Drought and waterlogging seriously affect the growth of plants and are considered severe constraints on agricultural and forestry productivity; their frequency and degree have increased over time due to global climate change. The morphology, photosynthetic activity, antioxidant enzyme system and hormone levels of plants could change in response to water stress.
- drought stress
- waterlogging stress
- plant morphology
- physiology and biochemistry
1. Introduction
2. Morphological Structure Responses to Water Stress in Plants
The response of plants to water stress is mainly reflected in leaves and roots, and their external morphological characteristics and internal anatomical structure can best reflect the adaptability to adverse environments [16,17,18,19][16][17][18][19] (Table 1). Leaves are the most variable organs in long-term adaptation to the environment. They react similarly under drought and waterlogging stress, showing signs of etiolation, atrophy, curling, senescence and even abscission [20,21][20][21]. In some cases, stress resulted in stunted leaf growth and reduced leaf number and area [22,23,24][22][23][24] (Figure 1).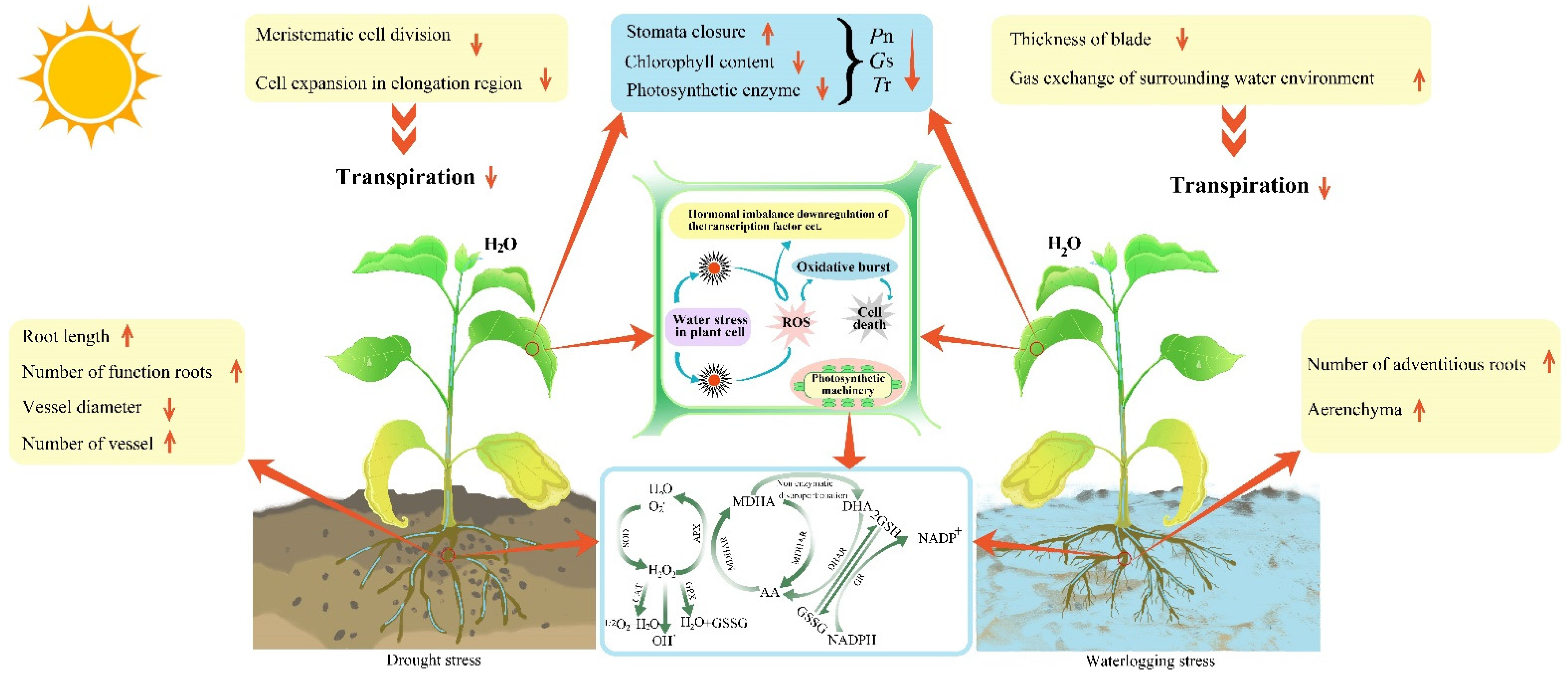
2.1. Morphological Structure Responses to Drought Stress
Drought can limit plant growth by inhibiting the cell division of leaf meristematic tissue and cell expansion in elongation areas, as well as inducing complex changes in leaf thickness, palisade tissue and spongy tissue during adaptation [25,26,27][25][26][27]. Rueda et al. [28] found that the conifers (water-holding capacity of plants) could be improved by increasing the thickness of leaves and decreasing the thickness of palisade tissue and spongy tissue in drought environments. However, Zheng et al. [29] found that Lycium barbarum increased the thickness of palisade tissue and reduced the thickness of spongy tissue, inhibiting transpiration and preventing tissue from excessive dehydration. The above results presented that the internal structure of the leaf changes resulted in transpiration reduction, as well as photosynthetic rate. The root is an important organ for plants to fix and absorb substances from the soil. Drought stress reduces the stele area, vessel diameter and secondary root cortex cells and increases the number of vessels in the stele to facilitate water flow [30,31,32][30][31][32]. To improve water retention and drought resistance, plants not only extend the root system by increasing the number of functional roots, but also increase the water-absorbing capacity of the root sheath [33,34][33][34]. Furthermore, plants improve resistance by changing the root structure (such as root hair and root density) to influence root spatial distribution, soil fixation and nutrient absorption [35,36,37][35][36][37]. Therefore, plants could improve water absorption capacity by changing root length and internal structure under drought stress conditions.2.2. Morphological Structure Responses to Waterlogging Stress
The main response symptoms of leaves to waterlogging stress are curling, yellowing, wilting, falling off, rotting, etc. Leaves have two kinds of adaptation to waterlogging stress: one is to increase the thickness, while the other is to reduce the thickness. For the former, the water loss is reduced and the water holding capacity of plants is improved by increasing palisade tissue and spongy tissue, as well as the decrease in leaf and stomata size [38,39,40][38][39][40]. The latter takes place because leaves cannot complete morphogenesis normally due to lack of water and nutrition [41]. Thereby, some plants thin their leaves or form special leaves to promote the infiltration ability of CO2 and inorganic nutrients into the leaves [42[42][43],43], and improve gas exchange to restore and maintain respiration under waterlogging stress [44,45][44][45]. Therefore, the internal anatomy variation of the leaf is to adjust the stomata and improve transpiration under waterlogging stress, but the reason is uncertain and further study is needed. Aerenchyma forming in the adventitious roots are the most obvious adaptation features under waterlogging stress. Meanwhile, the epithelial cell wall keratinizes gradually under a waterlogged