Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Rodrigo Petersen Saadi.
Transcatheter aortic valve replacement (TAVR) is a well-established treatment option for patients with severe symptomatic aortic stenosis (AS) whose procedural efficacy and safety have been continuously improving. Appropriate preprocedural planning, including aortic valve annulus measurements, transcatheter heart valve choice, and possible procedural complication anticipation is mandatory for a successful procedure. The gold standard for preoperative planning is still to perform a multi-detector computed angiotomography (MDCT), which provides all the information required. The planning can be performed manually, using semi-automated and automated software.
- TAVR
- sizing
- planning
- MDCT
- 3D echocardiography
- MRI
1
1. Introduction
Transcatheter aortic valve replacement (TAVR) has risen as a less invasive alternative for treating severe symptomatic aortic stenosis (AS) in patients at all surgical risk scores [1][2][3][4][5][6][7]. Since Alain Cribier pioneered the TAVR procedure in 2002, the advances in this field have been outstanding. Newer generation devices and different types of self and balloon-expandable valves are being released every year, and the results are remarkable improvements in procedural efficacy and safety [8].
One of the key points for a successful TAVR is carefully preprocedural planning, including accurate aortic root and aortic valve diameters measurements. Aortic angulation; aortic annulus minimum, medium and maximum diameters, area, and perimeter; left ventricle outflow tract minimum, medium and maximum diameters, area, and perimeter; sinus of Valsalva diameters; right and left coronary arteries height; sinotubular junction diameters, area, and perimeter; ascending aorta diameters; calcification distribution pattern; and C-arm angulation are all relevant information to perform a procedure properly [9].
The measurements of the aortic root and ascending aorta are used to choose the appropriate transcatheter heart valve type and size and foresee possible procedure-related complications, such as coronary artery obstruction, aortic annulus, and sinotubular junction rupture, paravalvular leak, valve embolization, and pacemaker implantation need. The size of the transcatheter heart valve that will be implanted is chosen based on the aortic annulus perimeter for the self-expandable platforms, and on the aortic annulus area for the balloon-expandable ones [10][11]. Undersizing the aortic annulus may cause paravalvular leak or valve embolization, whereas oversizing may reduce prosthesis durability, cause annulus rupture, and conduction issues leading to pacemaker implantation [12][13][14].
Initially, TAVR sizing was made using 2D echocardiography and/or during the procedure, using graduated pigtail catheters (Figure 1). However, the planning strategy evolved dramatically, especially when 3D technology started to be used since 2D echo frequently undersized the aortic annulus. The current gold standard method to perform valve measurements and preprocedural TAVR planning is the multi-detector computed angiotomography (MDCT), with an appropriate TAVR protocol [15][16]. MDCT measurements can be performed manually, using semi-automated software, or using automated software. Furthermore, the aortic annulus may be measured by magnetic resonance imaging and 3D transesophageal echocardiography. The possibility of a 3D image (MDCT or 3D echo) allowed a more precise measurement.
