Echinacea purpurea (L.) Moench (EP)is a perennial herbaceous flowering plant, commonly known as purple coneflower and it belongs to the Asteraceae family. The Echinacea genus is originally from North America, in the United States, and its species are widely distributed throughout. There are nine different species of Echinacea, but only three of them are used as medicinal plants with wide therapeutic uses: Echinacea purpurea (L.) Moench, Echinacea pallida (Nutt.) Nutt. and Echinacea angustifolia DC. Several significant groups of bioactive compounds with pharmacological activities have been isolated from Echinacea species. Numerous beneficial effects have been demonstrated about these compounds.
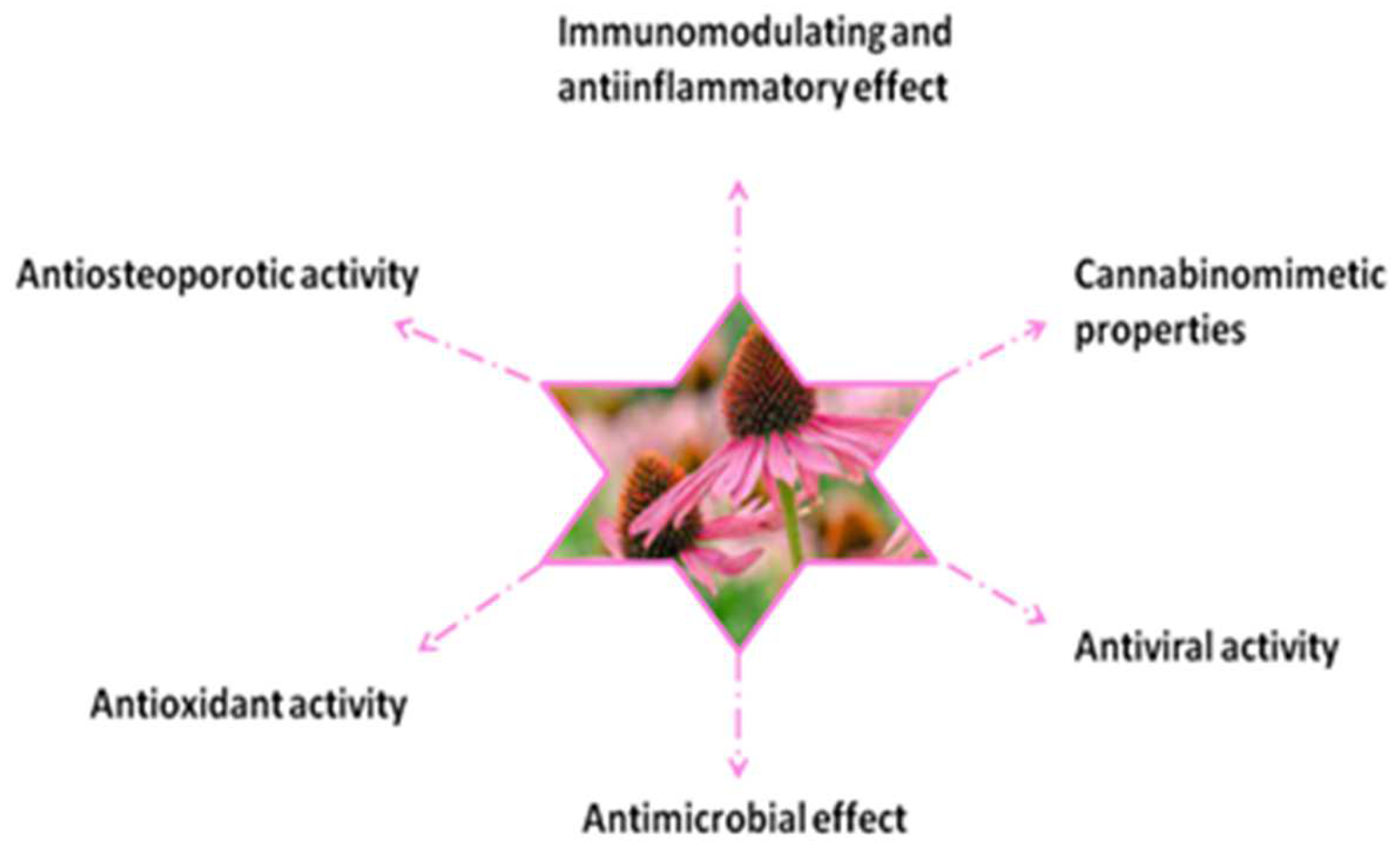
- Echinacea purpurea (L.) Moench
- bioactive compounds
- immunomodulatory
- cannabinomimetic
- anti-inflammatory
- antiviral
- antimicrobial
- antioxidant effect
1. Introduction
2. Bioactive Compounds of Echinacea purpurea (L.) Moench
Several significant groups of bioactive compounds, with pharmacological activities, have been isolated from Echinacea species. The most important components of Echinacea purpurea (L.) Moench are alkylamides, polysaccharides, glycoproteins, flavonoids and phenolic compounds, which include [22][14] derivates of caffeic acid, like caffeic acid, chicoric acid, caftaric acid, chlorogenic acid and echinacoside, Figure 2, [23[15][16],24], whose amounts vary based on the plant's sections. In addition to these components, the weresearchers also identified thatphylloxanthobilins, β-phellandrene, acetaldehyde, dimethyl sulfide, camphene, hexanal, α-pinene and limonene are present in all plant tissues, regardless of species. Fatty acids, aldehydes and terpenoidsare constituents whose presence depend on the parts of plants used [25,26,27,28,29,30,31,32,33,34,35,36,37,38][17][18][19][20][21][22][23][24][25][26][27][28][29][30].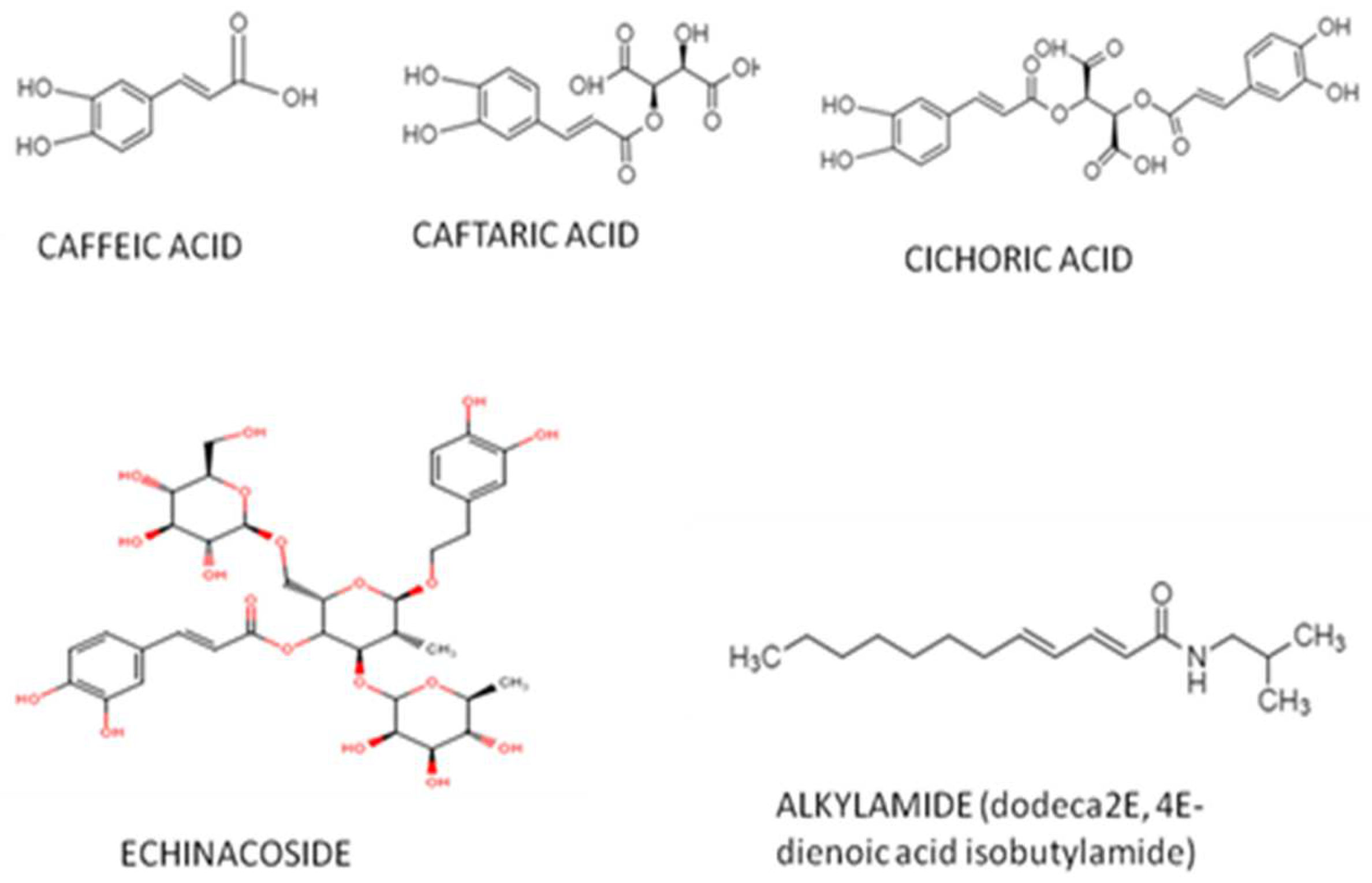
|
Storage Condition |
Storage Temperature |
|---|
