Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Rita Xu and Version 2 by Rita Xu.
The feeding of domestic animals with diets in which polyphenols are present is increasingly attracting the attention of nutritionists and scientists.
Free radical oxygen molecules are formed during aerobic cellular metabolism, containing one or more unpaired electrons. Free radicals can bind to various molecules and damage membranes, nucleic acids, and proteins. In recent years, the plants used in feed have served as sources of different bioactive compounds for animals. In addition, nutrient compounds play a very important role in protecting against the effects of free radicals.
- plant polyphenols
- reproduction
- antioxidant activity
1. Introduction
Free radical oxygen molecules are formed during aerobic cellular metabolism, containing one or more unpaired electrons. Free radicals can bind to various molecules and damage membranes, nucleic acids, and proteins [1]. In recent years, the plants used in feed have served as sources of different bioactive compounds for animals [2]. In addition, nutrient compounds play a very important role in protecting against the effects of free radicals [3][4]. An antioxidant dietary supplement can improve an animal’s productive status by altering metabolic processes. ROS (reactive oxygen species) production is a common physiological process in various organs, including the testes, which can lead to male infertility, although antioxidants can help [51][62]. It has been reported that antioxidants might be beneficial against the detrimental effects of leukocyte-derived ROS on sperm motility [73] (shown for vitamins C and E, dimethylsulfoxide, catalase, hypotaurine, N-acetylcysteine, and reduced glutathione) and function [84] (shown for ethylcysteine and vitamin E) due to the association of ROS overproduction with male infertility.
The reproductive rate significantly limits the livestock production efficiency. In the last two decades, there has been a growing interest in the use of herbal supplements in animal husbandry. Namely, the addition of herbs and their derivatives has improved animal health. This improvement is attributed to secondary plant metabolites or polyphenols. The findings to date confirm that polyphenols have antioxidant, immunomodulatory, antimutagenic, and anti-inflammatory effects. By restricting the use of antibiotics in livestock production, the use of plant polyphenols is increasingly being resorted to. The low intestinal absorption and low concentration in the target cells reduce their antioxidant effect. Researchers will look at previous research on the bioavailability of polyphenols in reproductive organs [95].
There is evidence of negative effects of feed with high levels of phytochemicals and polyphenols on animal homeostasis, especially on sheep reproduction [106] associated with a high rate of infertility, affecting embryo survival and fetal development. In 1982–1983, an analogous result, i.e., serious fertility disturbances indicating estrogenic stimulation, was obtained after feeding cows with red clover plants rich in isoflavones, a subclass of polyphenols [117]. Similar results were also reported in other studies in which domestic animals were fed with soybean or linseed [106].
There are few studies that provide detailed measurements of the effects of foods rich in polyphenols that can act as phytoestrogens on the reproduction of domestic animals. In the study by Mustonen et al. [128], two groups of sheep of equal size were monitored in an experiment. In another study [139], the effects of condensed tannins and saponin in animal fed supplementation on reproductive performance in Barki ewes were analyzed. The question of the presence, diversity and optimal concentration of polyphenols in traditional ruminant nutrition is raised. The answer to this question was given in the study by Fraisse et al. [1410], who analyzed the composition of chemicals in mountain pasture grass. This favorite nutrition of ruminants is genetically and epigenetically selected or optimized, together with rumen microflora, which helps and directs the digestion and absorption of nutrients. The presence of phytoestrogens in the milk of cows fed a polyphenol-rich diet was also studied [1511], and it was found that their concentration was much higher when the animals were fed by legumes.
Immune dysfunction is caused by various factors, including changes in relevant immune regulators and environmental stress, and nutrition may play an essential role in immunity by interfering with proinflammatory cytokine synthesis, immune cell regulation, and gene expression [1612]. Polyphenols can promote immunity to foreign pathogens via various pathways. Namely, different immune cells can express multiple types of receptors that recognize and allow the cellular uptake of polyphenols, thereby activating signaling pathways and initiating immune responses [1612]. Furthermore, the polyphenols can induce epigenetic changes in cells, and they can be used to regulate intestinal mucosal immune responses, allergic diseases, and antitumor immunity.
