Uranium was discovered in 1789 by the German chemist Martin Heinrich Klaproth. It is the most known and used actinide element mainly because of its usage in nuclear fuel processing; however, the application potential of uranium compounds is much broader, stretching, e.g., into the field of organometallic synthesis, catalysis and beyond. Moreover, uranium is one of the few naturally occurring actinides, whereas the other members, with the exception of thorium, are considered to be human-made, despite their natural occurrence in traces as a result of uranium and thorium spontaneous fissions. In nature, uranium occurs as three main isotopes, where the non-fissile 238U is the most abundant, encompassing over 99% of the available uranium resources.
- uranium
- nuclear energy
- Nuclear fuel cycle
1. Introduction
2. Role of Uranium in the Nuclear Fuel Cycle
Uranium is mainly used in the nuclear fuel cycle sketched in Figure 2, an industrial process involving various activities to produce electricity from uranium in nuclear power reactors, i.e., the progression of nuclear fuel from creation to disposal. Typically, uranium is employed in the form of UO2. During uranium decay, fissile plutonium is produced. As spent fuel can be reprocessed and recycled, this explains the presence of a plutonium admixture with uranium in the subsequent fuel cycle [14,93][14][36].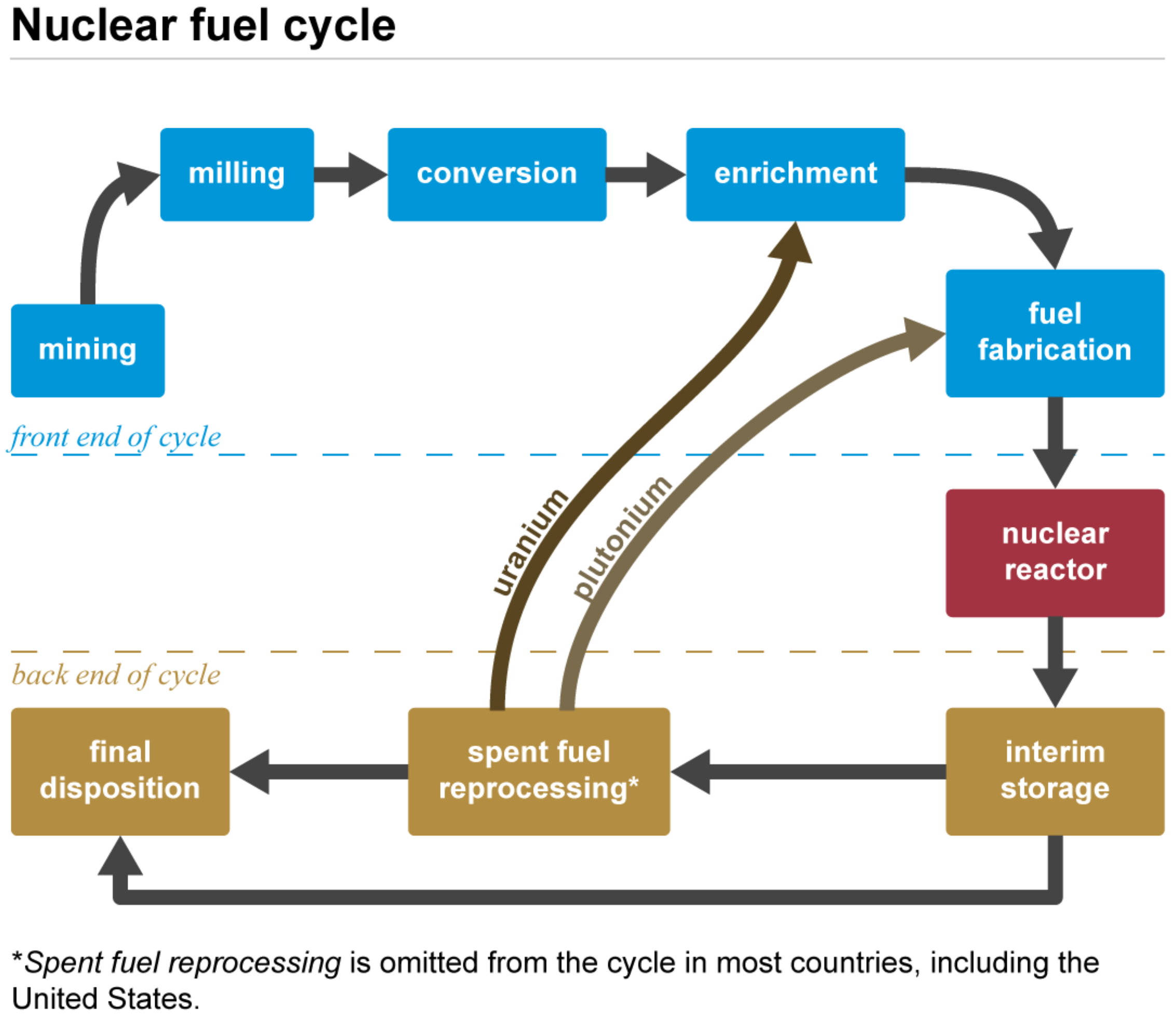
References
- Cotton, S. Introduction to the Actinides. In Lanthanide and Actinide Chemistry; John Wiley & Sons, Ltd.: West Sussex, UK, 2006; Chapter 9; pp. 145–153.