3. Biological and Pharmacological Effects of Echinacea purpurea (L.) Moench
Currently, the number of herbs that are subject to scientific studies is increasing. The well-known medicinal plants are intensively studied to obtain the most accurate data about the chemical composition, the pharmacological effects and the safety of use in therapy [73][65]. Table 2 summarizes the most important components identified in Echinacea purpurea (L.) Moench and the scientifically proven biological and pharmacological effects, according to the literature. It can be seen that most of the demonstrated effects are common to several compounds, such as the immunomodulatory, antioxidant or antimicrobial effects.|
Bioactive Compounds |
Biological and Pharmacological Effects | Alchylamides Concentration |
Chicoric Acid Concentration |
Ref. |
|
|---|---|---|---|---|---|
References | |||||
|
60 days in the dark |
5 °C |
||||
|
Alkylamides |
Anti-inflammatory | unchanged |
70% decrease |
||
|
60 days in the light |
20 °C |
65% decrease |
unchanged |
|
Immunomodulatory |
||
|
Modulation of macrophages |
||
|
Reduction of NO and tumor necrosis factor -α |
||
|
Mediators of antiviral immunity |
||
|
Cannabinoid receptor type 2 |
||
|
Polysaccharides |
Antitumoral |
[82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90] |
|
Antioxidant |
||
|
Antimicrobial |
||
|
Antifungal |
||
|
Antiviral |
||
|
Immunomodulatory |
||
|
Hypoglycemic |
||
|
Hepatoprotective |
||
|
Gastrointestinal-protective |
||
|
Antidiabetic |
||
|
Glycoproteins |
Immunomodulatory |
|
|
Flavonoids |
Antioxidant activity |
|
|
Anti-inflammatory |
||
|
Anti-ulcer activity |
||
|
Antiallergic |
||
|
Antiviral |
||
|
Caffeic acid derivatives |
Antioxidant activity |
[110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118] |
|
Antiosteoporotic activity |
||
|
Anti-inflammatory |
||
|
Antimicrobial |
||
|
Anti-tumoral |
||
|
Neuroprotective action |
References
- Salmeron-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376.