Indeed, the balance between the harms and benefits of the use of polyphenols could argue for the use of polyphenols in animals used for meat production, as possible negative effects disappear at the end of the animal’s production cycle [106]. The reproductive outcome may also be affected by improper polyphenol intake. Incorrect ingestion can affect offspring (due to changes affecting gene expression or programming) and the future health of offspring. These genetic changes can result not only from parenteral exposure but also from the use of assisted reproductive techniques (ART) [106]. Genetic changes can be observed during parental exposure, as well as with the use of ART. Polyphenols are used as antioxidants and antibacterial compounds in ART, which certainly includes protection in gamete or embryo breeding. The simultaneous addition of green tea polyphenol, IGF-1, and glucose to cattle in a maturation medium increased the intracellular glutathione concentration in oocytes after in vitro maturation and improved the embryonic development and blastocyst quality [1713].
The positive characteristics of polyphenol applications in vivo and in vitro should be further investigated before they are systematically used in practice as supplements to the basic livestock diet.
2. Polyphenols and Their Benefits
“Let food be your medicine, and medicine your food”, said Hippocrates more than 2000 years ago, showing that the benefits of natural sources of healthy food have been appreciated since ancient times [1814]. Plant foods, including fruits and vegetables containing active substances, play a key role in human and animal health. Plants synthesize polyphenols under stressful conditions during adaptation to their environment. Polyphenols are an important source of active substances in pharmaceutical products [1915]. The widespread use of polyphenols as secondary metabolites is an essential part of animal and human nutrition and is of great interest to scientists because of their biological properties. In the last few decades, scientists have been paying close attention to the health benefits of polyphenols [2016][2117]. Although the beneficial effects of polyphenols in both humans and animals have been confirmed, there are concerns regarding the potential health hazards of excessive polyphenol consumption [2218][2319]. The most vulnerable groups are pregnant animals and their fetuses [2420]. Therefore, it is essential to understand the impacts of plant polyphenol consumption on reproductive health. In plants, these compounds are usually synthesized as defenses against physiological and environmental stimuli. More attention has been paid in recent years to the benefits of polyphenols to human and animal health. This has been observed based on the chemical and biological activity and the obtained results [2521][2622][2723]. Polyphenols have an advantage over other substances due to their good availability, low toxicity, and specific activity, while their biggest disadvantage is their fast metabolism and low bioavailability. A complex mixture of polyphenols