environment to promote oxygen capture by underwater tissue, and enhance waterlogging tolerance [46,47][46][47]. Yamauchi et al. [48] found that there are a lot of root hairs in the adventitious roots, the surface area is large, and the cuticle of the adventitious root is thin, but the aerenchyma is well developed, which can improve the oxygen content of waterlogging-tolerant plants. Moreover, lignified and embolized vascular bundle cortical cells contribute to long-distance oxygen diffusion to the root tips, and block the entry of soil toxins into plants effectively. For instance, Ranathunge et al. [49] found that rice promoted the early formation and increased lignin deposition in both the internal and external epidermis of roots, and prevented ion penetration more effectively under waterlogged conditions. Abiko et al. [50] found that waterlogging-tolerant teosinte formed adventitious roots and produced larger aerenchyma, a stronger lignified vascular bundle cell barrier, and the transport of oxygen from stem base to root tip was better than normal maize under a waterlogging environment. Therefore, the ways of producing adventitious roots are diverse in different types of plants under waterlogging stress, and strong waterlogging-tolerant plants are more likely to have the ability to form adventitious roots. It has been indicated that roots could improve adaptability by creating air cavities in the aerenchyma to expand storage space, and block the entry of soil toxins into plants.|
Treatment |
Root |
Reference |
Leaf |
Reference |
|---|---|---|---|---|
|
Drought stress |
Root system lengthens; functional root number increases; distribution breadth increases. |
Wilting; crimping; stomatal closure. |
||
|
Area of the stele reduces; number of vascular bundles increases but their diameter reduces. |
Thickness of spongy tissue decreases; vascular bundles increase. |
|||
|
Waterlogging stress |
Number of roots decreases; root activity decreases; adventitious roots are generated. |
Etiolation; wilting; abscission; stomatal closure. |
||
|
Aerenchyma is formed in adventitious roots; size of the stele reduces. |
Blade thickness is reduced; number and area of leaves decreases. |
3. Photosynthetic Characteristics of Plant Responses to Water Stress
3.1. Photosynthetic Characteristics of Plant Responses to Drought Stress
To maintain photosynthesis, plants form a series of defense mechanisms to protect their photosynthetic organs from damage in the process of adapting to water stress [70,71][70][71]. For most plants, light water stress can control stomata and transpiration, directly regulate leaf water potential, and self-repair after a return to a normal water supply; some plants even increase photosynthesis [72,73][72][73]. For example, light drought stress usually leads to a stomatal conductance and transpiration increase, while moderate and severe drought stress results in a net photosynthetic rate (Pn), stomatal conductance (Gs) and transpiration rate (Tr) decrease. However, the intercellular carbon dioxide concentration (Ci) shows a different trend. Ci increases or decreases with the deepening of stress, while the stomatal limit (Ls) first increases and then decreases. These results indicate that the decrease in Pn under drought stress is mainly caused by nonstomatal factors [74,75][74][75]. Most nonstomatal factors, including chlorophyll content, photosynthetic enzyme activity and active oxygen metabolism, are induced by moderate and severe drought stress. Drought not only inhibits the formation of chlorophyll directly [76,77][76][77], but also causes difficulty in absorbing mineral elements from the soil, causing leaf nutrient deficiency (for example, leaf etiolation) [78,79][78][79] (Figure 1). The regulation of photosynthetic enzymes is a very complicated process. Light drought stress may slightly affect the photosynthetic carboxylation efficiency, but it can inhibit the activity of RuBPCase, which may result in a decrease in the photosynthetic carboxylation efficiency under severe drought stress [80].3.2. Photosynthetic Characteristics of Plant Responses to Waterlogging Stress