- Xin, X.; Douair, I.; Zhao, Y.; Wang, S.; Maron, L.; Zhu, C. Dinitrogen Cleavage by a Heterometallic Cluster Featuring Multiple Uranium-Rhodium Bonds. J. Am. Chem. Soc. 2020, 142, 15004–15011.
- Tsoureas, N.; Mansikkamäki, A.; Layfield, R.A. Uranium(IV) cyclobutadienyl sandwich compounds: Synthesis, structure and chemical bonding. Chem. Commun. 2020, 56, 944–947.
- Guo, F.S.; Tsoureas, N.; Huang, G.Z.; Tong, M.L.; Mansikkamäki, A.; Layfield, R.A. Isolation of a Perfectly Linear Uranium(II) Metallocene. Angew. Chem. Int. Ed. 2020, 59, 2299–2303.
- Boreen, M.A.; McCabe, K.N.; Lohrey, T.D.; Watt, F.A.; Maron, L.; Hohloch, S.; Arnold, J. Uranium Metallocene Azides, Isocyanates, and Their Borane-Capped Lewis Adducts. Inorg. Chem. 2020, 591, 8580–8588.
- Boreen, M.A.; Lohrey, T.D.; Rao, G.; Britt, R.D.; Maron, L.; Arnold, J. A Uranium Tri-Rhenium Triple Inverse Sandwich Compound. J. Am. Chem. Soc. 2019, 141, 5144–5148.
- Boronski, J.T.; Doyle, L.R.; Seed, J.A.; Wooles, A.J.; Liddle, S.T. f-Element Half-Sandwich Complexes: A Tetrasilylcyclobutadienyl-Uranium(IV)-Tris(tetrahydroborate) Anion Pianostool Complex. Angew. Chem. Int. Ed. 2019, 59, 295–299.
- Terrani, K.A.; Jolly, B.C.; Harp, J.M. Uranium nitride tristructural-isotropic fuel particle. J. Nucl. Mater. 2020, 531, 152034.
- Hartline, D.R.; Meyer, K. From chemical curiosities and trophy molecules to uranium-based catalysis: Developments for uranium catalysis as a new facet in molecular uranium chemistry. JACS Au 2021, 1, 698–709.
- Feng, G.; Zhang, M.; Shao, D.; Wang, X.; Wang, S.; Maron, L.; Zhu, C. Transition-metal-bridged bimetallic clusters with multiple uranium–metal bonds. Nat. Chem. 2019, 11, 248–253.
- Das, D.; Joshi, M.; Kannan, S.; Kumar, M.; Ghanty, T.K.; Vincent, T.; Manohar, S.; Kaushik, C. A Combined Experimental and Quantum Chemical Studies on the Structure and Binding Preferences of Picolinamide Based Ligands with Uranyl Nitrate. Polyhedron 2019, 171, 486–492.
- Lu, G.; Haes, A.J.; Forbes, T.Z. Detection and Identification of Solids, Surfaces, and Solutions of Uranium Using Vibrational Spectroscopy. Coord. Chem. Rev. 2018, 374, 314–344.