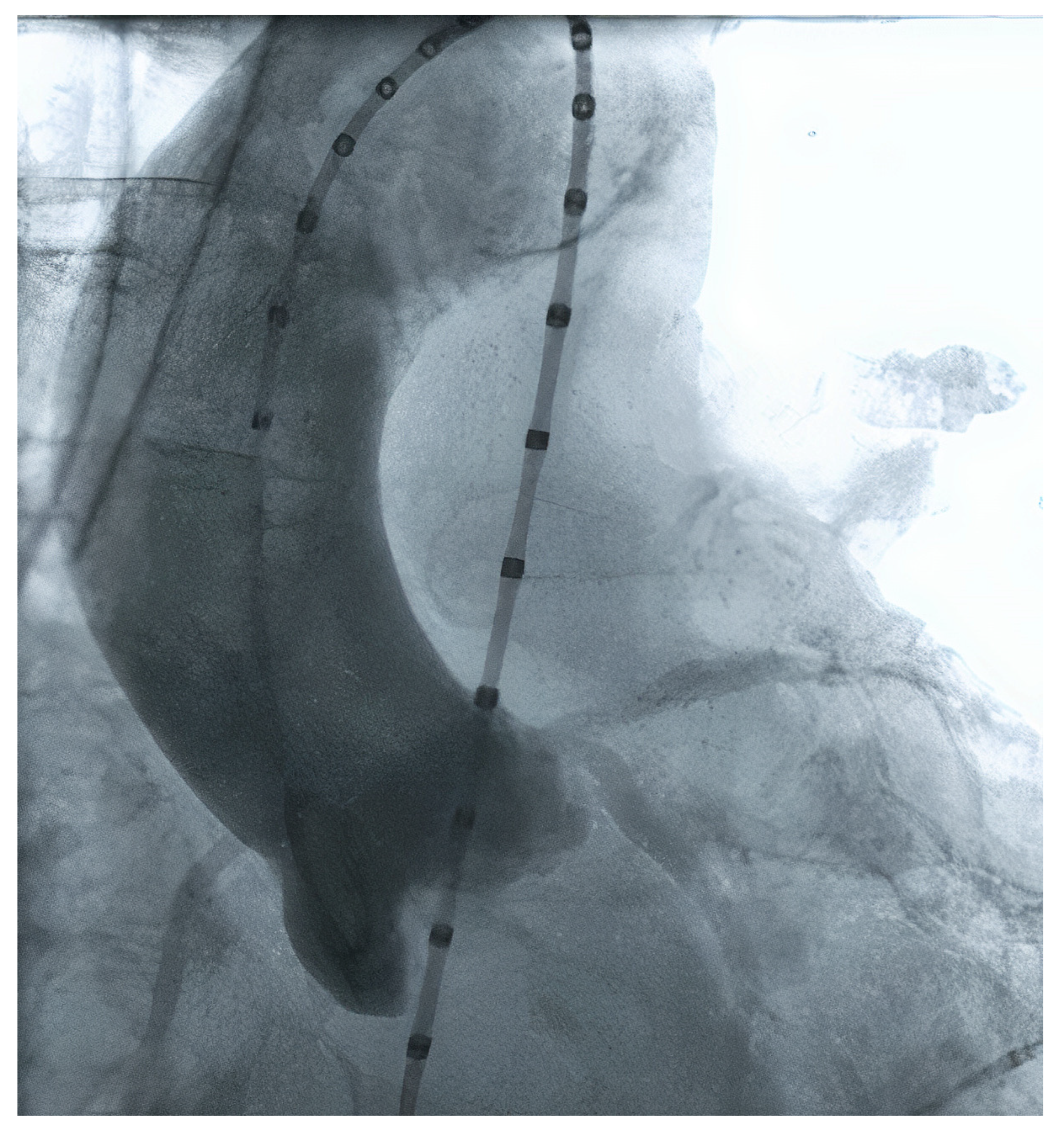
Figure 1. Aortic valve measurement using contrast injection from a pigtail catheter.
2. MDCT Acquisition
The key component for well-acquired MDCT images is an ECG-synchronized MDCT that covers at least the aortic root, followed by non-ECG synchronized images of the aorta, iliac, and femoral vasculature [29][17]. The ECG-synchronized MDCT of the aortic root is important, because the aortic annulus undergo conformable change throughout the cardiac cycle, being bigger and circular in systole, and oval in diastole. The goal is to measure the greatest possible annular dimension, which can be found during the cardiac systole (20–40% of the cardiac cycle) [31,32][18][19].
Regarding radiation, a tube potential of 100 kV is usually indicated for patients weighing <90 kg or with a body mass index (BMI) <30 kg/m2, whereas a tube potential of 120 kV is indicated for patients weighing >90 kg and with BMI >30 kg/m2.