Under waterlogging stress, both stomatal and nonstomatal factors inhibit photosynthesis. For stomatal factors, the chemical signals from roots are transferred to the ground, forcing the stomata of leaves to close, and reducing the photosynthetic rate by decreasing the absorption capacity of the photosynthetic substrate CO2 [81,82,83][81][82][83]; Another aspect of stomatal conductance increasing is the supply of CO2, which increases the amount of assimilates to maintain growth under waterlogging. For non-stomatal factors, there is the anaerobic respiration of the plant under hypoxic surroundings. Lactic acid and ethanol are produced, which break the balance of active oxygen metabolism, degrade chlorophyll and damage the photosynthetic apparatus, producing excess excitation energy and causing photoinhibition [84,85][84][85]. For severe waterlogging-tolerant plants, the stomata closed quickly due to the stress reaction of plants at the initial stage. For poor waterlogging-tolerant plants, leaf carbohydrates may accumulate rapidly within a few days, because root anaerobic respiration restrains sugar transfer from the stem to the root by reducing sugar consumption in the root, and the accumulation of photoassimilated products in leaves can form a negative feedback inhibition to the photosynthetic rate.4. Antioxidant System of Plant Responses to Water Stress
Under normal physiological activities, plants produce reactive oxygen species (ROS), such as superoxide anion radicals (O2−), singlet oxygen (O2), hydroxyl radicals (·OH) and hydrogen peroxide (H2O2), as signal transmitters to regulate gene and protein expression in plant cells, and the production and elimination of ROS are always in a state of dynamic equilibrium [86]. When the plant is stressed, the balance will be broken, the physiological and biochemical functions of the plant cell membrane will be disturbed, and the production of reactive oxygen species will increase [87]. Plants have similar responses to drought and waterlogging, and both stresses activate the antioxidant defense system of plants to avoid cell damage. The components of the antioxidant defense system are enzymatic and nonenzymatic antioxidants. The enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), glutathione reductase (GR), dehydroascorbate reductase glutathione (DHAR) and monodehydroascorbic acid reductase (MDHAR). The nonenzymatic antioxidants are glutathione (GSH), ascorbic acid (AA) (both water soluble), carotenoids and tocopherols (lipid soluble). Both components counteract the harm caused by reactive oxygen species [88,89,90,91][88][89][90][91]. The response of antioxidant enzymes in plants to water stress is mainly related to tolerance and the level of stress. The activity of SOD in leaves and roots of the same species increases with an increasing level of water stress. Furthermore, the disproportionation conversion of O2− to H2O2 increases and the content of O2− decreases. POD and CAT decompose H2O2 to H2O, inhibit the accumulation of H2O2 effectively, protect plants from oxidative damage, and reduce the toxic effect on plants caused by water stress [92]. This mechanism has been demonstrated in mosses [93], trifoliate orange seedlings [94], and tobacco [95]. There are different antioxidant enzyme activities in different tolerant varieties under the same water stress. The adaptive mechanism of plants is a very complicated process, and there are no fixed rules to follow. For example, the SOD activity of Poa pratensis and Festuca arundinacea increased briefly and then decreased, while the CAT activity of F. arundinacea decreased with increasing drought stress [96]. The SOD activity of the drought-sensitive cultivar Trifolium repens was inhibited under stress, but there was no significant change in the drought-tolerant cultivar Debut, which may be related to its higher ability to mitigate oxidative damage [97]. These results showed that plants could increase the activity of antioxidant enzymes to cope with adverse environments, but the dynamic changes across individuals and stress degrees.References
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The threat of the combined effect of biotic and abiotic stress factors in forestry under a changing climate. Front. Plant Sci. 2020, 11, 601009.