is found in food, and various factors affect their diversity, primarily environmental (e.g., rain, pedological soil composition, sun exposure) and biochemical (e.g., storage conditions, degree of maturity, and method of preparation) factors [95]. Glycosides and aglycones of polyphenols are the most important plant secondary metabolites in both human and animal nutrition, having significant health effects [2824][2925]. A review of the literature on polyphenols, which includes more than 20,000 papers, confirmed that a significant proportion of these molecules have inhibitory activity against enzymes, as well as antitumor, anti-inflammatory, antibacterial, and antifungal activities [3026][3127], reflecting the extensive benefits of polyphenols for the animal community, including improvements in memory and cognition in animals and humans [3228]. The bioavailability and kinetics of different polyphenols are very variable, so the knowledge of the fate of these compounds is quite unclear. In addition, based on their intensive metabolism in the gut and liver [3329][3430] of the parent compound, circulating metabolites very often differ from the parent compound, which further complicates the study of in vitro biological activity in animal models. From the above, it can be concluded that understanding the kinetics and bioavailability of polyphenols is crucial to know and understand the health benefits of these compounds.3. Division of Polyphenols and Their Sources in the Diet
The term “polyphenol” is used for compounds synthesized exclusively by the shikimin–phenylpropanoid and shikimin–polyketide pathways, which are constructed of more than one phenolic moiety and do not exhibit fundamental nitrogen functionality [3531]. Polyphenols are plants or synthetic compounds consisting of one or more phenolic units. Most of them are glycosylated or can bind to other phenols. In addition, they can conjugate with other compounds such as glucuronic acid, galacturonic acid, or glutathione during metabolism [3632]. The diversity and wide distribution of polyphenols in plants have led to different methods of categorizing these natural compounds [3531][3632]. Polyphenols are classified according to their source of origin, biological function, and chemical structure. In addition, most polyphenols in plants are present in the form of glycosides with different carbohydrate units and acylated carbohydrates at different positions of the polyphenolic scaffold. The classification of polyphenols in this entry is based on the chemical structure of the aglycone. Thus, polyphenols are divided into two main groups, namely flavonoids and non-flavonoids [3531].3.1. Flavonoids
The class of flavonoids contains more than 4000 low molecular weight secondary plant metabolites. They are formed from aromatic amino acids [3632][3733]. Flavonoids are classified into flavonols, flavones, flavanols, flavanones, anthocyanins, isoflavones, and proanthocyanidins (Figure 1) [3733][3834].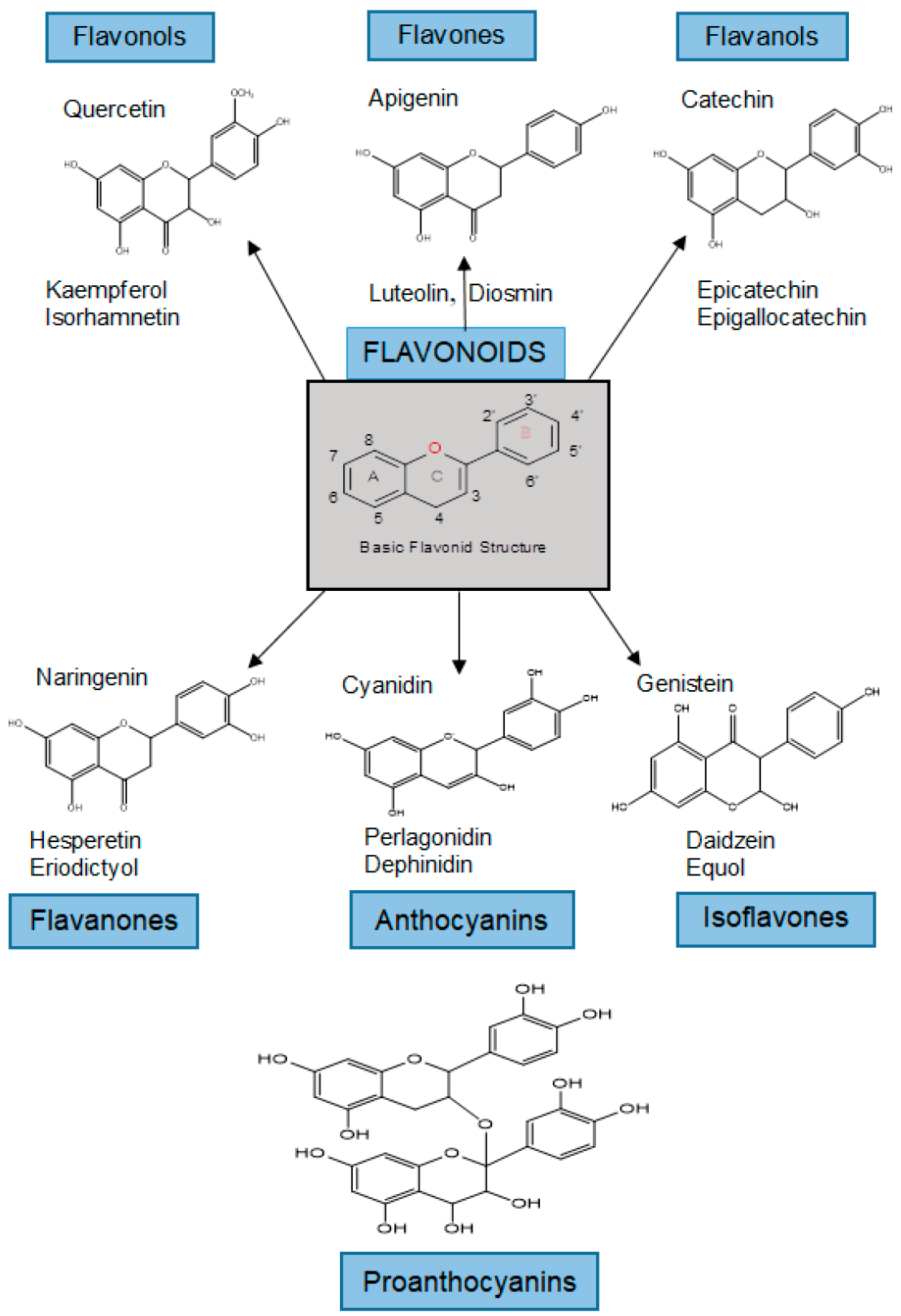
Figure 1. Classification and examples of structures of flavonoids.
