Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Cecilia Bucci.
Ras-related C3 botulinum toxin substrate 1 (Rac1) is a member of the family of the typical Rho guanosine triphosphate phosphohydrolases (GTPases), which are known for their role in several cellular processes such as cytoskeleton organization, gene expression regulation, and cell migration. The small GTPases of the Rho family regulate many aspects of actin dynamics, but are functionally connected to many other cellular processes. Rac1, a member of this family, besides its known function in the regulation of actin cytoskeleton, plays a key role in the production of reactive oxygen species, in gene transcription, in DNA repair, and also has been proven to have specific roles in neurons.
- Rac1
- GTPase
- Rab proteins
1. Rac1 and Rabs: Interactions and Regulations of Their Functions
Physical interactions between Rac1 and members of the Rab family have been reported [55][1]. In fact, Rac1 binds strongly to Rab7a wt and to the Rab7a Q67L constitutively-active mutant, but very weakly to the Rab7a T22N dominant negative mutant, indicating that Rac1 binds preferentially to the GTP-bound form of Rab7a [55][1]. Interestingly, the GTP-bound form of Rac1 affects Rab7a activity also through one of its effectors, called Armus, which is a Rab7a GAP [17][2]. Furthermore, it has also been proven that Rab7a is able to regulate Rac1 activity, although the molecular mechanism is not known yet [56,57][3][4]. These data suggest that the coordination between Rac1 and Rab proteins is accomplished at different levels in order to control finely their functions.
Furthermore, Rab23 has been proven to affect Rac1 by regulating a Rac1 GEF, Tiam1 [58][5]. Indeed, GTP-bound Rab23 interacts with β1 integrin, which then recruits Tiam1 and activates Rac1 [58][5]. Binding of Rab23 to Tiam1 is dependent on the presence of β1 integrin [58][5]. Furthermore, Rab5 activity is regulated by Rac1 through a GEF [59][6]. In fact, active Rac1 interacts with alsin, a Rab5 GEF, and a Rac1 effector and activates the Rab GTPase [59][6]. All together, these data demonstrate that one important level of regulation is based on modulating the activity of GTPase modulators such as GEFs and GAPs.
So far, only a few direct interactions between Rac1 and Rab proteins and/or their regulators have been discovered, but clearly, this could be a general mechanism for their coordination responsible for cellular altered functions and possibly diseases.
2. Rac1 in Cancer: Role of Rac1 and Rabs in Cell Migration and Metastasis
Cancer is a complex group of diseases characterized by uncontrolled cell growth. Excess of Rac1 activity has been linked to several cancer types such as breast cancer, colorectal cancer, gastric cancer, prostate cancer, and cervical cancer [60,61,62,63,64,65][7][8][9][10][11][12]. In particular, Rac1 is involved in several phases of cancer development, as it can promote cancer initiation, cancer progression, and metastases through its role in gene transcription, cell cycle progression, neovascularization, cell adhesion, migration, and invasion [56,66,67,68,69][3][13][14][15][16]. Moreover, mutations of Rac1 could determine a pathological state as Rac1 stimulates macropinocytosis, a fluid-phase endocytosis driven by actin-based protrusion of the plasma membrane [19][17]. Macropinocytosis relies on large organelles called macropinosomes that allow internalization of extracellular material that it is subsequently used by cancer cells to increase their metabolism, therefore contributing to the growth of the tumor [70][18].
The most important feature of cancer, negatively related to rate of survival, is the ability of cancer cells to spread to other parts of the body through a process called metastasis, which is characterized by cell migration and invasion. Cell migration is a process that consists of the formation of cell protrusions such as lamellipodia and filopodia and new adhesion sites at the front of the cell (leading edge), contraction of the cell body, and detachment of adhesions at the rear [71,72][19][20]. Several studies have demonstrated that Rac1 is involved in the regulation of cell migration and invasion and that Rab proteins can collaborate in this process [17,56,58,73,74,75,76,77,78,79][2][3][5][21][22][23][24][25][26][27].