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin Wound Healing and Phytomedicine: A Review. Ski. Pharmacol. Physiol. 2014, 27, 303–310.
- Hegedűs, C.; Muresan, M.; Badale, A.; Bombicz, M.; Varga, B.; Szilágyi, A.; Sinka, D.; Bácskay, I.; Popoviciu, M.; Magyar, I.; et al. SIRT1 Activation by Equisetum arvense L. (Horsetail) Modulates Insulin Sensitivity in Streptozotocin Induced Diabetic Rats. Molecules 2020, 25, 2541.
- Stanisavljević, I.; Stojičević, S.; Veličković, D.; Veljković, V.; Lazic, M. Antioxidant and Antimicrobial Activities of Echinacea (Echinacea purpurea L.) Extracts Obtained by Classical and Ultrasound Extraction. Chin. J. Chem. Eng. 2009, 17, 478–483.
- Kaya, M.; Merdivan, M.; Tashakkori, P.; Erdem, P.; Anderson, J.L. Analysis of Echinacea flower volatile constituents by HS-SPME-GC/MS using laboratory-prepared and commercial SPME fibers. J. Essent. Oil Res. 2018, 31, 91–98.
- Lekar’, A.V.; Borisenko, S.N.; Filonova, O.V.; Vetrova, E.V.; Maksimenko, E.V.; Borisenko, N.I.; Minkin, V.I. Extraction of caftaric and cichoric acids from Echinacea purpurea L. in subcritical water. Russ. J. Phys. Chem. B 2013, 7, 968–975.
- Nadaf, M.; Joharchi, M.R.; Amiri, M.S. Ethnomedicinal uses of plants for the treatment of nervous disorders at the herbal markets of Bojnord, North Khorasan Province, Iran. J. Phytomedicine 2018, 9, 153–163.
- Cao, C.; Kindscher, K. The Medicinal Chemistry of Echinacea Species. Echinacea 2016, 127–145.
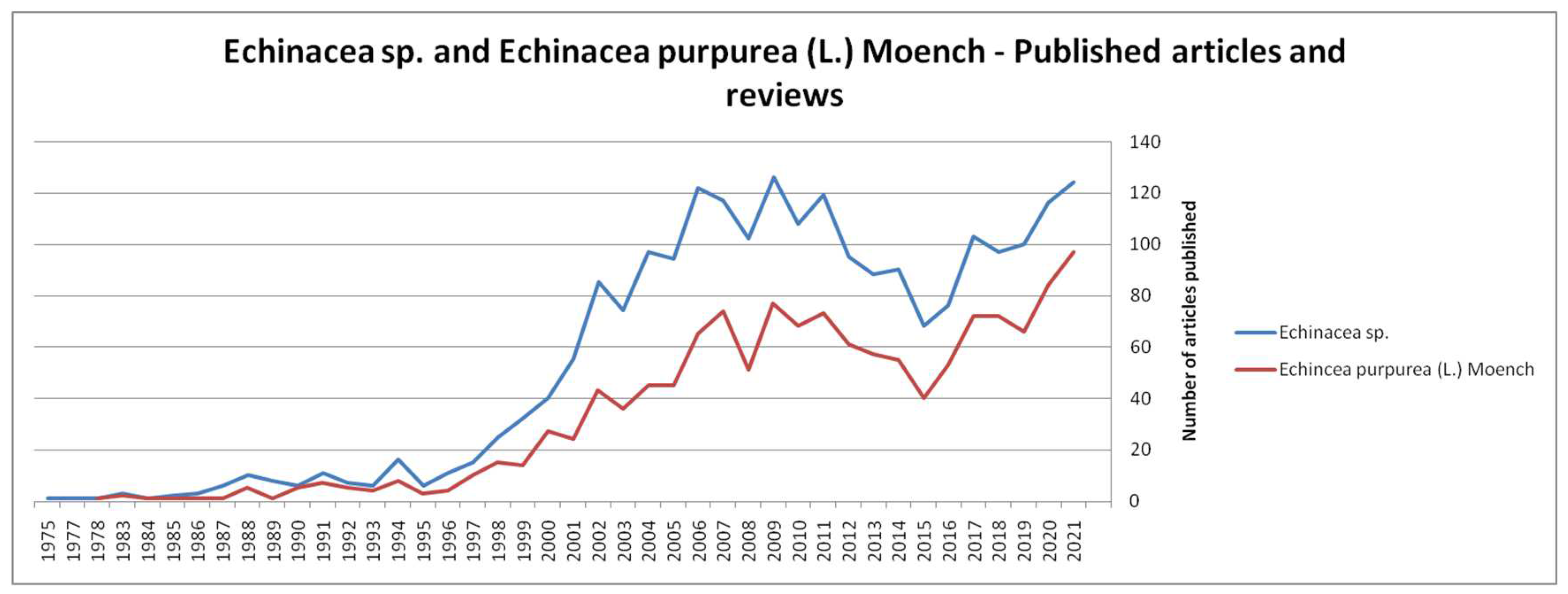
- Scopus Database. Available online: https://www-scopus-com.am.e-nformation.ro/results/results.uri?sort=plf-f&src=s&st1=Echinacea&sid=1265d6dbcfd9127a51f79e0747ea36dd&sot=b&sdt=b&sl=24&s=TITLE-ABS-KEY%28Echinacea%29&origin=searchbasic&editSaveSearch=&yearFrom=Before+1960&yearTo=Present (accessed on 9 March 2022).