3.2. Non-Flavonoids
Non-flavonoids can be classified into lower molecular weight compounds such as phenolic acids, lignans, and stilbenes, and more complex structures such as tannins (Figure 2).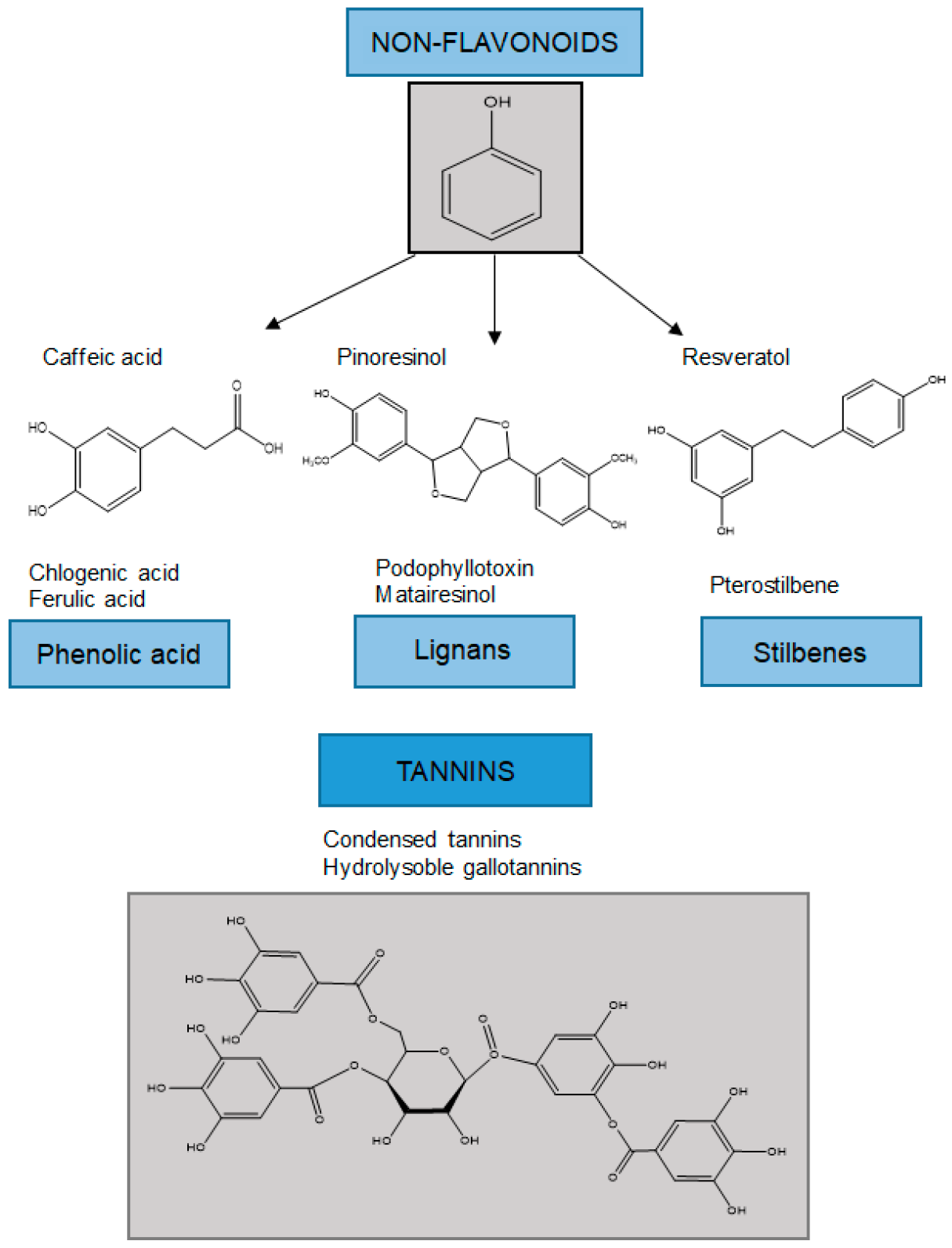
Figure 2. Classification of non-flavonoids and examples of their chemical structures.