Intravenous contrast administration is mandatory. Optimal images require high intra-arterial opacification, and attenuation values should exceed 250 Hounsfield units. MDCT data should be reconstructed as an axial, thin-sliced multiphasic data set, with <1 mm slice thickness. Reconstruction intervals should be spaced at <10% intervals across the acquired portion of the cardiac cycle [29][17].
A 3D multiplanar reconstruction (MPR) of the aorta, aortic valve, and its structures is mandatory to perform TAVR planning (Figure 32).
Figure 32.
A MPR reconstruction from MDCT images using the Horos
®
software.
23. Available Methods
Many different kinds of software can be used to make appropriate MDCT measurements of the aortic root, coronary ostia, and optimal angiographic deployment projections: manual, semi-automated, and automated. The manual measures are the most used by operators since they can be done by cheap or free software, such as Horos® and Osirix®.
2.1. Manual Sizing
3.1. Manual Sizing
The manual TAVR sizing is usually made using the 3D MPR tool of Osirix® or Horos® software. In the MPR mode, wthe researchers have three correlated images: coronal, sagittal, and transversal. The goal is to perfectly align the virtual aortic annulus, which corresponds to the base of the three aortic cusps. The manual method does not provide information about the steps needed for sizing, and there is no automated report. In 2019, a consensus on MDCT imaging on TAVR describing the main steps was published [29][17]. This consensus provides further and detailed information about MDCT manual preprocedural planning. The manual measurement takes more time than the semi-automated and automated measures, and its learning curve is bigger. However, when used by experienced professionals, it may provide all the information necessary to perform a safe TAVR procedure.
There are some studies comparing the variability of measurement by different observers. These articles found a strong agreement for aortic annulus and coronary arteries height assessment for experienced observers (at least 2 years of experience) [33,34][20][21].
Furthermore, Knobloch et al. and Le Couteulx et al. reported interobserver variability in MDCTs evaluated by observers with different levels of expertise. In the Le Couteulx et al. study, Observer 1 was an expert, whereas Observer 2 was a resident physician with 6 months of practice, and Observer 3 was a trained resident physician with starting experience. Intra- and inter-observer reproducibility were excellent for all aortic annulus dimensions, with an intraclass correlation coefficient ranging, respectively, from 0.84 to 0.98 and from 0.82 to 0.97. Agreement for selection of prosthesis size was almost perfect between the two most experienced observers (k = 0.82) and substantial with the inexperienced observer (k = 0.67) [35][22]. In the Knobloch et al. study, Observer 1 was a radiologist with 6 years of experience, Observer 2 was a laboratory technician with 3 years of experience, and Observer 3 was a medical student with no experience. Intra-observer variability did not differ significantly. However, significant differences were found in mean inter-observer variance (p < 0.001). They advocate that multi-reader paradigms led to significantly increased precision compared with single readers with different levels of experience [36][23].
2.2. Semi-Automated and Automated Sizing
3.2. Semi-Automated and Automated Sizing
Semi-automated software are broadly used by TAVR companies and operators around the globe. The most commonly utilized software is the 3MensioValves (3mensio Medical Imaging BV, Maastricht, The Netherlands). However, the drawback of 3MensioVales software is its high cost, preventing its broad use (Figure 43). There is another semi-automated software called ProSizeAV, which is actually a plugin to be used with Horos® or Osirix®. However, this plugin does not have CE or FDA approval, and there are no data proving its efficacy (Figure 54).