- Web of Science Database. Available online: https://www-webofscience-com.am.e-nformation.ro/wos/woscc/summary/cc619678-4982-4090-8c17-ca37d828cff1-3348de15/relevance/1 (accessed on 10 March 2022).
- Mistríková, I.; Vaverková, Š. Morphology and anatomy of Echinacea purpurea, E. angustifolia, E. pallida and Parthenium integrifolium. Biologia 2007, 62, 2–5.
- Awang, D.V.C.; Kindack, D.G. Herbal medicine: Echinacea. Can. Pharm. J. 1991, 124, 512–516.
- McGregor, R. The taxonomy of the genus Echinacea (Compositae). University of Kansas. Sci. Bull. 1968, 48, 113–142.
- Harborne, J.B.; Williams, C.A. Phytochemistry of the Genus Echinacea, Echinacea; CRC Press: Boca Raton, FL, USA, 2004; pp. 71–88.
- Attarzadeh, M.; Balouchi, H.; Rajaie, M.; Dehnavi, M.M.; Salehi, A. Improving growth and phenolic compounds of Echinacea purpurea root by integrating biological and chemical resources of phosphorus under water deficit stress. Ind. Crop. Prod. 2020, 154, 112763.
- Bruni, R.; Brighenti, V.; Caesar, L.K.; Bertelli, D.; Cech, N.B.; Pellati, F. Analytical methods for the study of bioactive compounds from medicinally used Echinacea species. J. Pharm. Biomed. Anal. 2018, 160, 443–477.
- Pallag, A.; Bungau, S.; Tit, M.D.; Jurca, T.M.; Sirbu, V.; Honiges, A.; Horhogea, C. Comparative Study of Polyphenols, Flavonoids and Chlorophylls in Equisetum arvense L. Populations. Revista Chimie 2016, 67, 350–353.
- Banica, F.; Bungau, S.; Tit, D.M.; Behl, T.; Otrisal, P.; Nechifor, A.C.; Gitea, D.; Pavel, F.-M.; Nemeth, S. Determination of the Total Polyphenols Content and Antioxidant Activity of Echinacea Purpurea Extracts Using Newly Manufactured Glassy Carbon Electrodes Modified with Carbon Nanotubes. Processes 2020, 8, 833.
- Bauer, R.; Wagner, H.; Hikano, H.; Farnsworth, N.R. Echinacea species as potential immunostimulatory drugs. Econ. Med. Plant Res. 1991, 5, 253–321.
- Abdelmohsen, M.M.; Nafiz, N.M.; Seif el Nasr, M.M. Microwave assisted extraction of bio-active compounds (phenolics and alkamides) from Echinacea purpurea. Int. J. Pharma Pharma Sci. 2014, 6, 265–268.
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954.
- de Oliveira, B.G.; Santos, L.F.F.; Pianetti, G.A.; César, I.C. A Rapid UPLC Method for the Simultaneous Quantitation of Caffeic Acid Derivatives in Dried Extracts of Echinacea Purpurea. J. Chromatogr. Sci. 2021, 59, 439–444.
- Vendramin, V.; Viel, A.; Vincenzi, S. Caftaric Acid Isolation from Unripe Grape: A “Green” Alternative for Hydroxycinnamic Acids Recovery. Molecules 2021, 26, 1148.
- Ramezannezhad, R.; Aghdasi, M.; Fatemi, M. Enhanced production of cichoric acid in cell suspension culture of Echinacea purpurea by silver nanoparticle elicitation. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 139, 261–273.
- Attarzadeh, M.; Balouchi, H.; Rajaie, M.; Dehnavi, M.M.; Salehi, A. Growth and nutrient content of Echinacea purpurea as affected by the combination of phosphorus with arbuscular mycorrhizal fungus and Pseudomonas florescent bacterium under different irrigation regimes. J. Environ. Manag. 2018, 231, 182–188.
- Maggini, V.; De Leo, M.; Granchi, C.; Tuccinardi, T.; Mengoni, A.; Gallo, E.R.; Biffi, S.; Fani, R.; Pistelli, L.; Firenzuoli, F.; et al. The influence of Echinacea purpurea leaf microbiota on chicoric acid level. Sci. Rep. 2019, 9, 1–11.
- Tabar, R.S.; Moieni, A.; Monfared, S.R. Improving biomass and chicoric acid content in hairy roots of Echinacea purpurea L. Biologia 2019, 74, 941–951.
- Dalby-Brown, L.; Barsett, H.; Landbo, A.-K.R.; Meyer, A.A.S.; Mølgaard, P. Synergistic Antioxidative Effects of Alkamides, Caffeic Acid Derivatives, and Polysaccharide Fractions from Echinacea purpurea on in Vitro Oxidation of Human Low-Density Lipoproteins. J. Agric. Food Chem. 2005, 53, 9413–9423.
- Kakimov, A.; Muratbayev, A.; Zharykbasova, K.; Amanzholov, S.; Mirasheva, G.; Kassymov, S.; Utegenova, A.; Jumazhanova, M.; Shariati, M.A. Heavy metals analysis, GCMS-QP quantification of flavonoids, amino acids and saponins, analysis of tannins and organoleptic properties of powder and tincture of Echinacea purpurea (L.) and Rhapónticum carthamoídes. Potravinarstvo Slovak J. Food Sci. 2021, 15, 330–339.
- Nyalambisa, M.; Oyemitan, I.; Matewu, R.; Oyedeji, O.; Oluwafemi, O.; Songca, S.P.; Nkeh-Chungag, B.N. Volatile constituents and biological activities of the leaf and root of Echinacea species from South Africa. Saudi Pharm. J. 2016, 25, 381–386.