- Gupta, A.; Rico-Medina, A.; Cano-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269.
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87.
- Du, W.; FitzGerald, G.J.; Clark, M.; Hou, X.Y. Health impacts of floods. Prehosp. Disaster Med. 2010, 25, 265–272.
- Shi, W.; Wang, M.; Liu, Y. Crop yield and production responses to climate disasters in China. Sci. Total Environ. 2021, 750, 141147.
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250.
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Responses of carbon metabolism and antioxidant system of summer maize to waterlogging at different stages. J. Agron. Crop Sci. 2018, 204, 505–514.
- Wang, X.; Huang, M.; Zhou, Q.; Cai, J.; Dai, T.B.; Cao, W.X.; Jiang, D. Physiological and proteomic mechanisms of waterlogging priming improves tolerance to waterlogging stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2016, 132, 175–182.
- Zhu, M.K.; Chen, G.P.; Zhang, J.L.; Zhang, Y.J.; Xie, Q.L.; Zhao, Z.P.; Pan, Y.; Hu, Z.L. The abiotic stress-responsive NAC-type transcription factor SlNAC4 regulates salt and drought tolerance and stress-related genes in tomato (Solanum lycopersicum). Plant Cell Rep. 2014, 33, 1851–1863.
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Boldt, J.K. Effect of drought and carbon dioxide on nutrient uptake and levels of nutrient-uptake proteins in roots of barley. Am. J. Bot. 2020, 107, 1401–1409.
- Jiao, P.P.; Wu, Z.H.; Wang, X.; Jiang, Z.B.; Wang, Y.Q.; Liu, H.; Qin, R.; Li, Z.J. Short-term transcriptomic responses of Populus euphratica roots and leaves to drought stress. J. Forestry Res. 2021, 32, 841–853.
- Zhao, Q.; Guo, J.; Shu, M.; Wang, P.; Hu, S. Impacts of drought and nitrogen enrichment on leaf nutrient resorption and root nutrient allocation in four Tibetan plant species. Sci. Total Environ. 2020, 723, 138106.
- Hartman, S.; Liu, Z.; van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Zhang, H.; van Dongen, N.; Bosman, F.; Bassel, G.W. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019, 10, 4020.
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71.
- Coutinho, I.D.; Henning, L.M.; Dopp, S.A.; Nepomuceno, A.; Moraes, L.A.; Marcolino-Gomes, J.; Richter, C.; Schwalbe, H.; Colnago, L.A. Flooded soybean metabolomic analysis reveals important primary and secondary metabolites involved in the hypoxia stress response and tolerance. Environ. Exp. Bot. 2018, 153, 176–187.
- Cal, A.J.; Sanciangco, M.; Rebolledo, M.C.; Luquet, D.; Torres, R.O.; McNally, K.L.; Henry, A. Leaf morphology, rather than plant water status, underlies genetic variation of rice leaf rolling under drought. Plant Cell Environ. 2019, 42, 1532–1544.
- Liu, W.S.; Zheng, L.; Qi, D.H. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175.
