2. Gene Expression Profiling
It was not long ago that the diagnosis of liquid tumors rested on morphology, immunohistochemical analyses, and cytogenetics. The original diffuse large B-cell lymphoma (DLBCL) molecular subclassification followed the advent of DNA microarrays, a technology that allows the analysis of thousands of expressed genes simultaneously; see
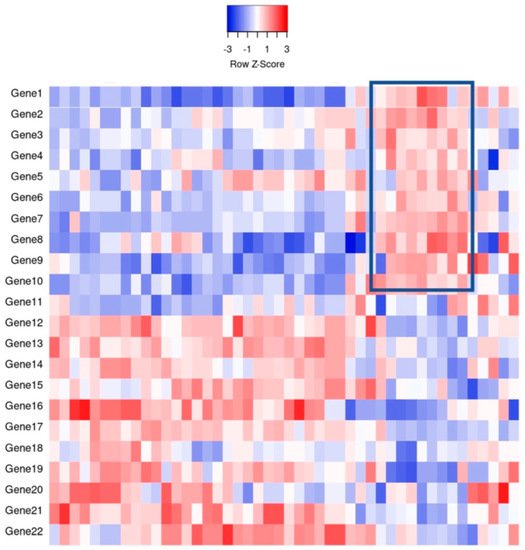
Figure 1 for a theoretical example
[2]. This DNA microarray-based technology allowed for transcriptional gene pattern expression (e.g., Lymphochip by Alizadeh et al.
[3]) analysis under defined conditions that delineated the seminal molecular study classifying a liquid cancer DLBCL into the so-called cell of origin (COO) subtypes
[4]. This accomplishment resulted in the subtyping of approximately 80–85% of all DLBCL cases and importantly showed subtype prognostic values that were greater than that of the standard clinical predictor, the International Prognostic Index (IPI)
[4]. In addition, this critical work paved the way for immunohistochemical (IHC) determination of COO.
Figure 1. An example of gene expression profiling comparing the relative gene expression levels and grouping of cases by clusters. For example, the indicated boxed area indicates that Genes 1–10 have a relative increase in expression, thereby potentially clustering cases together for classification purposes.
Although the study population was small by current standards, with imperfect subtyping, the classification was adopted and continues today as the standard of diagnostic care, recognized within the World Health Organization’s Revised Fourth Edition of the Classification of Tumors of the Hematopoietic and Lymphoid Tissues
[5] as germinal center B-cell like (GCB) and activated B-cell like (ABC), with the remainder left as unclassified. Novelty is never without controversy as other approaches, including an unbiased a priori approach which used supervised machine learning to analyze GEP, did not find molecular correlates of COO to be independently prognostic
[6]. Nevertheless, the same group reported differing GEP signatures that predicted response to CHOP chemotherapy (cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisone). Indeed, in that vein, groups like Rosenwald et al.
[7] reported cell specific and non-cell specific genetic signatures that differed depending on response to standard chemotherapies. Regardless of molecular prognostication and subtyping, molecular investigation was certain to provide increasing identification of precision therapeutic targets based on biochemical pathways
[4][6][7][8][4,6,7,8].
Despite the reasonable success of standard CHOP and R-CHOP, and the growing number of precision therapies, heterogeneity remained an issue, as evidenced by inconsistent treatment responses and relapses despite COO subtyping. Although gaining favor and resolving power, it was thought perhaps that the transcriptionally based GEP was an inadequate representation of underlying aberrant genetic programming (e.g., a DNA repair protein with single nucleotide polymorphism). However, practically, other technical hurdles, such as the logistical necessity for fresh tissue specimens, stood in the way of immediate clinical adoption of GEP in the subcategorization of DLBCLs. Initially, this was a difficult step to overcome, however several novel testing options were eventually able to surmount this issue, including Nanostring
[9], HTG
[10], and Roche
[11]. These options allowed the use of formalin fixed paraffin embedded (FFPE) tissues, which was in keeping with standard pathologic workflow and also allowed for an increase in analytical case numbers to be studied.