3.2.1. Phenolic Acids
Phenolic acids are abundant in food and are divided into two classes: benzoic acid derivatives and cinnamic acid derivatives. Phenolic acids and flavonoids are the most abundant polyphenols in foods—they account for about one-third and two-thirds of the total sources, respectively [3632][3733]. The content of hydroxybenzoic acid in edible plants is generally low, except in some red fruits, black radish, and onions, which may have concentrations of several tens of mg/kg fresh mass. Hydroxycinnamic acids are more common than hydroxybenzoic acids and consist mainly of p-coumaric, caffeic, ferulic, synaptic, chlorogenic, and rosmarinic acids [3834].3.2.2. Stilbenes
Stilbenes are a small and important class of non-flavonoid polyphenols characterized by a 14-carbon skeleton. They are built of two benzene rings connected by an ethylene bridge (Figure 1) [3733]. In the central part of the structure, two aromatic rings are connected to ethene, and ethene hydrogen can be in the cis and trans positions. In nature, stilbenes occur most frequently in the form of trans stereoisomers. To date, more than 400 different stilbene compounds have been identified, most of which are derived from trans resveratrol (3,5,4′-trihydroxy-trans stilbene). Due to the complexity of qualitative–quantitative stilbene analysis, most studies have focused on simple stilbenes, such as resveratrol, piceid, pterostilbene, and piceatannol. The knowledge on stilbenes mainly relates to the protection of plants against biotic (phytopathogenic microorganisms and herbivores) and abiotic (e.g., radiation and tropospheric ozone) stress. In this way, they repel attacks by having a direct toxic effect on the pathogen, while on the other hand they act as antioxidants and protect the cells from oxidative stress [3733].3.2.3. Lignans
Lignans are diphenolic compounds that contain a 2,3-dibenzylbutane structure formed by the dimerization of two cinnamic acid residues (Figure 1). The richest source is flaxseed, which contains secoisolariciresinol and matairesinol. One of the most common forms of lignans is secoisolariciresinol (2-(4-hydroxy-3-methoxybenzyl)-3-(3-metoxybenzyl)butene-1-4-diol) diglycoside. Seicoisolariciresinol and matairesinol ingested with food are converted by the intestinal microflora into mammalian lignans, enterodiol and enterolactone, which are absorbed via the enterohepatic circulation. Mammalian lignans have a chemical structure similar to natural estrogen, and are thought to act as selective modulators of estrogen receptors and to have anticancer activity [4036].References
- Aladag, I.; Eyibilen, A.; Guven, M.; Atis, O.; Erkorkmaz, U. Role of oxidative stress in hearing impairment in patients with type two diabetes mellitus. J. Laryngol. Otol. 2009, 123, 957–963. O’Connell, A.R.; Demmers, K.J.; Smaill, B.; Reader, K.L.; Juengel, J.L. Early embryo loss, introduction morphology, and effect of previous immunization against androstenedione in the ewe. Theriogenology 2016, 86, 1285–1293.
- Pellerrini, N.M.; Serfini, B.; Colombi, D.D.; Rio, S.T.; Salvotore, R.A.; Bionchii, W.O. Total antioxidant capacity of plant foods, beverages and oils consumed in italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. Sinclair, S. Male infertility: Nutritional and environmental considerations. Altern. Med. Rev. 2000, 5, 28–38.
- Sikora, E.; Ewa, C.; Toploska, K. The sources of natural antioxidants. Acta Sci. Pol. Technol. Alimen. 2008, 7, 5–17. Baker, H.W.; Brindle, J.; Irvine, D.S.; Aitken, R.J. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil. Steril. 1996, 65, 411–419.
- Elwan, A.M.H.; Dawood, D.H.; El-Shafei, S.A.M.E.; El-Rahman, A.A.E.A.; Abdel-Latif, S.A.; Mohany, M.; Alqahtani, F.; Alqahtani, S.; Al-Rejaie, S.S. The Potential Role of Citrus limon Powder as a Natural Feed Supplement to Boost the Productive Performance, Antioxidant Status, and Blood Biochemistry of Growing Rabbits. Animals 2019, 9, 426. Akiyama, M. In Vivo scavenging effect of ethycysteine on reactive oxygen species in human semen. Nihon Hinyokika Gakkai. Zassh. 1999, 90, 421–428.
- O’Connell, A.R.; Demmers, K.J.; Smaill, B.; Reader, K.L.; Juengel, J.L. Early embryo loss, introduction morphology, and effect of previous immunization against androstenedione in the ewe. Theriogenology 2016, 86, 1285–1293. D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342.