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Flaig, I.C.; Rillig, M.C. Root trait responses to drought are more heterogeneous than leaf trait responses. Funct. Ecol. 2020, 34, 2224–2235.
- Pedersen, O.; Sauter, M.; Colmer, T.D.; Nakazono, M. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol. 2021, 229, 42–49.
- Patharkar, O.R.; Walker, J.C. Connections between abscission, dehiscence, pathogen defense, drought tolerance, and senescence. Plant Sci. 2019, 284, 25–29.
- Bhusal, N.; Kim, H.S.; Han, S.G.; Yoon, T.M. Photosynthetic traits and plant-water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111.
- Wei, W.L.; Li, D.H.; Wang, L.H.; Ding, X.; Zhang, Y.X.; Gao, Y.; Zhang, X.R. Morpho-anatomical and physiological responses to waterlogging of sesame (Sesamum indicum L.). Plant Sci. 2013, 208, 102–111.
- Fang, Y.J.; Xiong, L.Z. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 2015, 72, 673–689.
- Nadal, M.; Roig-Oliver, M.; Bota, J.; Flexas, J. Leaf age-dependent elastic adjustment and photosynthetic performance under drought stress in Arbutus unedo seedlings. Flora 2020, 271, 151662.
- Nelissen, H.; Sun, X.H.; Rymen, B.; Jikumaru, Y.; Kojima, M.; Takebayashi, Y.; Abbeloos, R.; Demuynck, K.; Storme, V.; Vuylsteke, M. The reduction in maize leaf growth under mild drought affects the transition between cell division and cell expansion and cannot be restored by elevated gibberellic acid levels. Plant Biotechnol. J. 2018, 16, 615–627.
- Binks, O.; Meir, P.; Rowland, L.; Costa, A.C.; Vasconcelos, S.S.; Oliveira, A.A.R.; Ferreira, L.; Mencuccini, M. Limited acclimation in leaf anatomy to experimental drought in tropical rainforest trees. Tree Physiol. 2016, 36, 1550–1561.
- Meng, D.; Dong, B.; Niu, L.; Song, Z.; Wang, L.; Amin, R.; Cao, H.; Li, H.; Yang, Q.; Fu, Y. The pigeon pea CcCIPK14-CcCBL1 pair positively modulates drought tolerance by enhancing flavonoid biosynthesis. Plant J. 2021, 106, 1278–1297.
- Rueda, M.; Godoy, O.; Hawkins, B.A. Spatial and evolutionary parallelism between shade and drought tolerance explains the distributions of conifers in the conterminous United States. Global Ecol. Biogeogr. 2017, 26, 31–42.
- Zheng, G.Q.; Zhang, L.; Zheng, G.B.; Zhang, Y.P.; Wang, J.; Hu, Z.H. Effects of irrigation amount on leaf structure, photosynthetic physiology, and fruit yield of Lycium barbarum in arid area. Ying Yong Sheng Tai Xue Bao 2010, 21, 2806–2813.
- De Bauw, P.; Vandamme, E.; Lupembe, A.; Mwakasege, L.; Senthilkumar, K.; Drame, K.N.; Merckx, R. Anatomical root responses of rice to combined phosphorus and water stress-relations to tolerance and breeding opportunities. Funct. Plant Biol. 2019, 46, 1009–1022.
- Thangthong, N.; Jogloy, S.; Punjansing, T.; Kvien, C.K.; Kesmala, T.; Vorasoot, N. Changes in root anatomy of peanut (Arachis hypogaea L.) under different durations of early season drought. Agronomy 2019, 9, 215.
- Hazman, M.; Brown, K.M. Progressive drought alters architectural and anatomical traits of rice roots. Rice 2018, 11, 1–16.
- Lee, D.K.; Jung, H.; Jang, G.; Jeong, J.S.; Kim, Y.S.; Ha, S.H.; Do Choi, Y.; Kim, J.K. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol. 2016, 172, 575–588.
- Pierret, A.; Maeght, J.L.; Clement, C.; Montoroi, J.P.; Hartmann, C.; Gonkhamdee, S. Understanding deep roots and their functions in ecosystems: An advocacy for more unconventional research. Ann. Bot. 2016, 118, 621–635.
- Henry, A.; Gowda, V.R.P.; Torres, R.O.; McNally, K.L.; Serraj, R. Variation in root system architecture and drought response in rice (Oryza sativa): Phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crop Res. 2011, 120, 205–214.