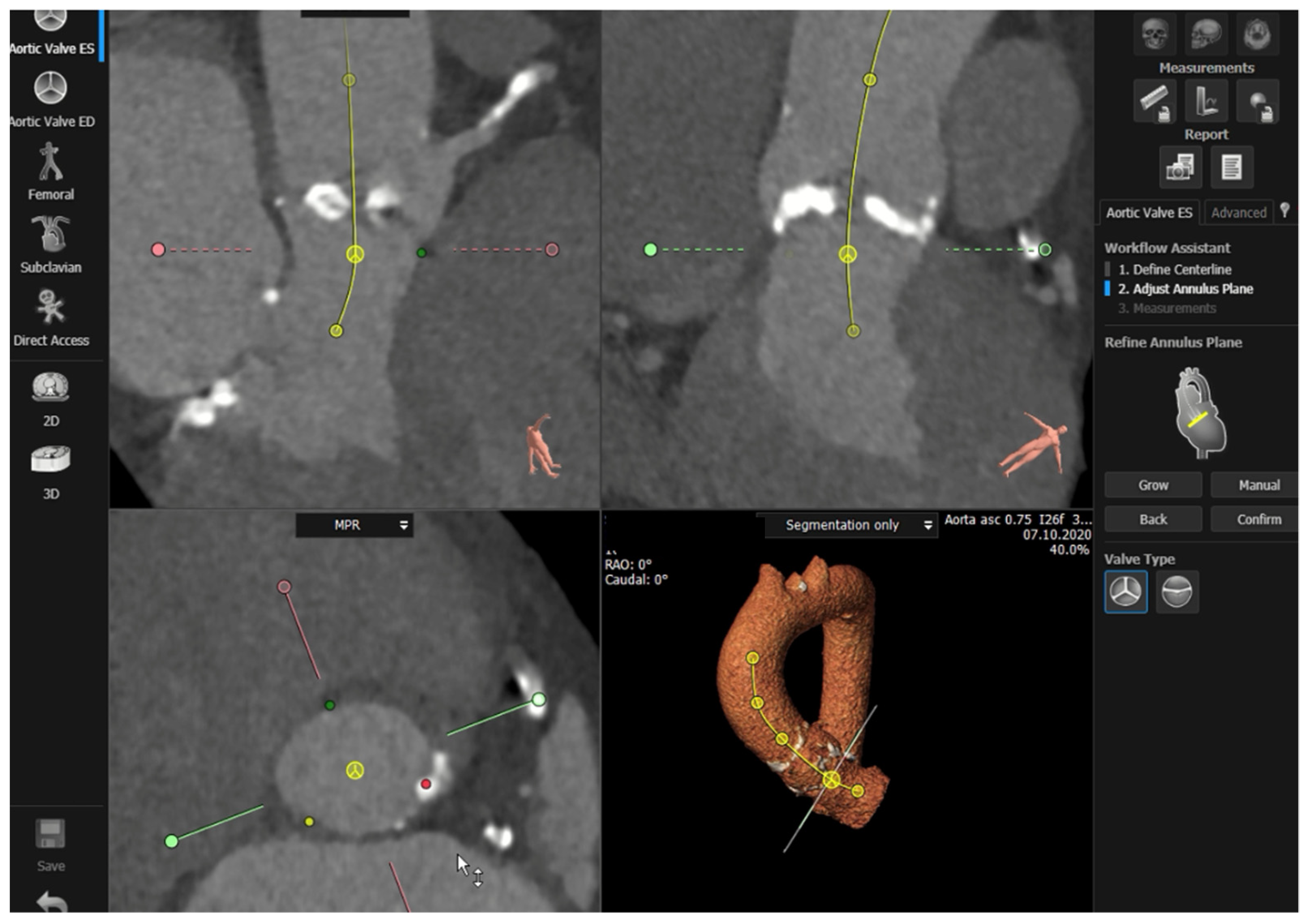
Figure 43.
Measurements performed using the 3MensioValves.