- Balciunaite, G.; Haimi, P.-J.; Mikniene, Z.; Savickas, G.; Ragazinskiene, O.; Juodziukyniene, N.; Baniulis, D.; Pangonyte, D. Identification of Echinacea Purpurea (L.) Moench Root LysM Lectin with Nephrotoxic Properties. Toxins 2020, 12, 88.
- Kumar, K.M.; Ramaiah, S. Pharmacological importance of Echinacea purpurea. Int. J. Pharm. Biol. Sci. 2011, 2, 305–314.
- Liu, C.-Z.; Abbasi, B.H.; Gao, M.; Murch, A.S.J.; Saxena, P.K. Caffeic Acid Derivatives Production by Hairy Root Cultures of Echinacea purpurea. J. Agric. Food Chem. 2006, 54, 8456–8460.
- Erkoyuncu, M.T.; Yorgancilar, M. Optimization of callus cultures at Echinacea purpurea L. for the amount of caffeic acid derivatives. Electron. J. Biotechnol. 2021, 51, 17–27.
- Pellati, F.; Benvenuti, S.; Magro, L.; Melegari, M.; Soragni, F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. J. Pharm. Biomed. Anal. 2004, 35, 289–301.
- Thygesen, L.; Thulin, J.; Mortensen, A.; Skibsted, L.H.; Molgaard, P. Antioxidant activity of cichoric acid and alkamides from Echinacea purpurea, alone and in combination. Food Chem. 2007, 101, 74–81.
- Jukić, H.; Habeš, S.; Aldžić, A.; Durgo, K.; Kosalec, I. Antioxidant and prooxidant activities of phenolic compounds of the extracts of Echinacea purpurea (L.). Bull. Chem. Technol. Bosnia Herzeg. 2015, 44, 43–52.
- Kim, H.-O.; Durance, T.D.; Scaman, C.H.; Kitts, D.D. Retention of Caffeic Acid Derivatives in Dried Echinacea purpurea. J. Agric. Food Chem. 2000, 48, 4182–4186.
- Bergeron, C.; Livesey, J.F.; Awang, D.V.C.; Arnason, J.T.; Rana, J.; Baum, B.R.; Letchamo, W. A quantitative HPLC method for the quality assurance of Echinacea Products on the North American market. Phytochem. Anal. 2000, 11, 207–215.
- Brown, P.N.; Chan, M.; Betz, J.M. Optimization and single-laboratory validation study of a high-performance liquid chromatography (HPLC) method for the determination of phenolic Echinacea constituents. Anal. Bioanal. Chem. 2010, 397, 1883–1892.
- Chiou, S.-Y.; Sung, J.-M.; Huang, P.-W.; Lin, S.-D. Antioxidant, Antidiabetic, and Antihypertensive Properties of Echinacea purpurea Flower Extract and Caffeic Acid Derivatives Using In Vitro Models. J. Med. Food 2017, 20, 171–179.
- Geng, X.; Tian, X.; Tu, P.; Pu, X. Neuroprotective effects of echinacoside in the mouse MPTP model of Parkinson's disease. Eur. J. Pharmacol. 2007, 564, 66–74.
- Bauer, R. . Z. fur arztliche Fortbild. 1996, 90.
- Hou, R.; Xu, T.; Li, Q.; Yang, F.; Wang, C.; Huang, T.; Hao, Z. Polysaccharide from Echinacea purpurea reduce the oxidant stress in vitro and in vivo. Int. J. Biol. Macromol. 2020, 149, 41–50.
- Karg, C.A.; Wang, P.; Vollmar, A.M.; Moser, S. Re-opening the stage for Echinacea research—Characterization of phylloxanthobilins as a novel anti-oxidative compound class in Echinacea purpurea. Phytomedicine 2019, 60, 152969.
- Karg, C.A.; Wang, P.; Kluibenschedl, F.; Müller, A.P.D.T.; Allmendinger, L.; Vollmar, A.M.; Moser, S. Phylloxanthobilins are Abundant Linear Tetrapyrroles from Chlorophyll Breakdown with Activities Against Cancer Cells. Eur. J. Org. Chem. 2020, 2020, 4499–4509.
- Mazza, G.; Cottrell, T. Volatile Components of Roots, Stems, Leaves, and Flowers of Echinacea Species. J. Agric. Food Chem. 1999, 47, 3081–3085.
- Luo, X.-B.; Chen, B.; Yao, S.-Z.; Zeng, J.-G. Simultaneous analysis of caffeic acid derivatives and alkamides in roots and extracts of Echinacea purpurea by high-performance liquid chromatography–photodiode array detection–electrospray mass spectrometry. J. Chromatogr. A 2002, 986, 73–81.
- Thomsen, M.O.; Fretté, X.C.; Christensen, K.B.; Christensen, L.P.; Grevsen, K. Seasonal Variations in the Concentrations of Lipophilic Compounds and Phenolic Acids in the Roots of Echinacea purpurea and Echinacea pallida. J. Agric. Food Chem. 2012, 60, 12131–12141.
- Coelho, J.; Barros, L.; Dias, M.I.; Finimundy, T.C.; Amaral, J.S.; Alves, M.J.; Calhelha, R.C.; Santos, P.F.; Ferreira, I.C. Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions. Pharmaceuticals 2020, 13, 125.
- Meyer, S.A. Echinacea. Elsevier 2005, 2, 116.
- Billah, M.; Hosen, B.; Khan, F.; Niaz, K. Echinacea Book: Nonvitamin and Nonmineral Nutritional Supplements, 1st ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 205–210.
- Birt, D.F.; Widrlechner, M.P.; Lalone, C.; Wu, L.; Bae, J.; Solco, A.K.; Kraus, G.; Murphy, P.; Wurtele, E.; Leng, Q.; et al. Echinacea in infection. Am. J. Clin. Nutr. 2008, 87, 488S–492S.