- Tanaka, N.; Kato, M.; Tomioka, R.; Kurata, R.; Fukao, Y.; Aoyama, T.; Maeshima, M. Characteristics of a root hair-less line of Arabidopsis thaliana under physiological stresses. J. Exp. Bot. 2014, 65, 1497–1512.
- Strock, C.F.; Burridge, J.D.; Niemiec, M.D.; Brown, K.M.; Lynch, J.P. Root metaxylem and architecture phenotypes integrate to regulate water use under drought stress. Plant Cell Environ. 2021, 44, 49–67.
- Yin, D.M.; Luo, H.L. Anatomical responses to waterlogging in Chrysanthemum zawadskii. Sci. Hortic. 2012, 146, 86–91.
- Tahira, M.H.; Muhammad, A.; Muhammad, S.A.A.; Riffat, B.; Sana, F. Anatomical and physiological adaptations in aquatic ecotypes of Cyperus alopecuroides Rottb. under saline and waterlogged conditions. Aquat. Bot. 2014, 116, 60–68.
- Zuniga, F.A.; Bustos, S.A.; Alves, F.; Martinez, V.; Smith, R.C. Physiological and morphological responses to permanent and intermittent waterlogging in seedlings of four evergreen trees of temperate swamp forests. Tree Physiol. 2017, 37, 779–789.
- Fan, C.F.; Yang, Y.F. Water affects morphogenesis of growing aquatic plant leaves. Phys. Rev. Lett. 2020, 124, 038003.
- Colmer, T.D.; Pedersen, O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: Gas films improve CO2 and O2 exchange. New Phytol. 2008, 177, 918–926.
- Brodersen, K.E.; Hammer, K.J.; Schrameyer, V.; Floytrup, A.; Rasheed, M.A.; Ralph, P.J.; Kuhl, M.; Pedersen, O. Sediment resuspension and deposition on seagrass leaves impedes internal plant aeration and promotes phytotoxic H2S intrusion. Front. Plant Sci. 2017, 8, 657.
- Lawson, J.R.; Fryirs, K.A.; Leishman, M.R. Interactive effects of waterlogging and atmospheric CO2 concentration on gas exchange, growth and functional traits of Australian riparian tree seedlings. Ecohydrology 2017, 10, e1803.
- Mommer, L.; Visser, E.J. Underwater photosynthesis in flooded terrestrial plants: A matter of leaf plasticity. Ann Bot. 2005, 96, 581–589.
- Ayi, Q.L.; Zeng, B.; Liu, J.H.; Li, S.Q.; Bodegom, P.M.; Cornelissen, J.H.C. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann. Bot. 2016, 118, 675–683.
- Pedersen, O.; Nakayama, Y.; Yasue, H.; Kurokawa, Y.; Takahashi, H.; Floytrup, A.H.; Omori, F.; Mano, Y.; Colmer, T.D.; Nakazono, M. Lateral roots, in addition to adventitious roots, form a barrier to radial oxygen loss in Zea nicaraguensis and a chromosome segment introgression line in maize. New Phytol. 2021, 229, 94–105.
- Yamauchi, T.; Abe, F.; Tsutsumi, N.; Nakazono, M. Root cortex provides a venue for gas-space formation and is essential for plant adaptation to waterlogging. Front. Plant Sci. 2019, 10, 259.
- Ranathunge, K.; Lin, J.; Steudle, E.; Schreiber, L. Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice (Oryza sativa L.) roots. Plant Cell Environ. 2011, 34, 1223–1240.
- Abiko, T.; Kotula, L.; Shiono, K.; Malik, A.I.; Colmer, T.D.; Nakazono, M. Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ. 2012, 35, 1618–1630.
- Djanaguiraman, M.; Prasad, P.V.V.; Kumari, J.; Rengel, Z. Root length and root lipid composition contribute to drought tolerance of winter and spring wheat. Plant Soil 2019, 439, 57–73.
- Song, H.; Li, Y.B.; Zhou, L.; Xu, Z.Z.; Zhou, G.S. Maize leaf functional responses to drought episode and rewatering. Agr. For. Meteorol. 2018, 249, 57–70.