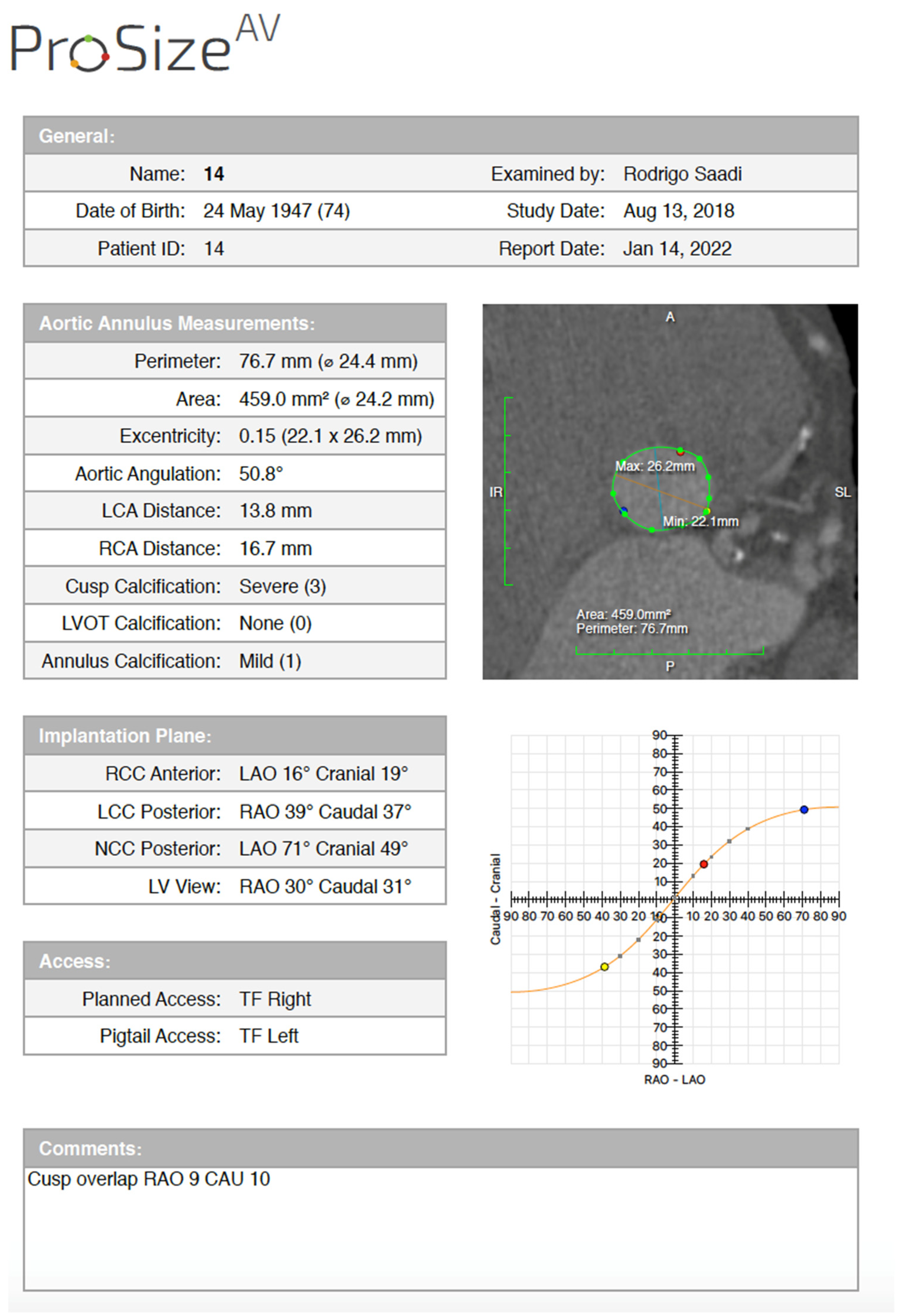
Figure 54.
ProSizeAV report.
There is another available semi-automated (syngo. viaVB20A, Siemens, Munich, Germany) software. In 2018, Horehledova et al. compared the Siemens manual and semi-automated software and demonstrated an excellent inter-software agreement (ICC = 0.93; range 0.90–0.95). The time needed for evaluation using semi-automatic assessment (3 min 24 s) was significantly lower (p < 0.001) compared with a fully manual approach (6 min 31 s) [37][24].