- Sinclair, S. Male infertility: Nutritional and environmental considerations. Altern. Med. Rev. 2000, 5, 28–38. Hashem, N.M.; Gonzalez-Bulnes, A.; Simal-Gandara, J. Polyphenols in Farm. Source of Reproductive Gain or Waste? Antioxidants 2020, 9, 1023.
- Baker, H.W.; Brindle, J.; Irvine, D.S.; Aitken, R.J. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil. Steril. 1996, 65, 411–419. Kallela, K.; Heinonen, K.; Saloniemi, H. Plant oestrogens; the cause of decreased fertility in cows. A case report. Nord. Vet. 1984, 36, 124–129.
- Akiyama, M. In Vivo scavenging effect of ethycysteine on reactive oxygen species in human semen. Nihon Hinyokika Gakkai. Zassh. 1999, 90, 421–428. Mustonen, E.; Taponen, S.; Andersson, M.; Sukura, A.; Katila, T.; Taponen, J. Fertility and growth of nulliparous ewes after feeding red clover silage with high phyto-oestrogen concentrations. Animal 2014, 8, 1699–1705.
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. Baheg, R.M.A.; El-Bahrawy, K.A.; El-Azrak, K.M.; Samak, M.A.; Sallam, S.M. Effect of condensed tannins and saponin supplementation on reproductive performance in Barki ewes. Egypt. J. Nutr. Feed. 2017, 20, 197–210.
- Hashem, N.M.; Gonzalez-Bulnes, A.; Simal-Gandara, J. Polyphenols in Farm. Source of Reproductive Gain or Waste? Antioxidants 2020, 9, 1023. Fraisse, D.; Carnat, A.; Viala, D.; Pradel, P.; Besle, J.-M.; Coulon, J.-B.; Felgines, C.; Lamaison, J.-L. Polyphenolic Composition of a Permanent Pasture: Variations Related to the Period of Harvesting. J. Sci. Food Agric. 2007, 87, 2427–2435.
- Kallela, K.; Heinonen, K.; Saloniemi, H. Plant oestrogens; the cause of decreased fertility in cows. A case report. Nord. Vet. 1984, 36, 124–129. Andersen, C.; Nielsen, T.S.; Purup, S.; Kristensen, T.; Eriksen, J.; Søegaard, K.; Sørensen, J.; Frette, X.C. Phyto-oestrogens in herbage and milk from cows grazing white clover, red clover, lucerne or chicory-rich pastures. Animal 2009, 3, 1189–1195.
- Mustonen, E.; Taponen, S.; Andersson, M.; Sukura, A.; Katila, T.; Taponen, J. Fertility and growth of nulliparous ewes after feeding red clover silage with high phyto-oestrogen concentrations. Animal 2014, 8, 1699–1705. Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074.
- Baheg, R.M.A.; El-Bahrawy, K.A.; El-Azrak, K.M.; Samak, M.A.; Sallam, S.M. Effect of condensed tannins and saponin supplementation on reproductive performance in Barki ewes. Egypt. J. Nutr. Feed. 2017, 20, 197–210. Piras, A.-R.; Menéndez-Blanco, I.; Soto-Heras, S.; Catalá, M.-G.; Izquierdo, D.; Bogliolo, L.; Paramio, M.-T. Resveratrol supplementation during in vitro maturation improves embryo development of prepubertal goat oocytes selected by brilliant cresyl blue staining. J. Reprod. Dev. 2019, 65, 113–120.
- Fraisse, D.; Carnat, A.; Viala, D.; Pradel, P.; Besle, J.-M.; Coulon, J.-B.; Felgines, C.; Lamaison, J.-L. Polyphenolic Composition of a Permanent Pasture: Variations Related to the Period of Harvesting. J. Sci. Food Agric. 2007, 87, 2427–2435. Wegener, G. Let Food Be Thy Medicine, and Medicine Be Thy Food: Hippocrates Revisited. Acta Neuropsychiatr. 2014, 26, 1–3.
- Andersen, C.; Nielsen, T.S.; Purup, S.; Kristensen, T.; Eriksen, J.; Søegaard, K.; Sørensen, J.; Frette, X.C. Phyto-oestrogens in herbage and milk from cows grazing white clover, red clover, lucerne or chicory-rich pastures. Animal 2009, 3, 1189–1195. Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-Radical Scavenging Activity Relationships of Flavonoids. Croat. Chem. Acta 2003, 76, 55–61.
