Reproductive dysfunction is often characterized by malfunction of the reproductive tissues, which may lead to disruption of the synergistic rhythm that should bring about a progression of sexual events and the conception of new life. This may therefore result in the sexual dysfunction and infertility that can be seen in couples having prolonged biological difficulty in reproducing their offspring after having unrestricted sexual intercourse for at least twelve months. Several factors have been implicated in the cause and progression of reproductive dysfunction, including poor nutrition, drug side effects, disease states, and toxicant ingestion. A well-known food additive that has been found to be potent at initiating reproductive anomalies in males is monosodium glutamate (MSG).
- antioxidant enzymes
- monosodium glutamate
- reactive oxygen species
1. Introduction
2. Mechanism of MSG-Induced Testicular Alteration
The action of MSG on the male reproductive morphology and function may be as a result of its diverse influence on cells, thereby initiating spermatogenic alterations, oxidative damage, histological alteration, and gonadotropin imbalance which may eventually culminate into reproductive abnormalities in the males as shown in Figure 1.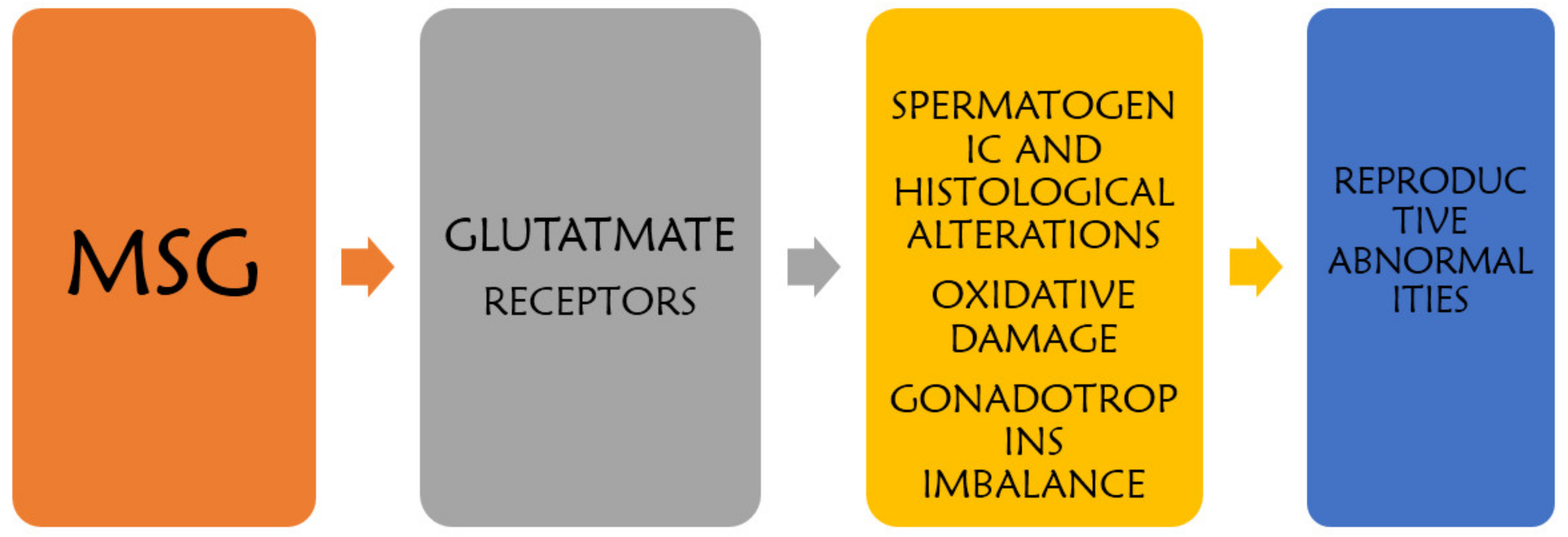
2.1. Oxidative Stress
The organs of the reproductive system are targets of reactive oxygen species (ROS) because of adipose tissue present in these organs [13][9]. Studies have revealed an increase in the lipid peroxidation (malondialdehyde, MDA) and decreased antioxidant activity (reduced glutathione, GSH) as well as noticeable increase in testicular oxidative stress and a corresponding reduction in antioxidant/antioxidant enzyme activities after MSG administration [7,13,19][7][9][10]. Increased production of free radicals caused by MSG could lead to lipid peroxidation and sperm membrane dysfunction, sperm DNA damage, and impaired sperm movement. The abundance of unsaturated fat (plasma membrane) and low levels of antioxidants (cytoplasm) make the testes and sperm cells susceptible to oxidative stress [20][11]. Patients with asthenozoospermia were found to have a high ROS generation in seminal plasma as well as sperm membrane damage mediated by MSG [7,19][7][10]. The direct implication of this ROS-induced damage on membrane integrity are impaired sperm motility and viability [20][11]. Therefore, therapeutic agents such as antioxidants may be useful in reversing MSG-induced reproductive toxicity.2.2. Neurotoxicity
The neurotoxic effect of MSG causes excitotoxicity in the brain through disruption of the hypothalamic–pituitary-axis pathway (HPA) [13][9]. Glutamate is an excitatory neurotransmitter, and a high influx of neuron intracellular calcium caused by high glutamate may lead to neuronal death. HPA disruption may reduce levels of sex hormones, including testosterone, follicle-stimulating hormone, and luteinizing hormone. This ultimately leads to alterations in sperm quality [21][12]. Spermatogenesis is totally dependent on the sex hormones and androgen-dependent organs of the reproductive system, which include the prostate gland, epididymis and seminal vesicles. Any androgen hormone (i.e., testosterone, luteinizing hormone, and follicle-stimulating hormone) disorder will therefore have a negative impact on the reproductive tissues [7].2.3. Histomorphological Alterations