- Palumbo, C.T.; Scopelliti, R.; Zivkovic, I.; Mazzanti, M. C-H Bond Activation by an Isolated Dinuclear U(III)/U(IV) Nitride. J. Am. Chem. Soc. 2020, 142, 3149–3157.
- Loveland, W.D.; Morrissey, D.J.; Seaborg, G.T. Modern Nuclear Chemistry; John Wiley & Sons: New York, NY, USA, 2017.
- Navrotsky, A.; Shvareva, T.; Guo, X.; Burns, P. Thermodynamics of uranium minerals and related materials. In Uranium: Cradle to Grave; Burns, P.C., Sigmin, G.E., Eds.; Mineralogical Association of Canada: Winnipeg, MB, Canada, 2013; pp. 147–164.
- Olander, D.R. The theory of uranium enrichment by the gas centrifuge. Prog. Nucl. Energy 1981, 8, 1–33.
- Mazher, A.K. A review of uranium economics. Int. J. Nucl. Gov. Econ. Ecol. 2009, 2, 337–361.
- Edwards, C.; Oliver, A. Uranium processing: A review of current methods and technology. JOM 2000, 52, 12–20.
- Ho, M.; Obbard, E.; Burr, P.A.; Yeoh, G. A review on the development of nuclear power reactors. Energy Procedia 2019, 160, 459–466.
- Gorman-Lewis, D.; Burns, P.C.; Fein, J.B. Review of uranyl mineral solubility measurements. J. Chem. Thermodyn. 2008, 40, 335–352.
- Krivovichev, S.V.; Plášil, J. Mineralogy and crystallography of uranium. In Uranium: From Cradle to Grave; Burns, P.C., Sigmon, G.E., Eds.; Mineralogical Association of Canada Short Courses: Quebec, QC, Canada, 2013; Volume 43, pp. 15–119.
- Seyferth, D. Uranocene: The first member of a new class of organometallic derivatives of the f elements. Organometallics 2004, 23, 3562–3583.
- Boronski, J.T.; Liddle, S.T. The Emergence of Actinide Cyclobutadienyl Chemistry. Eur. J. Inorg. Chem. 2020, 2020, 2851–2861.
- Sessler, J.L.; Melfi, P.J.; Pantos, G.D. Uranium complexes of multidentate N-donor ligands. Coord. Chem. Rev. 2006, 250, 816–843.
- Van Horn, J.D.; Huang, H. Uranium (VI) bio-coordination chemistry from biochemical, solution and protein structural data. Coord. Chem. Rev. 2006, 250, 765–775.
- Yang, W.; Parker, T.G.; Sun, Z.M. Structural chemistry of uranium phosphonates. Coord. Chem. Rev. 2015, 303, 86–109.
- Dam, H.H.; Reinhoudt, D.N.; Verboom, W. Multicoordinate ligands for actinide/lanthanide separations. Chem. Soc. Rev. 2007, 36, 367–377.
- Nash, K.L.; Nilsson, M. Introduction to the reprocessing and recycling of spent nuclear fuels. In Reprocessing and Recycling of Spent Nuclear Fuel; Taylor, R., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Oxford, UK, 2015; pp. 3–25.
- Geist, A.; Adnet, J.M.; Bourg, S.; Ekberg, C.; Galan, H.; Guilbaud, P.; Miguirditchian, M.; Modolo, G.; Rhodes, C.; Taylor, R. An overview of solvent extraction processes developed in Europe for advanced nuclear fuel recycling, part 1—Heterogeneous recycling. Sep. Sci. Technol. 2021, 56, 1866–1881.
- Taylor, R.; Bodel, W.; Stamford, L.; Butler, G. A Review of Environmental and Economic Implications of Closing the Nuclear Fuel Cycle—Part One: Wastes and Environmental Impacts. Energies 2022, 15, 1433.
- Ewing, R.C.; Weber, W.J.; Clinard, F.W., Jr. Radiation effects in nuclear waste forms for high-level radioactive waste. Prog. Nucl. Energy 1995, 29, 63–127.
- Ewing, R.C.; Weber, W.J.; Lian, J. Nuclear waste disposal—Pyrochlore (A2B2O7): Nuclear waste form for the immobilization of plutonium and “minor” actinides. J. Appl. Phys. 2004, 95, 5949–5971.