Lou et al. also compared manual, semi-automated, and fully automated measurement of the aortic annulus using Siemens software. Semi-automated analysis required major correction in five patients (4.5%). Mean manual annulus area was significantly smaller than fully automated results (p < 0.001), but similar to semi-automated measurements. The frequency of concordant recommendations for valve size increased if a manual analysis was replaced by semi-automated method (60% agreement was improved to 82.4%; 95% confidence interval for the difference [69.1–83.4%]) [38][25].
References
- Kapadia, S.R.; Leon, M.B.; Makkar, R.R.; Tuzcu, E.M.; Svensson, L.G.; Kodali, S.; Webb, J.G.; Mack, M.J.; Douglas, P.S.; Thourani, V.H.; et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2485–2491.
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Sondergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2017, 376, 1321–1331.
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1695–1705.
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1706–1715.
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2016, 363, 1511–1520.
- Barker, C.M.; Reardon, M.J. The CoreValve US Pivotal Trial. Semin. Thorac. Cardiovasc. Surg. 2014, 26, 179–186.
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Herrmann, H.C.; Doshi, D.; Cohen, D.J.; Pichard, A.D.; Kapadia, S.; Dewey, T.; Babaliaros, V.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620.
- Généreux, P.; Head, S.J.; Wood, D.A.; Kodali, S.K.; Williams, M.R.; Paradis, J.M.; Spaziano, M.; Kappetein, A.P.; Webb, J.G.; Cribier, A.; et al. Transcatheter aortic valve implantation 10-year anniversary: Review of current evidence and clinical implications. Eur. Heart J. 2012, 33, 2388–2400.
- Blanke, P.; Euringer, W.; Baumann, T.; Reinöhl, J.; Schlensak, C.; Langer, M.; Pache, G. Combined assessment of aortic root anatomy and aortoiliac vasculature with dual-source CT as a screening tool in patients evaluated for transcatheter aortic valve implantation. Am. J. Roentgenol. 2010, 195, 872–881.
- Schwarz, F.; Lange, P.; Zinsser, D.; Greif, M.; Boekstegers, P.; Schmitz, C.; Reiser, M.F.; Kupatt, C.; Becker, H.C. CT-Angiography–Based Evaluation of the Aortic Annulus for Prosthesis Sizing in Transcatheter Aortic Valve Implantation (TAVI)–Predictive Value and Optimal Thresholds for Major Anatomic Parameters. PLoS ONE 2014, 9, e103481.
- Kasel, A.M.; Cassese, S.; Bleiziffer, S.; Amaki, M.; Hahn, R.T.; Kastrati, A.; Sengupta, P.P. Standardized imaging for aortic annular sizing: Implications for transcatheter valve selection. JACC Cardiovasc. Imaging 2013, 6, 249–262.
- Horehledova, B.; Mihl, C.; Hendriks, B.M.F.; Eijsvoogel, N.G.; Vainer, J.; Veenstra, L.F.; Wildberger, J.E.; Das, M. Do CTA measurements of annular diameter, perimeter and area result in different TAVI prosthesis sizes? Int. J. Cardiovasc. Imaging 2018, 34, 1819–1829.
- Bleakley, C.; Monaghan, M.J. The Pivotal Role of Imaging in TAVR Procedures. Curr. Cardiol. Rep. 2018, 20, 9.
- Latsios, G.; Gerckens, U.; Buellesfeld, L.; Mueller, R.; John, D.; Yuecel, S.; Syring, J.; Sauren, B.; Grube, E. “Device landing zone” calcification, assessed by MSCT, as a predictive factor for pacemaker implantation after TAVI. Catheter. Cardiovasc. Interv. 2010, 76, 431–439.