Among Rab proteins, Rab5a is responsible for the regulation of the trafficking between the plasma membrane and early endosomes [80,81][28][29]. However, Rab5a is required also for the activation of Rac1 and, in turn, for the regulation of the actin cytoskeletal organization [82][30] (Figure 1a). In particular, Rab5a is able to regulate the formation of integrin and adhesion complexes and, in turn, controls Rac1 activity and the organization of actin structures [73][21]. Therefore, Rab5a silencing reduces the number and size of protrusions and decreases cancer cell motility and invasion, which is important for metastasis and tumor spread [73][21]. Another isoform of Rab5, Rab5c, has been linked to the regulation of Rac1 activity dependent on EGF and therefore to cell migration [74][22]. Similar to Rab5a, Rab5c depletion determines the presence of fewer focal adhesion foci, less membrane ruffles, and less cell migration [74][22]. In fact, Rab5c is responsible for recruiting Rac1 at the plasma membrane, where it promotes the formation of lamellipodia [74][22]. In line with this, Rab5c silencing affects the abundance of Rac1 in the membrane fraction when compared to control cells [74][22]. Moreover, upon stimulation with EGF, Rab5c-depleted cells show less phosphorylated, active, AKT and, in turn, PI3 kinases [74][22]. This leads to a reduction of EGF-stimulated Rac1 activity in Rab5c-depleted cells compared to control cells, although lower Rac1 activity has been detected also at steady state upon Rab5c silencing [74][22] (Figure 1c).
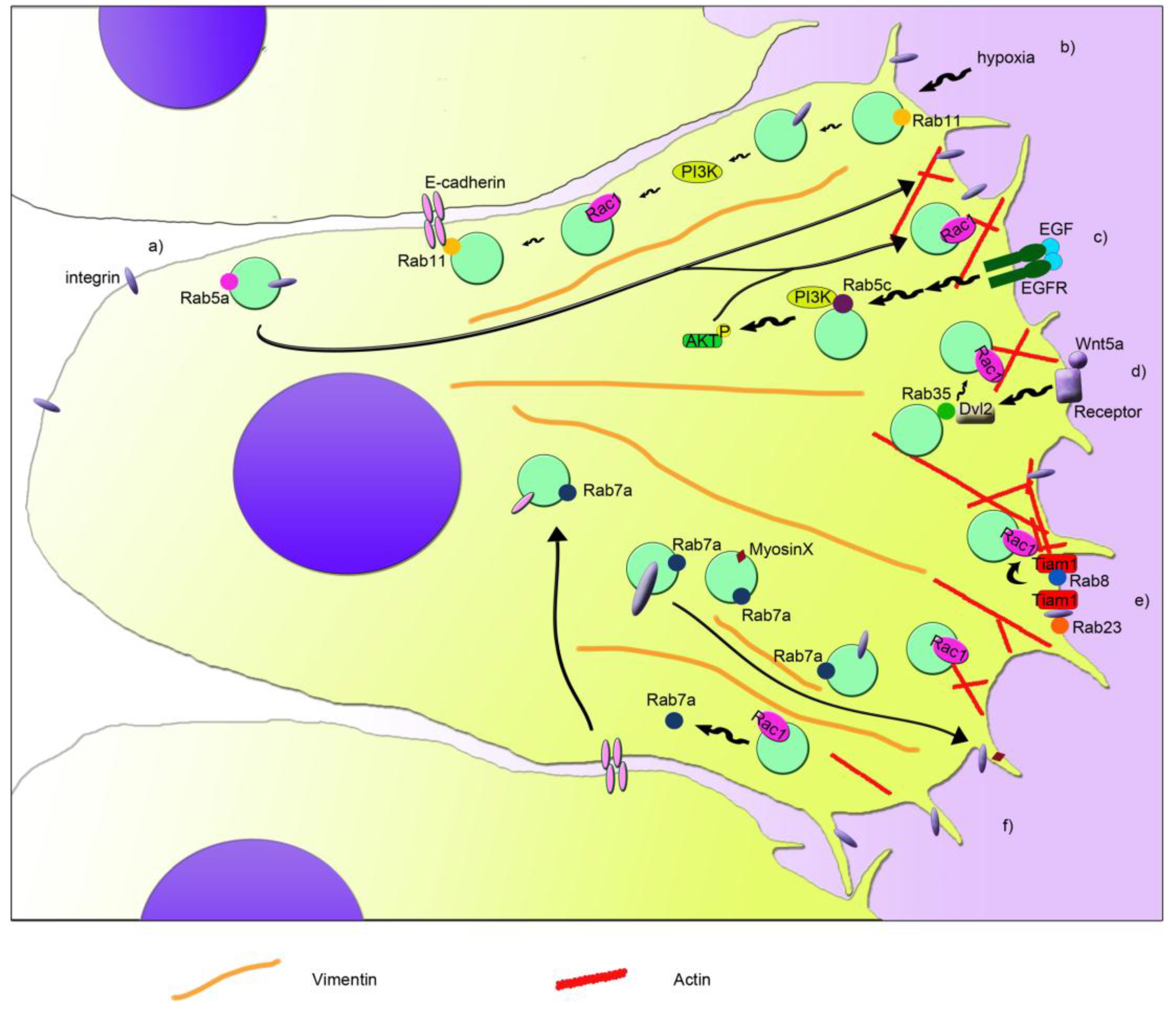
Figure 1. Schematic model that depicts the coordination of Rab proteins and Rac1 in cell migration. Different pathways are indicated with letters. (a) Rab5a regulates the internalization and recycling of integrins from the rear edge to the leading edge. This regulation is important for the formation of new adhesions at the front of the cell, for Rac1 activation, and for actin filament (in red) reorganization. (b) Rab11 interacts with E-cadherin and recruits and activates Rac1 at the plasma membrane. In hypoxia, αvβ3 integrin and PI3K are activated through the action of Rab11, leading to Rac1 activation and cell migration. (c) Cell stimulation with EGF and its binding to EGFR modulates