Alterations of the testicular histopathology such as spermatogenic arrest, low sperm production, and edema have previously been reported [7,10,11,13][7][9][13][14]. Meanwhile, another study observed no overt histopathological changes in the MSG-treated animals [15]. Low spermatogonia levels have been linked with maturation arrest in MSG-exposed animals, and this correlates with a low level of testosterone leading to inhibition of spermatogenesis [7,10,11,12,14,15,16][7][13][14][15][16][17][18]. Other studies, however, observed improved testicular histopathology after the administration of selenium, vitamin E, and curcumin, respectively [7,22][7][19]. Treatments such as graviola extract, vitamin C, vitamin E, camel milk, propolis, quince extract, and curcumin have proven to provide protective effects against MSG-induced histomorphological testicular toxicities [17,18,19,20,21,22][10][11][12][19][20][21].2.4. Glutamate Receptor Dysfunction
Another mechanism of MSG-induced male reproductive toxicity is via glutamate receptors, as MSG directly affects the glutamate transporter on the epithelium of seminiferous tubules. Glutamate receptors are found in different organs and tissues, including the endocrine glands, hypothalamus, thymus, ovaries, liver, kidney, and testis. The testis has been found to exhibit morphological alterations subsequent to MSG treatment due to mal-expression of glutamate receptor in the testis [7,10,21][7][12][13].2.5. Brief Clinically Observed Adverse Effect of MSG
Clinical trials conducted in the past have revealed the interplay between MSG and hunger and food intake. In one study, 32 volunteers were screened for the effect of MSG on food intake. It was observed that those who consumed soup containing MSG had increased hunger and food intake when compared to those who took soup without MSG [8,23][8][22]. In another study involving 100 French men given an MSG-added diet, an obvious increase in food intake was noticed [8,24][8][23]. There have been clinical reports on the direct relationship between MSG intake and obesity in humans [8,25,26,27][8][24][25][26]. Clinical trials have also shown that the consumption of MSG could result in certain allergic reactions in humans [8].References
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 10th ed.; Harcourt International Edition; W.B. Saunder Company: Philadelphia, PA, USA, 2000; pp. 279–281.
- Ganong, W.F. Review of Medical Physiology, 20th ed.; Lange Medical Books/McGraw-Hill Medical Publishing Division: London, UK, 2001; p. 543.
- Kandeel, F.R.; Koussa, V.K.; Swerdloff, R.S. Male sexual function and its disorders: physiology, pathophysiology, clinical investigation, and treatment. Endocr. Rev. 2001, 22, 342.
- Kayode, O.T.; Yakubu, M.T. Parquetina nigrescens leaves: Chemical profile and effects of its Aqueous Extract on the Physical and Biochemical Parameters of Sexual Behaviour of Male Rats. J. Integr. Med. 2017, 15, 64–76.
- Harchegani, A.B.; Irandoost, A.; Mirnamniha, M.; Rahmani, H.; Tahmasbpour, E.; Shahriary, A. Possible Mechanisms for The Effects of Calcium Deficiency on Male Infertility. Int. J. Fertil. Steril. 2019, 12, 267–272.
- Bera, T.K.; Kar, S.K.; Yadav, P.K.; Mukherjee, P.; Yadav, S.; Joshi, B. Effects of monosodium glutamate on human health: A systematic review. World J. Pharm. Sci. 2017, 5, 139–144.
- Hamza, R.Z.; AL-Harbi, M.S. Monosodium glutamate induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicol. Report 2014, 1, 1037–1045.
- Kazmi, Z.; Fatima, I.; Perveen, S.; Malik, S.S. Monosodium glutamate: Review on clinical reports. Int. J. Food Prop. 2017, 20 (Suppl. 2), 1807–1815.
- Hanipah, E.N.A.; Yahya, N.J.; Ajik, E.M.; Yusoff, N.A.; Taib, I.S. Monosodium Glutamate Induced Oxidative Stress in Accessory Reproductive Organs of Male Sprague-Dawley Rats. J. Sains Kesihat. Malays. 2018, 16, 67–73.