- McEachern, R.J.; Taylor, P. A review of the oxidation of uranium dioxide at temperatures below 400 °C. J. Nucl. Mater. 1998, 254, 87–121.
- Belle, J. Oxygen and uranium diffusion in uranium dioxide (a review). J. Nucl. Mater. 1969, 30, 3–15.
- Yoo, T.S.; Herrmann, S.D.; Yoon, S.J.; Marsden, K.C. Analysis and Modeling of Oxide Reduction Processes for Uranium Oxides. J. Nucl. Mater. 2021, 545, 152625.
- Crossland, I. Nuclear Fuel Cycle Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2012.
- Wilson, P.D. The Nuclear Fuel Cycle from Ore to Wastes; Oxford Science Publications: Oxford, UK, 1996.
- Leybros, A.; Hung, L.; Hertz, A.; Hartmann, D.; Grandjean, A.; Boutin, O. Supercritical CO2 extraction of uranium from natural ore using organophosphorus extractants. Chem. Eng. J. 2017, 316, 196–203.
- Prabhat, P.; Rao, A.; Mishra, V.G.; Shah, D.J.; Kumar, P.; Tomar, B.S. Direct extraction of uranium from yellow cake and ore matrices using supercritical carbon dioxide. Radiochim. Acta 2020, 108, 769–777.
- Zachariasen, W. The Crystal Structure of U2F9. J. Chem. Phys. 1948, 16, 425.
- Zachariasen, W. Crystal Chemical Studies of the 5f-Series of Elements. XI. The Crystal Structure of α-UF5 and of β-UF5. Acta Crystallogr. 1949, 2, 296–298.
- Cotton, S. Binary Compounds of the Actinides. In Lanthanide and Actinide Chemistry; John Wiley & Sons, Ltd.: West Sussex, UK, 2006; Chapter 10; pp. 155–172.
- Peluzo, B.M.; Galvão, B.R. Theoretical study on the structure and reactions of uranium fluorides. J. Mol. Model. 2018, 24, 197.
- Krass, A.S. Uranium Enrichment and Nuclear Weapon Proliferation; Oxford University Press: Oxford, UK, 1983.
- Katoh, Y.; Vasudevamurthy, G.; Nozawa, T.; Snead, L.L. Properties of zirconium carbide for nuclear fuel applications. J. Nucl. Mater. 2013, 441, 718–742.
- McDeavitt, S.; Abraham, D.; Park, J. Evaluation of stainless steel–zirconium alloys as high-level nuclear waste forms. J. Nucl. Mater. 1998, 257, 21–34.
- Ali, K.; Ghosh, P.; Arya, A. A DFT study of structural, elastic and lattice dynamical properties of Fe2Zr and FeZr2 intermetallics. J. Alloys Compd. 2017, 723, 611–619.
- Basdevant, J.L.; Rich, J. Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology; Springer Science & Business Media: New York, NY, USA, 2005.
- Streit, M.; Ingold, F. Nitrides as a nuclear fuel option. J. Eur. Ceram. Soc. 2005, 25, 2687–2692.
- Hunt, R.D.; Yustein, J.T.; Andrews, L. Matrix infrared spectra of NUN formed by the insertion of uranium atoms into molecular nitrogen. J. Chem. Phys. 1993, 98, 6070–6074.
- Liu, G.; Zhang, C.; Ciborowski, S.M.; Asthana, A.; Cheng, L.; Bowen, K.H. Mapping the electronic structure of the uranium (VI) dinitride molecule, UN2. J. Phys. Chem. A 2020, 124, 6486–6492.
- Kushto, G.P.; Souter, P.F.; Andrews, L.; Neurock, M. A matrix isolation FT–IR and quasirelativistic density functional theory investigation of the reaction products of laser-ablated uranium atoms with NO, NO2 and N2O. J. Chem. Phys. 1998, 106, 5894–5903.
- Zhou, M.; Andrews, L. Infrared spectra and pseudopotential calculations for NUO+, NUO, and NThO in solid neon. J. Chem. Phys. 1999, 111, 11044–11049.
- Wang, X.; Andrews, L.; Vlaisavljevich, B.; Gagliardi, L. Combined Triple and Double Bonds to Uranium: The N≡U=N-H Uranimine Nitride Molecule Prepared in Solid Argon. Inorg. Chem. 2011, 50, 3826–3831.