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. Visioli, F.; Alarcon-De-La-Lastra, C.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and Human Health: A Prospectus. Crit. Rev. Food. Sci. Nutr. 2011, 51, 524–546.
- Piras, A.-R.; Menéndez-Blanco, I.; Soto-Heras, S.; Catalá, M.-G.; Izquierdo, D.; Bogliolo, L.; Paramio, M.-T. Resveratrol supplementation during in vitro maturation improves embryo development of prepubertal goat oocytes selected by brilliant cresyl blue staining. J. Reprod. Dev. 2019, 65, 113–120. Lu, C.; Zhu, W.; Shen, C.-L.; Gao, W. Green Tea Polyphenols Reduce Body Weight in Rats by Modulating Obesity-Related Genes. PLoS ONE 2012, 7, e38332.
- Wegener, G. Let Food Be Thy Medicine, and Medicine Be Thy Food: Hippocrates Revisited. Acta Neuropsychiatr. 2014, 26, 1–3. Pinent, M.; Blay, M.; Serrano, J.; Ardévol, A. Effects of Flavanols on the Enteroendocrine System: Repercussions on Food Intake. Crit. Rev. Food Sci. Nutr. 2017, 57, 326–334.
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-Radical Scavenging Activity Relationships of Flavonoids. Croat. Chem. Acta 2003, 76, 55–61. Chavarro, J.E.; Toth, T.L.; Sadio, S.M.; Hauser, R. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum. Reprod. 2008, 23, 2584–2590.
- Visioli, F.; Alarcon-De-La-Lastra, C.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and Human Health: A Prospectus. Crit. Rev. Food. Sci. Nutr. 2011, 51, 524–546. Zielinsky, P.; Piccoli, A.P., Jr.; Manica, J.L.; Nicoloso, L.H.; Menezes, H.; Busato, A.; Moraes, M.R.; Silva, J.; Bender, L.; Pizzato, P.; et al. Maternal consumption of polyphenol-rich foods in late pregnancy and fetal ductus arteriosus flow dynamics. J. Perinatol. 2010, 30, 17–21.
- Lu, C.; Zhu, W.; Shen, C.-L.; Gao, W. Green Tea Polyphenols Reduce Body Weight in Rats by Modulating Obesity-Related Genes. PLoS ONE 2012, 7, e38332. Jacobsen, B.K.; Jaceldo-Siegl, K.; Knutsen, S.F.; Fan, J.; Oda, K.; Fraser, G.E. Soy isoflavone intake and the likelihood of ever becoming a mother: The Adventist Health Study-2. Int. J. Womens Health 2014, 6, 377–384.
- Pinent, M.; Blay, M.; Serrano, J.; Ardévol, A. Effects of Flavanols on the Enteroendocrine System: Repercussions on Food Intake. Crit. Rev. Food Sci. Nutr. 2017, 57, 326–334. Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211.
- Chavarro, J.E.; Toth, T.L.; Sadio, S.M.; Hauser, R. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum. Reprod. 2008, 23, 2584–2590. Zhang, H.; Tsao, R. Dietary Polyphenols. Oxidative Stress Antioxidant Anti-Inflammatory Effects. Cur. Opinion. Food Sci. 2016, 8, 33–42.
- Zielinsky, P.; Piccoli, A.P., Jr.; Manica, J.L.; Nicoloso, L.H.; Menezes, H.; Busato, A.; Moraes, M.R.; Silva, J.; Bender, L.; Pizzato, P.; et al. Maternal consumption of polyphenol-rich foods in late pregnancy and fetal ductus arteriosus flow dynamics. J. Perinatol. 2010, 30, 17–21. Amić, D.; Davidović-Amić, D.; Bešlo, D.; Lučić, B.; Trinajstić, N. Prediction of pK Values, Half-Lives, and Electronic Spectra of Flavylium Salts from Molecular Structure. J. Chem. Inf. Comput. Sci. 1999, 39, 967–973.