- Hayashida, K.; Bouvier, E.; Lefèvre, T.; Hovasse, T.; Morice, M.C.; Chevalier, B.; Romano, M.; Garot, P.; Mylotte, D.; Farge, A.; et al. Impact of CT-guided valve sizing on post-procedural aortic regurgitation in transcatheter aortic valve implantation. EuroIntervention 2012, 8, 546–555.
- Lehmkuhl, L.; Foldyna, B.; Von Aspern, K.; Lücke, C.; Grothoff, M.; Nitzsche, S.; Kempfert, J.; Haensig, M.; Rastan, A.; Walther, T.; et al. Inter-individual variance and cardiac cycle dependency of aortic root dimensions and shape as assessed by ECG-gated multi-slice computed tomography in patients with severe aortic stenosis prior to transcatheter aortic valve implantation: Is it crucial for. Int. J. Cardiovasc. Imaging 2013, 29, 693–703.
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Norgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2019, 13, 1–20.
- Suchá, D.; Tuncay, V.; Prakken, N.H.J.; Leiner, T.; Van Ooijen, P.M.A.; Oudkerk, M.; Budde, R.P.J. Does the aortic annulus undergo conformational change throughout the cardiac cycle? A systematic review. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1307–1317.
- Jurencak, T.; Turek, J.; Kietselaer, B.L.J.H.; Mihl, C.; Kok, M.; van Ommen, V.G.V.A.; van Garsse, L.A.F.M.; Nijssen, E.C.; Wildberger, J.E.; Das, M. MDCT evaluation of aortic root and aortic valve prior to TAVI. What is the optimal imaging time point in the cardiac cycle? Eur. Radiol. 2015, 25, 1975–1983.
- Schmidkonz, C.; Marwan, M.; Klinghammer, L.; Mitschke, M.; Schuhbaeck, A. Interobserver variability of CT angiography for evaluation of aortic annulus dimensions prior to transcatheter aortic valve implantation. Eur. J. Radiol. 2014, 83, 1672–1678.
- Schuhbaeck, A.; Achenbach, S.; Pflederer, T.; Marwan, M.; Schmid, J.; Nef, H.; Rixe, J.; Hecker, F.; Schneider, C.; Lell, M.; et al. Reproducibility of aortic annulus measurements by computed tomography. Eur. Radiol. 2014, 24, 1878–1888.
- Le Couteulx, S.; Caudron, J.; Dubourg, B.; Cauchois, G.; Dupré, M.; Michelin, P.; Durand, E.; Eltchaninoff, H.; Dacher, J.N. Multidetector computed tomography sizing of aortic annulus prior to transcatheter aortic valve replacement (TAVR): Variability and impact of observer experience. Diagn. Interv. Imaging 2018, 99, 279–289.
- Knobloch, G.; Sweetman, S.; Bartels, C.; Raval, A.; Gimelli, G.; Jacobson, K.; Lozonschi, L.; Kohmoto, T.; Osaki, S.; François, C.; et al. Inter- and intra-observer repeatability of aortic annulus measurements on screening CT for transcatheter aortic valve replacement (TAVR): Implications for appropriate device sizing. Eur. J. Radiol. 2018, 105, 209–215.
- Horehledova, B.; Mihl, C.; Schwemmer, C.; Hendriks, B.M.F.; Eijsvoogel, N.G.; Kietselaer, B.L.J.H.; Wildberger, J.E.; Das, M. Aortic root evaluation prior to transcatheter aortic valve implantation—Correlation of manual and semi-automatic measurements. PLoS ONE 2018, 13, e0199732.
- Lou, J.; Obuchowski, N.A.; Krishnaswamy, A.; Popovic, Z.; Flamm, S.D.; Kapadia, S.R.; Svensson, L.G.; Bolen, M.A.; Desai, M.Y.; Halliburton, S.S.; et al. Manual, semiautomated, and fully automated measurement of the aortic annulus for planning of transcatheter aortic valve replacement (TAVR/TAVI): Analysis of interchangeability. J. Cardiovasc. Comput. Tomogr. 2015, 9, 42–49.
More