- Chen, M.J.; Zhu, X.F.; Zhang, Y.; Du, Z.H.; Chen, X.B.; Kong, X.R.; Sun, W.J.; Chen, C.S. Drought stress modify cuticle of tender tea leaf and mature leaf for transpiration barrier enhancement through common and distinct modes. Sci. Rep. 2020, 10, 6696.
- Canales, F.J.; Rispail, N.; Garcia, T.O.; Arbona, V.; Perez, L.A.; Prats, E. Drought resistance in oat involves ABA-mediated modulation of transpiration and root hydraulic conductivity. Environ. Exp. Bot. 2021, 182, 104333.
- Xiao, S.; Liu, L.T.; Zhang, Y.J.; Sun, H.C.; Zhang, K.; Bai, Z.Y.; Dong, H.Z.; Li, C.D. Fine root and root hair morphology of cotton under drought stress revealed with RhizoPot. J. Agron. Crop Sci. 2020, 206, 679–693.
- Zhang, J.S.; Zhang, H.; Srivastava, A.K.; Pan, Y.J.; Bai, J.J.; Fang, J.J.; Shi, H.Z.; Zhu, J.K. Knockdown of rice MicroRNA166 Confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 2018, 176, 2082–2094.
- Ouyang, W.J.; Struik, P.C.; Yin, X.Y.; Yang, J.C. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205.
- Palta, J.A.; Ganjeali, A.; Turner, N.C.; Siddique, K.H.M. Effects of transient subsurface waterlogging on root growth, plant biomass and yield of chickpea. Agr. Water Manag. 2010, 97, 1469–1476.
- Domisch, T.; Qian, J.; Sondej, I.; Martz, F.; Lehto, T.; Piirainen, S.; Finer, L.; Silvennoinen, R.; Repo, T. Here comes the flood! Stress effects of continuous and interval waterlogging periods during the growing season on Scots pine saplings. Tree Physiol. 2020, 40, 869–885.
- Dresboll, D.B.; Thorup, K.K. Spatial variation in root system activity of tomato (Solanum lycopersicum L.) in response to short and long-term waterlogging as determined by N-15 uptake. Plant Soil 2012, 357, 161–172.
- Pan, D.L.; Wang, G.; Wang, T.; Jia, Z.H.; Guo, Z.R.; Zhang, J.Y. AdRAP2.3, a novel ethylene response factor vii from Actinidia deliciosa, enhances waterlogging resistance in transgenic tobacco through improving expression levels of PDC and ADH genes. Int. J. Mol. Sci. 2019, 20, 1189.
- Yin, D.M.; Chen, S.M.; Chen, F.D.; Guan, Z.Y.; Fang, W.M. Morpho-anatomical and physiological responses of two Dendranthema species to waterlogging. Environ. Exp. Bot. 2010, 68, 122–130.
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of winter crops at early and late stages: Impacts on leaf physiology, growth and yield. Front. Plant Sci. 2018, 9, 1863.
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034.
- Celedonio, R.P.D.; Abeledo, L.G.; Mantese, A.I.; Miralles, D.J. Differential root and shoot biomass recovery in wheat and barley with transient waterlogging during preflowering. Plant Soil 2017, 417, 481–498.
- Zhang, X.C.; Shabala, S.; Koutoulis, A.; Shabala, L.; Johnson, P.; Hayes, D.; Nichols, D.S.; Zhou, M.X. Waterlogging tolerance in barley is associated with faster aerenchyma formation in adventitious roots. Plant Soil 2015, 394, 355–372.
- Sundgren, T.K.; Uhlen, A.K.; Lillemo, M.; Briese, C.; Wojciechowski, T. Rapid seedling establishment and a narrow root stele promotes waterlogging tolerance in spring wheat. J. Plant Physiol. 2018, 227, 45–55.
- Mommer, L.; Pons, T.L.; Wolters-Arts, M.; Venema, J.H.; Visser, E.J. Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiol. 2005, 139, 497–508.
- Challabathula, D.; Zhang, Q.; Bartels, D. Protection of photosynthesis in desiccation-tolerant resurrection plants. J. Plant Physiol. 2018, 227, 84–92.
- Yang, X.; Li, Y.; Chen, H.; Huang, J.; Zhang, Y.; Qi, M.; Liu, Y.; Li, T. Photosynthetic response mechanism of soil salinity-induced cross-tolerance to subsequent drought stress in tomato plants. Plants 2020, 9, 363.
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531.
