; Sato, K.; Hashimoto, K.; Wakasaki, T.; Matsuo, M.; et al. Genomic Sequencing of Cancer-related Genes in Sinonasal Squamous Cell Carcinoma and Coexisting Inverted Papilloma. Anticancer Res. 2021, 41, 71–79.
- Wang, H.; Li, H.; Hu, L.; Wang, J.; Liu, Q.; Wang, D.; Sun, X. Overexpression of FoxM1 in Sinonasal Inverted Papilloma and Associated Squamous Cell Carcinoma. Am. J. Rhinol. Allergy 2019, 33, 706–715.
- Udager, A.M.; Rolland, D.C.M.; McHugh, J.B.; Betz, B.L.; Murga-Zamalloa, C.; Carey, T.E.; Marentette, L.J.; Hermsen, M.A.; DuRoss, K.E.; Lim, M.S.; et al. High-Frequency Targetable EGFR Mutations in Sinonasal Squamous Cell Carcinomas Arising from Inverted Sinonasal Papilloma. Cancer Res. 2015, 75, 2600–2606.
- Cabal, V.N.; Menendez, M.; Vivanco, B.; Potes-Ares, S.; Riobello, C.; Suarez-Fernandez, L.; Garcia-Marin, R.; Blanco-Lorenzo, V.; Lopez, F.; Alvarez-Marcos, C.; et al. EGFR mutation and HPV infection in sinonasal inverted papilloma and squamous cell carcinoma. Rhinology 2020, 58, 368–376.
- Mirza, S.; Bradley, P.J.; Acharya, A.; Stacey, M.; Jones, N.S. Sinonasal inverted papillomas: Recurrence, and synchronous and metachronous malignancy. J. Laryngol. Otol. 2007, 121, 857–864.
- Kim, D.Y.; Hong, S.L.; Lee, C.H.; Jin, H.R.; Kang, J.M.; Lee, B.J.; Moon, I.J.; Chung, S.K.; Rha, K.S.; Cho, S.H.; et al. Inverted papilloma of the nasal cavity and paranasal sinuses: A Korean multicenter study. Laryngoscope 2012, 122, 487–494.
- Yu, H.X.; Liu, G. Malignant transformation of sinonasal inverted papilloma: A retrospective analysis of 32 cases. Oncol. Lett. 2014, 8, 2637–2641.
- Kuan, E.C.; Frederick, J.W.; Palma Diaz, M.F.; Lim, D.W.; Suh, J.D. Complete response of skull base inverted papilloma to chemotherapy: Case report. Allergy Rhinol. (Provid. R.I.) 2017, 8, 105–108.
- Adriaensen, G.F.; Lim, K.H.; Georgalas, C.; Reinartz, S.M.; Fokkens, W.J. Challenges in the Management of Inverted Papilloma: A Review of 72 Revision Cases. Laryngoscope 2016, 126, 322–328.
More