- Rahman, A.N.A.; Khalil, A.A.; Abdallah, H.; ElHady, M. The effects of the dietary supplementation of Echinacea purpurea extract and/or vitamin C on the intestinal histomorphology, phagocytic activity, and gene expression of the Nile tilapia. Fish Shellfish Immunol. 2018, 82, 312–318.
- Barrett, B. Medicinal properties of Echinacea: A critical review. Phytomedicine 2003, 10, 66–86.
- Senchina, D.S.; Martin, A.E.; Buss, J.E.; Kohut, M.L. Effects of Echinacea extracts on macrophage antiviral activities. Phytotherapy Res. 2009, 24, 810–816.
- Soon, S.L.; Crawford, R.I. Recurrent erythema nodosum associated with echinacea herbal therapy. J. Am. Acad. Dermatol. 2001, 44, 298–299.
- Cupp, M.J.; Davis, J. Toxicology and Clinical Pharmacology of Herbal Products; Humana Press: Totowa, NJ, SUA, 2000; pp. 85–93.
- Aucoin, M.; Cooley, K.; Saunders, P.R.; Carè, J.; Anheyer, D.; Medina, D.N.; Cardozo, V.; Remy, D.; Hannan, N.; Garber, A. The effect of Echinacea spp. on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: A rapid review. Adv. Integr. Med. 2020, 7, 203–217.
- Weishaupt, R.; Bächler, A.; Feldhaus, S.; Lang, G.; Klein, P.; Schoop, R. Safety and Dose-Dependent Effects of Echinacea for the Treatment of Acute Cold Episodes in Children: A Multicenter, Randomized, Open-Label Clinical Trial. Children 2020, 7, 292.
- Elek, F.; Eszter, D.; Rebeka, K.; Szende, V.; Melinda, U.; Eszter, L.-Z. Mapping of Echinacea-based food supplements on the Romania market and qualitative evaluation of the most commonly used products. Bull. Med Sci. 2020, 93, 111–123.
- Chen, X.-L.; Zhang, J.-J.; Chen, R.; Li, Q.-L.; Yang, Y.-S.; Wu, H. An Uncommon Plant Growth Regulator, Diethyl Aminoethyl Hexanoate, Is Highly Effective in Tissue Cultures of the Important Medicinal Plant Purple Coneflower (Echinacea purpurea L.). BioMed Res. Int. 2013, 2013, 1–12.
- Stuart, D.L.; Wills, R.B.H. Alkylamide and Cichoric Acid Levels inEchinacea purpureaTissues During Plant Growth. J. Herbs Spices Med. Plants 2000, 7, 91–101.
- Thomas, A. Regulatory Aspects of Herbal Medicine; Academic Press: Cambridge, MA, USA, 2017; pp. 165–178.
- Waidyanatha, S.; Pierfelice, J.; Cristy, T.; Mutlu, E.; Burback, B.; Rider, C.V.; Ryan, K. A strategy for test article selection and phytochemical characterization of Echinacea purpurea extract for safety testing. Food Chem. Toxicol. 2020, 137, 111125.
- Rios, M.Y.; Olivo, H.F. Natural and Synthetic Alkamides. Stud. Nat. Prod. Chem. 2014, 43, 79–121.
- Woelkart, K.; Koidl, C.; Grisold, A.; Gangemi, J.D.; Turner, R.B.; Marth, E.; Bauer, R. Bioavailability and Pharmacokinetics of Alkamides From the Roots of Echinacea angustifolia in Humans. J. Clin. Pharmacol. 2005, 45, 683–689.
- Woelkart, K.; Dittrich, P.; Beubler, E.; Pinl, F.; Schoop, R.; Suter, A.; Bauer, R. Pharmacokinetics of the Main Alkamides after Administration of three Different Echinacea purpurea Preparations in Humans. Planta Medica 2008, 74, 651–656.
- Saeidnia, S.; Manayi, A.; Vazirian, M. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63–72.
- Matthias, A.; Banbury, L.; Stevenson, L.M.; Bone, K.M.; Leach, D.N.; Lehmann, R.P. Alkylamides from Echinacea Modulate Induced Immune Responses in Macrophages. Immunol. Investig. 2007, 36, 117–130.
- Mudge, E.; Lopes-Lutz, D.; Brown, P.; Schieber, A. Analysis of Alkylamides in Echinacea Plant Materials and Dietary Supplements by Ultrafast Liquid Chromatography with Diode Array and Mass Spectrometric Detection. J. Agric. Food Chem. 2011, 59, 8086–8094.
- Cech, N.B.; Kandhi, V.; Davis, J.M.; Hamilton, A.; Eads, D.; Laster, S.M. Echinacea and its alkylamides: Effects on the influenza A-induced secretion of cytokines, chemokines, and PGE2 from RAW 264.7 macrophage-like cells. Int. Immunopharmacol. 2010, 10, 1268–1278.
- Schumacher, A.; Friedberg, K. Untersuchungen zur Wirkung von Echinacea angustifolia auf die unspezifische zelluläre Immunantwort der Maus . Arzneimmittel-Forschung 1991, 41, 141–147.
- Shariatinia, Z. Natural Polysaccharides in Drug Delivery and Biomedical Applications; Academic Press: London, UK, 2019; Chapter 2; pp. 15–57.
- Balciunaite, G.; Juodsnukyte, J.; Savickas, A.; Ragazinskiene, O.; Siatkute, L.; Zvirblyte, G.; Mistiniene, E.; Savickiene, N. Fractionation and evaluation of proteins in roots of Echinacea purpurea (L.) Moench. Acta Pharm. 2015, 65, 473–479.