- Ding, L.; Lu, Z.; Gao, L.; Guo, S.; Shen, Q. Is Nitrogen a key determinant of water transport and photosynthesis in higher plants upon drought stress? Front. Plant Sci. 2018, 9, 1143.
- Killi, D.; Haworth, M. Diffusive and metabolic constraints to photosynthesis in quinoa during drought and salt stress. Plants 2017, 6, 49.
- Li, P.D.; Zhu, Y.F.; Song, X.L.; Song, F.P. Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum. Plant Physiol. Biochem. 2020, 155, 93–104.
- Tian, L.X.; Li, J.; Bi, W.S.; Zuo, S.Y.; Li, L.J.; Li, W.L.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) under field conditions. Agr. Water Manag. 2019, 218, 250–258.
- Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi. J. Biol. Sci. 2018, 25, 285–289.
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant. 2016, 158, 225–235.
- Smirnoff, N.; Colombe, S.V. Drought influences the activity of enzymes of the chloroplast hydrogen peroxide scavenging system. J. Exp. Bot. 1988, 39, 1097–1108.
- Bondada, B.R.; Oosterhuis, D.M. Canopy photosynthesis, specific leaf weight, and yield components of cotton under varying nitrogen supply. J. Plant Nutr. 2001, 24, 469–477.
- Parry, M.A.; Andralojc, P.J.; Khan, S.; Lea, P.J.; Keys, A.J. Rubisco activity: Effects of drought stress. Ann Bot. 2002, 89, 833–839.
- Pereira, T.S.; Lobato, A.K.S.; Alves, G.A.R.; Ferreira, R.N.; Silva, O.N.; Martins, A.P.; Pereira, E.S.; Sampaio, L.S. Tolerance to waterlogging in young Euterpe oleracea plants. Photosynthetica 2014, 52, 186–192.
- Horiguchi, G.; Nemoto, K.; Yokoyama, T.; Hirotsu, N. Photosynthetic acclimation of terrestrial and submerged leaves in the amphibious plant Hygrophila difformis. AoB Plants 2019, 11, plz009.
- De Pedro, L.F.; Mignolli, F.; Scartazza, A.; Colavita, J.P.M.; Bouzo, C.A.; Vidoz, M.L. Maintenance of photosynthetic capacity in flooded tomato plants with reduced ethylene sensitivity. Physiol. Plant. 2020, 170, 202–217.
- Hole, D.J.; Cobb, B.G.; Hole, P.S.; Drew, M.C. Enhancement of anaerobic respiration in root tips of Zea mays following low-oxygen (hypoxic) acclimation. Plant Physiol. 1992, 99, 213–218.
- Fan, X.; Zhang, Z.; Gao, H.; Yang, C.; Liu, M.; Li, Y.; Li, P. Photoinhibition-like damage to the photosynthetic apparatus in plant leaves induced by submergence treatment in the dark. PLoS ONE 2014, 9, e89067.
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236.
- Polle, A. Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol. 2001, 126, 445–462.
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467.
- Wu, S.W.; Hu, C.X.; Tan, Q.L.; Xu, S.J.; Sun, X.C. Nitric oxide mediates molybdenum-induced antioxidant defense in wheat under drought stress. Front Plant Sci. 2017, 8, 1085.
- Ahmad, S.; Kamran, M.; Ding, R.X.; Meng, X.P.; Wang, H.Q.; Ahmad, I.; Fahad, S.; Han, Q.F. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. Peer J. 2019, 7, e7793.
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94.
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577.
- Rajinder SDhindsa, W.M. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91.
- Huang, Y.M.; Zou, Y.N.; Wu, Q.S. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep.-UK 2017, 7, 42335.
- Zhang, H.H.; Xu, N.; Teng, Z.Y.; Wang, J.R.; Ma, S.L.; Wu, X.Y.; Li, X.; Sun, G.Y. 2-Cys Prx plays a critical role in scavenging H2O2 and protecting photosynthetic function in leaves of tobacco seedlings under drought stress. J. Plant Interact. 2019, 14, 119–128.
- Jiang, Y.; Huang, B. Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci. 2001, 41, 436.
- Vaseva, I.; Akiscan, Y.; Simova-Stoilova, L.; Kostadinova, A.; Nenkova, R.; Anders, I.; Feller, U.; Demirevska, K. Antioxidant response to drought in red and white clover. Acta Physiol. Plant. 2012, 34, 1689–1699.