- El-Sawy, H.B.I.; Soliman, M.M.; El-Shazly, S.A.; Ali, H.A. Protective effects of camel milk and vitamin E against monosodium glutamate induced biochemical and testicular dysfunctions. Prog. Nutr. 2018, 20, 76–85.
- Khaled, F.A.; Yousef, M.I.; Kamel, K.I. The protective role of propolis against the reproductive toxicity of mono-sodium glutamine in male rabbits. Int. J. Chem. Stud. 2016, 4, 4–9.
- Kianifard, D.; Gholamreza, S.V.; Farhad, R. Study of the protective effects of quince (Cydonia oblonga) leaf extract on fertility alterations and gonadal dysfunction induced by Monosodium glutamate in adult male wistar rats. Rom. J. Diabetes Nutr. Metab. Dis. 2015, 22, 375–384.
- Abd-Elaziz, A.M.S.; Ashoush, I.S. Effect of monosodium glutamate administration on the reproductive performance in male male albino rats. Egypy J. Basic Appl. Physiol. 2007, 6, 101–110.
- Das, R.S.; Ghosh, S.K. Long term effects of monosodium glutamate on spermatogenesis following neonatal exposure in albino mice—A histological study. Nepal Med. Coll. J. 2010, 12, 149–153.
- Igwebuike, U.M. The effects of oral administration of monosodium glutamaste (msg) on the testicular morphology and cauda epididymal sperm reserves of young and adult male rats. Vet. Arh. 2011, 81, 525–534.
- Fernandes, G.S.A.; Arena, A.C.; Campos, K.E.; Volpato, G.T.; Anselmo-Franci, J.A.; Damasceno, D.C.; Kempinas, W.G. Glutamate-induced obesity leads to decreased sperm reserves and acceleration of transit time in the epididymis of adult male rats. Reprod. Biol. Endocrinol. 2012, 10, 105.
- Iamsaard, S.; Sukhorum, W.; Samrid, R.; Yimdee, J.; Kanla, P.; Chaisiwamongkol, K.; Hipkaeo, W.; Fongmoon, D.; Kondo, H. The sensitivity of male rat reproductive organs to monosodium glutamate. Acta Med. Acad. 2014, 43, 3–9.
- Nosseir, N.S.; Ali, M.H.N.; Ebaid, H.M. A Histological and Morphometric Study of Monosodium Glutamate Toxic Effect on Testicular Structure and Potentiality of Recovery in Adult Albino Rats. Res. J. Biol. 2012, 2, 66–78.
- Sakr, S.A.; Bada, G.M. Protective Effect of Curcumin on Monosodium Glutamate-Induced Reproductive Toxicity in Male Albino Rats. Glob. J. Pharmacol. 2013, 7, 416–422.
- Abd-Ella, E.M.M.; Mohammed, A.M. Attenuation of Monosodium Glutamate-Induced Hepatic and Testicular Toxicity in Albino Rats by Annona Muricata Linn. (Annonaceae) Leaf Extract. IOSR J. Pharm. Biol. Sci. 2016, 11, 61–69.
- Ekaluo, U.B.; Ikpeme, E.V.; Ibiang, Y.B.; Amaechina, O.S. Attenuating role of vitamin C on sperm toxicity induced by monosodium glutamate in albino rats. J. Biol. Sci. 2013, 13, 298–301.
- Yeomans, M.R.; Gould, N.J.; Mobini, S.; Prescott, J. Acquired Flavor Acceptance and Intake Facilitated by Monosodium Glutamate in Humans. Physiol. Behav. 2008, 93, 958–966.
- Bellisle, F.; Monneuse, M.O.; Chabert, M.; Larue-Achagiotis, C.; Lanteaume, M.T.; Louis-Sylvestre, J. Monosodium Glutamate as a Palatability Enhancer in the European Diet. Physiol. Behav. 1991, 49, 869–873.
- He, K.; Zhao, L.; Daviglus, M.L.; Dyer, A.R.; Horn, L.; Garside, D.; Stamler, J. Association of Monosodium Glutamate Intake with Overweight in Chinese Adults: The INTERMAP Study. Obesity 2008, 16, 1875–1880.
- Shi, Z.; Luscombe-Marsh, N.D.; Wittert, G.A.; Yuan, B.; Dai, Y.; Pan, X.; Taylor, A.W. Monosodium Glutamate Is Not Associated with Obesity or a Greater Prevalence of Weight Gain over 5 Years: Findings from the Jiangsu Nutrition Study of Chinese Adults. Br. J. Nutar. 2010, 104, 457–463.
- Insawang, T.; Selmi, C.; Cha’on, U.; Pethlert, S.; Yongvanit, P.; Areejitranusorn, P.; Prasongwattana, V. Monosodium Glutamate (MSG) Intake Is Associated with the Prevalence of Metabolic Syndrome in a Rural Thai Population. Nutr. Metab. 2012, 9, 1.