Meanwhile, through whole exome sequencing and transcriptome sequencing, oncogenic drivers of DLBCL were mapped
[12]. These findings reported that the most unfavorable prognoses were DLBCL cases with
MYC aberrations along with MYC IHC over-expression. Other studies using mouse modelling also supported the molecular ideas emerging from GEP, showing oncogenic driver mutations in genes such as
EZH2 and
MYD88 that promoted lymphoma development
[13][14][13,14]. Simultaneously on the molecular subclassification side, other analytic hurdles, such as setup and integration of the multiple platforms (e.g., whole-exome sequencing and RNA sequencing), were required to adequately identify different types of genetic aberrations, including mutations, translocations, and copy number alterations
[15][16][15,16].
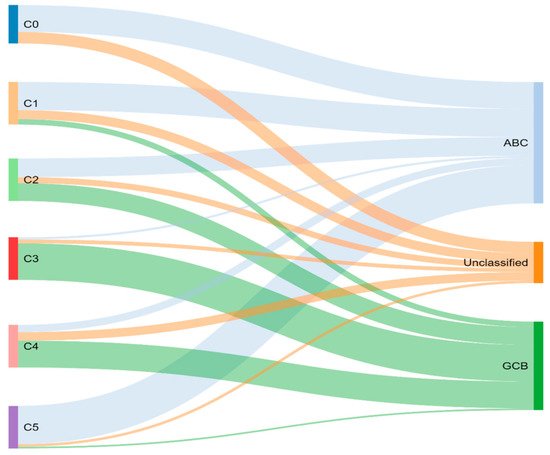
Through previously mentioned genomic methods, Chapuy et al.
[15] utilized clustering analytic methods to identify low-frequency alterations, captured recurrent mutations, somatic copy number alterations, and structural variants, and created five differential genetic signatures to further subtype DLBCL (
Figure 2). C1 was ABC-related, associated with
NOTCH2 mutations and favorable outcome; C2 was unrelated to GCB and ABC, had frequent biallelic
TP53 inactivation,
CDKN2A deletion, and poor outcomes; C3 was GCB-related, associated with
BCL2 translocation,
PTEN aberrations, epigenetic modifiers (
KMT2D,
CREBBP, and
EZH2) and unfavorable outcome; C4 was GCB-related, with BCR–PI3K, NF-κB, or RAS–JAK signal transducer, was an activator of transcription (BRAF and STAT3) pathway aberrations, histone gene mutations, cluster of differentiation proteins associated with immune evasion (CD83, CD70, and CD58), and had favorable outcomes; C5 was ABC related, gained
BCL2,
MYD88L265P,
CD79B,
PIM1, and
PRDM1 mutations, and had unfavorable outcome. The prognostic capability of the subtypes was also independent of the clinical gold standard IPI
[15].
Figure 2.
Chapuy and colleagues’ genetic cluster classification of DLBCL subtype, as compared to cell of origin. C0–C5 = cluster 0 to cluster 5.
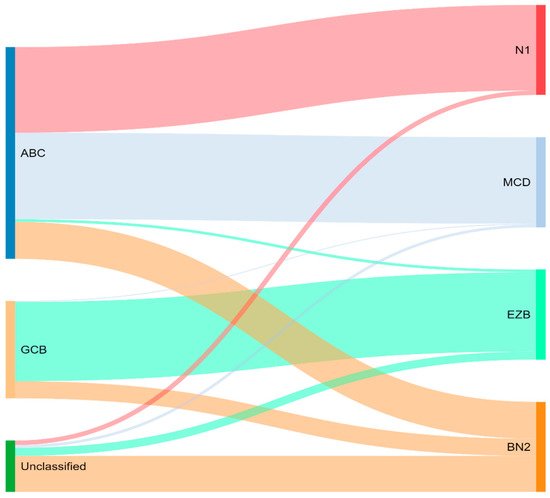
Concurrently, Schmitz et al.
[16] simultaneously characterized four DLBCL subsets via their GenClass algorithm, termed MCD (MYD88L265P, CD79B co-mutation), BN2 (BCL6 fusions or NOTCH2 mutation), N1 (NOTCH1 mutations), or EZB (EZH2 mutation or BCL2 translocation), based on a more homogenous set of genomic aberrations (
Figure 3). Interestingly, they were able to identify precision targets within the high-risk subtypes, showing, for example, that MCD could be more responsive to ibrutinib secondary to constitutive BCR signaling
[16].