- Cai, C.; Chen, Y.; Zhong, S.; Ji, B.; Wang, J.; Bai, X.; Shi, G. Anti-Inflammatory Activity of N-Butanol Extract from Ipomoea stolonifera In Vivo and In Vitro. PLoS ONE 2014, 9, e95931.
- Stimpel, M.; Proksch, A.; Wagner, H.; Lohmann-Matthes, M.L. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect. Immun. 1984, 46, 845–849.
- Sharma, S.; Anderson, M.; Schoop, S.; Hudson, J. Bactericidal and anti-inflammatory properties of a standardized Echinacea extract (Echinaforce®): Dual actions against respiratory bacteria. Phytomedicine 2010, 17, 563–568.
- Vazirian, M.; Dianat, S.; Manayi, A.; Ziari, R.; Mousazadeh, A.; Habibi, E.; Saeidnia, S.; Amanzadeh, Y. Anti-inflammatory effect, total polysaccharide, total phenolics content and antioxidant activity of the aqueous extract of three basidiomycetes. Res. J. Pharmacogn. 2014, 1, 15–21.
- Mazzio, E.A.; Soliman, K.F.A. In vitro screening for the tumoricidal properties of international medicinal herbs. Phytotherapy Res. 2008, 23, 385–398.
- Vickers, A. Botanical Medicines for the Treatment of Cancer: Rationale, Overview of Current Data, and Methodological Considerations for Phase I and II Trials. Cancer Investig. 2002, 20, 1069–1079.
- Voaden, D.J.; Jacobson, M. Tumor inhibitors. 3. Identification and synthesis of a oncolytic hydrocarbon from American coneflower roots. J. Med. Chem. 1972, 15, 619–623.
- Yang, G.; Li, K.; Liu, C.; Peng, P.; Bai, M.; Sun, J.; Li, Q.; Yang, Z.; Yang, Y.; Wu, H. A Comparison of the Immunostimulatory Effects of Polysaccharides from Tetraploid and Diploid Echinacea purpurea. BioMed Res. Int. 2018, 2018, 1–12.
- Yao, L.; Bai, L.; Tan, Y.; Sun, J.; Qu, Q.; Shi, D.; Guo, S.; Liu, C. The immunoregulatory effect of sulfated Echinacea purpurea polysaccharide on chicken bone marrow-derived dendritic cells. Int. J. Biol. Macromol. 2019, 139, 1123–1132.
- Tsai, Y.-L.; Chiu, C.-C.; Chen, J.Y.-F.; Chan, K.-C.; Lin, S.-D. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis. J. Ethnopharmacol. 2012, 143, 914–919.
- Abreu, R.M.; Ferreira, I.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.-J.R. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothienopyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806.
- Luettig, B.; Steinmüller, C.; Gifford, G.E.; Wagner, H.; Lohmann-Matthes, M.-L. Macrophage Activation by the Polysaccharide Arabinogalactan Isolated From Plant Cell Cultures of Echinacea purpurea. JNCI: J. Natl. Cancer Inst. 1989, 81, 669–675.
- Sharif, K.O.M.; Tufekci, E.F.; Ustaoglu, B.; Altunoglu, Y.C.; Zengin, G.; Llorent-Martínez, E.; Guney, K.; Baloglu, M.C. Anticancer and biological properties of leaf and flower extracts of Echinacea purpurea (L.) Moench. Food Biosci. 2021, 41, 101005.
- Jiang, W.; Zhu, H.; Xu, W.; Liu, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. Echinacea purpurea polysaccharide prepared by fractional precipitation prevents alcoholic liver injury in mice by protecting the intestinal barrier and regulating liver-related pathways. Int. J. Biol. Macromol. 2021, 187, 143–156.
- Xu, W.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Echinacea purpurea suppresses the cell survival and metastasis of hepatocellular carcinoma through regulating the PI3K/Akt pathway. Int. J. Biochem. Cell Biol. 2021, 142, 106115.
- Guiotto, P.; Woelkart, K.; Grabnar, I.; Voinovich, D.; Perissutti, B.; Invernizzi, S.; Granzotto, M.; Bauer, R. Pharmacokinetics and immunomodulatory effects of phytotherapeutic lozenges (bonbons) with Echinacea purpurea extract. Phytomedicine 2008, 15, 547–554.
- Del-Río-Navarro, B.E.; Espinosa-Rosales, F.J.; Flenady, V.; Sienra-Monge, J.J. Immunostimulants for preventing respiratory tract infection in children. Cochrane Database Syst. Rev. 2006, CD004974.
- Kim, H.-R.; Oh, S.-K.; Lim, W.; Lee, H.K.; Moon, B.-I.; Seoh, J.-Y. Immune Enhancing Effects of Echinacea purpurea Root Extract by Reducing Regulatory T Cell Number and Function. Nat. Prod. Commun. 2014, 9, 511–514.
- Bodinet, C.; Beuscher, N. Antiviral and Immunological Activity of Glycoproteins from Echinacea purpurea Radix. Planta Medica 1991, 57, A33–A34.
- Bergeron, C.; Gafner, S. Quantitative Analysis of the Polysaccharide and Glycoprotein Fractions in Echinacea purpurea. And Echinacea angustifolia. by HPLC-ELSD for Quality Control of Raw Material. Pharm. Biol. 2007, 45, 98–105.
- Kurkin, V.A.; Akushskaya, A.S.; Avdeeva, E.V.; Velmyaikina, E.I.; Daeva, E.D.; Kadentsev, V.I. Flavonoids from Echinacea purpurea. Russ. J. Bioorganic Chem. 2011, 37, 905–906.
- Agrawal, A.D. Pharmacological Activities of Flavonoids: A Review. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1394–1398.
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124.