- Jacobsen, B.K.; Jaceldo-Siegl, K.; Knutsen, S.F.; Fan, J.; Oda, K.; Fraser, G.E. Soy isoflavone intake and the likelihood of ever becoming a mother: The Adventist Health Study-2. Int. J. Womens Health 2014, 6, 377–384. Xiao, J. Phytochemicals in Food and Nutrition. Crit. Rev. Food Sci. Nutr. 2016, 56, S1–S3.
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food Prop. 2017, 20, 1700–1741.
- Zhang, H.; Tsao, R. Dietary Polyphenols. Oxidative Stress Antioxidant Anti-Inflammatory Effects. Cur. Opinion. Food Sci. 2016, 8, 33–42. Amić, D.; Davidović-Amić, D.; Bešlo, D.; Lučić, B.; Trinajstić, N. The Use of the Ordered Orthogonalized Multivariate Linear Regression in a Structure−Activity Study of Coumarin and Flavonoid Derivatives as Inhibitors of Aldose Reductase. J. Chem. Inf. Comput. Sci. 1997, 37, 581–586.
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Lučić, B.; Trinajstić, N. Prediction of pK Values, Half-Lives, and Electronic Spectra of Flavylium Salts from Molecular Structure. J. Chem. Inf. Comput. Sci. 1999, 39, 967–973. Rendeiro, C.; Vauzour, D.; Rattray, M.; Waffo-Téguo, P.; Mérillon, J.M.; Butler, L.T.; Williams, C.M.; Spencer, J.P. Dietary Levels of Pure Flavonoids Improve Spatial Memory Performance and Increase Hippocampal Brain-Derived Neurotrophic Factor. PLoS ONE 2013, 8, e63535.
- Xiao, J. Phytochemicals in Food and Nutrition. Crit. Rev. Food Sci. Nutr. 2016, 56, S1–S3. Amić, A.; Marković, Z.; Dimitrić Marković, J.M.; Lučić, B.; Stepanić, V.; Amić, D. The 2H+/2e- free radical scavenging mechanisms of uric acid: Thermodynamics of N-H bond cleavage. Comput. Theor. Chem. 2016, 1077, 2–10.
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food Prop. 2017, 20, 1700–1741. Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Lučić, B.; Trinajstić, N. The Use of the Ordered Orthogonalized Multivariate Linear Regression in a Structure−Activity Study of Coumarin and Flavonoid Derivatives as Inhibitors of Aldose Reductase. J. Chem. Inf. Comput. Sci. 1997, 37, 581–586. Tsao, R. Chemistry and biochemistry dierary polyphenols. Nutrients 2010, 2, 1231–1246.
- Rendeiro, C.; Vauzour, D.; Rattray, M.; Waffo-Téguo, P.; Mérillon, J.M.; Butler, L.T.; Williams, C.M.; Spencer, J.P. Dietary Levels of Pure Flavonoids Improve Spatial Memory Performance and Increase Hippocampal Brain-Derived Neurotrophic Factor. PLoS ONE 2013, 8, e63535. Ly, C.; Yockell-Lelievre, J.; Ferraro, Z.M.; Arnason, J.T.; Ferrier, J.; Gruslin, A. The effects of dietary polyphenols on reproductive health and early development. Hum. Reprod. Update 2015, 21, 228–248.
- Amić, A.; Marković, Z.; Dimitrić Marković, J.M.; Lučić, B.; Stepanić, V.; Amić, D. The 2H+/2e- free radical scavenging mechanisms of uric acid: Thermodynamics of N-H bond cleavage. Comput. Theor. Chem. 2016, 1077, 2–10. Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plant 2021, 10, 90.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278.
- Tsao, R. Chemistry and biochemistry dierary polyphenols. Nutrients 2010, 2, 1231–1246. Pal, S.; Saha, C. A Review on Structure–Affinity Relationship of Dietary Flavonoids with Serum Albumins. J. Biomol. Struct. 2014, 32, 1132–1147.
- Ly, C.; Yockell-Lelievre, J.; Ferraro, Z.M.; Arnason, J.T.; Ferrier, J.; Gruslin, A. The effects of dietary polyphenols on reproductive health and early development. Hum. Reprod. Update 2015, 21, 228–248. Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–9.
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plant 2021, 10, 90.
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278.
- Pal, S.; Saha, C. A Review on Structure–Affinity Relationship of Dietary Flavonoids with Serum Albumins. J. Biomol. Struct. 2014, 32, 1132–1147.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–9.
More