Figure 3. Schmitz and colleagues’ genetic classification of DLBCL subtype as compared to cell of origin. ABC = activated B-cell, GCB = germinal-center B-cell, N1 = notch 1, MCD = MYD88L265P, CD79B co-mutation, EZB = EZH2 mutation or BCL2 translocation, and BN2 = Notch 2.
To compare, the Chapuy C1, C3, and C5 clusters overlapped with the Schmitz GenClass BN2, EZB, and MCD groups, respectively. The Chapuy C2 and C4 subtypings did not overlap with any of the other Schmitz subtypings. This non-concordance was perhaps thought secondary to differences in bioinformatic analytic approaches.
For the last two decades, R-CHOP has been the standard of treatment in previously untreated DLBCL. With the molecular subtyping of DLBCL, it was proposed that a subtype specific treatment could improve response rates specially for patients who do not achieve complete remission or develop disease relapse (around 40% treated with R-CHOP)
[17]. Ibrutinib, a first-in-class oral covalent inhibitor of Bruton’s tyrosine kinase (BTK) showed some preferential activity in ABC DLBCL
[18]. In a randomized multicenter study
[19], the goal was to determine if addition of ibrutinib would improve efficacy of R-CHOP in ABC DLBCL. Interestingly, the addition of ibrutinib to R-CHOP improved event-free survival and overall survival in patients younger than 60 years. Unfortunately, older patients (>60 years of age) had increased serious adverse effects with ibrutinib plus R-CHOP. Of note, molecular subtyping increased median time to diagnosis by 27 days, which may have excluded patients necessitating immediate treatment. Th
eis study shows the potential of subtype specific treatment as well the need for reasonable turnaround diagnostic times if integrating molecular subtyping. Subsequently, the PHOENIX trial continued to demonstrate superior outcomes in younger patients treated with ibrutinib and R-CHOP, but also better overall survival in specific molecular subsets including the MCD and N1 subgroups compared to R-CHOP alone
[20]. Landsburg and colleagues found that ibrutinib monotherapy had a 60% response rate in relapsed/refractory patients with a non-germinal center, and MYC and BCL2 double expressor phenotype
[21].
The ROBUST study is a phase 3 clinical trial that compared the addition of lenalidomide to rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) therapy with R-CHOP therapy alone for treatment of activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL). ABC-type DLBCL has traditionally been shown to resist typical R-CHOP therapy, however, emerging phase 2 studies are demonstrating promise of the addition of lenalidomide to R-CHOP (R2-CHOP) in ABC-type therapy. The primary end point of the study was progression-free survival (PFS) of participants receiving R2-CHOP, compared to those receiving R-CHOP only. Although PFS was not met (hazard ratio 0.85), the median PFS was not reached for either group. PFS tended to favor R2-CHOP over placebo group in patients with higher-risk disease, but adverse events of R2-CHOP compared to placebo were neutropenia (60% vs. 48%), anemia (22% vs. 14%), thrombocytopenia (17% vs. 11%), and leukopenia (14% vs. 15%). Of note, ROBUST was the first phase 3 study to highlight biomarker identification of ABC patients and was able to demonstrate a consistent safety profile of R2-CHOP.
Initial hopes were high that the development of molecular GEP would have significant effects on prognostic DLBCL classification, leading to therapeutic tailoring. With the advent of GEP, studies naturally attempted retrospective gene expression profiling analyses on their DLBCL cohorts, unfortunately, with conflicting results. Davies et al. (REMoDL-B) were the first to show that GEP for therapeutic assignment was possible prospectively
[22]. However, their randomized phase 3 clinical trial results were disappointing, reporting that the addition of bortezomib to suppress the GEP apparently increased NF-kB gene expression to standard R-CHOP, and failed to improve survival in ABC DLBCLs. While the trial itself was not without criticism, the study lent some doubt as to whether GEP classification was a prognostic and therapeutic breakthrough…