- Dogan, Z.; Ergul, B.; Sarikaya, M.; Filik, L.; Gonultaş, M.A.; Hucumenoglu, S.; Can, M. The protective effect of Echinacea spp. (Echinacea angustifolia and Echinacea purpurea) in a rat colitis model induced by acetic acid. Pak. J. Pharm Sci. 2014, 27, 1827–1835.
- Lee, T.T.; Huang, C.C.; Shieh, X.H.; Chen, C.L.; Chen, L.J.; Yu, B. Flavonoid, Phenol and Polysaccharide Contents of Echinacea Purpurea, L. and Its Immunostimulant Capacity In Vitro. Int. J. Environ. Sci. Dev. 2010, 1.
- Speroni, E.; Govoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M. Anti-inflammatory and cicatrizing activity of Echinacea pallida Nutt. root extract. J. Ethnopharmacol. 2001, 79, 265–272.
- Ekeuku, S.O.; Pang, K.-L.; Chin, K.-Y. Effects of Caffeic Acid and Its Derivatives on Bone: A Systematic Review. Drug Des. Devel Ther. 2021, 15, 259–275.
- Zhang, P.; Tang, Y.; Li, N.-G.; Zhu, Y.; Duan, J.-A. Bioactivity and Chemical Synthesis of Caffeic Acid Phenethyl Ester and Its Derivatives. Molecules 2014, 19, 16458–16476.
- Yeğin, M.E.; Bilkay, U.; Tiftikçioğlu, Y.; Uyanikgil, Y.; Çavuşoğlu, T.; Ercan, G.; Gürdal, M. Altering effects of caffeic acid phenethyl ester (CAPE) and ischemia/reperfusion injury: An experimental study in a rat TRAM flap model. Eur. J. Plast. Surg. 2020, 43, 527–534.
- Wu, S.; Zhang, K.; Qin, H.; Niu, M.; Zhao, W.; Ye, M.; Zou, H.; Yang, Y. Caffeic acid phenethyl ester (CAPE) revisited: Covalent modulation of XPO1/CRM1 activities and implication for its mechanism of action. Chem. Biol. Drug Des. 2016, 89, 655–662.
- Wang, F.; Yang, J. A comparative study of caffeic acid and a novel caffeic acid conjugate SMND-309 on antioxidant properties in vitro. LWT 2012, 46, 239–244.
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Patents 2014, 24, 1257–1270.
- Chicca, A.; Adinolfi, B.; Martinotti, E.; Fogli, S.; Breschi, M.; Pellati, F.; Benvenuti, S.; Nieri, P. Cytotoxic effects of Echinacea root hexanic extracts on human cancer cell lines. J. Ethnopharmacol. 2007, 110, 148–153.
- Senica, M.; Mlinsek, G.; Veberic, R.; Mikulic-Petkovsek, M. Which Plant Part of Purple Coneflower (Echinacea purpurea (L.) Moench) Should be Used for Tea and Which for Tincture? J. Med. Food 2019, 22, 102–108.
- Wu, C.-H.; Murthy, H.N.; Hahn, E.-J.; Paek, K.-Y. Enhanced production of caftaric acid, chlorogenic acid and cichoric acid in suspension cultures of Echinacea purpurea by the manipulation of incubation temperature and photoperiod. Biochem. Eng. J. 2007, 36, 301–303.
- Chiellini, C.; Maida, I.; Maggini, V.; Bosi, E.; Mocali, S.; Emiliani, G.; Perrin, E.; Firenzuoli, F.; Mengoni, A.; Fani, R. Preliminary data on antibacterial activity of Echinacea purpurea-associated bacterial communities against Burkholderia cepacia complex strains, opportunistic pathogens of Cystic Fibrosis patients. Microbiol. Res. 2017, 196, 34–43.
- Tsai, Y.-L.; Chiou, S.-Y.; Chan, K.-C.; Sung, J.-M.; Lin, S.-D. Caffeic acid derivatives, total phenols, antioxidant and antimutagenic activities of Echinacea purpurea flower extracts. LWT 2012, 46, 169–176.
- Jiang, Z.; Wang, J.; Li, X.; Zhang, X. Echinacoside and Cistanche tubulosa (Schenk) R. wight ameliorate bisphenol A-induced testicular and sperm damage in rats through gonad axis regulated steroidogenic enzymes. J. Ethnopharmacol. 2016, 193, 321–328.
- Xing, X.X.; Liu, Z.J.; Han, B. Effects of Acteoside and Echinacoside on the Expression of the BMP2 in Rat Osteoblast. Prog. Vet. Med. 2011, 32, 45–48.
- Liu, J.; Yang, L.; Dong, Y.; Zhang, B.; Ma, X. Echinacoside, an Inestimable Natural Product in Treatment of Neurological and other Disorders. Molecules 2018, 23, 1213.
- Jia, C.; Shi, H.; Jin, W.; Zhang, K.; Jiang, Y.; Zhao, M.; Tu, P. Metabolism of Echinacoside, a Good Antioxidant, in Rats: Isolation and Identification of Its Biliary Metabolites. Drug Metab. Dispos. 2008, 37, 431–438.
- Pires, C.; Martins, N.; Carvalho, A.M.; Barros, L.; Ferreira, I.C. Phytopharmacologic preparations as predictors of plant bioactivity: A particular approach to Echinacea purpurea (L.) Moench antioxidant properties. Nutrition 2016, 32, 834–839.
- Zou, Z.; Dong, L.; Wang, H.; Niu, J.; Zou, M.; Wu, N.; Yu, D.; Wang, Y. Echinacoside induces apoptotic cancer cell death by inhibiting the nucleotide pool sanitizing enzyme MTH1. OncoTargets Ther. 2015, 8, 3649–3664.