activity of PI3K and AKT in a Rab5c-mediated manner. This in turn leads to increased Rac1 activity, membrane ruffles, and cell migration. (d) Wnt5a signaling induces the activation of Dvl2, which binds to Rab35 activating it. Active Rab35 increases Rac1 activity and therefore cell migration. (e) Rab8 activation induces Rac1 activation mediated by a Rac1 GEF called Tiam1, regulating cortical actin polymerization and focal adhesion reorganization. Furthermore, active Rab23 interacts with Tiam1, through its interaction with β1 integrin, regulating Rac1 activity and cell migration. (f) Rab7a regulates cell migration by modulating Rac1 activity, β1 integrin, and myosin X transport at the leading edge and the regulation of vimentin filament organization (in orange). Moreover, Rac1 regulates Rab7a activity and in turn E-cadherin degradation in lysosomes, and therefore cell-cell contacts.
Interestingly, also the interplay between Rac1 and Rab7a, which is localized mainly to late endosomes and regulates the late steps of endocytosis [83[31][32][33],84,85], has an important role in cell migration and metastasis [17,55,56][1][2][3]. Indeed, Rab7a interacts with Rac1 [55][1], and they can affect each other’s activation state [17,56][2][3]. Depletion of Rab7a induces a lower activation of Rac1, which in coordination with other effects on the activation of β1 integrin, organization of vimentin filament, and trafficking of myosin X, determines a negative effect on migration, formation of protrusions, adhesion, and spreading [56][3], similarly to Rab5a and Rab5c (Figure 1f). On the contrary, Rac1 activation determines a lower amount of active Rab7a by acting on Rac1-effector armus, which is a bona fide GAP for Rab7a [17][2].
The coordination of Rac1 and Rab7a in epithelial cells is important for cell-cell adhesion and for collective cell migration, epithelial-mesenchymal transition (EMT), and metastasis. Integrated signaling among Arf6, Rac1, and Rab7a is necessary for regulating E-cadherin degradation, which in turn, leads to the loss of cell-cell contacts [17][2]. Arf6 is able to activate both Rac1 and Rab7a, leading to internalization of E-cadherin, which becomes mainly perinuclear. Nevertheless, active Rac1 is able to regulate Rab7a’s activation state through its effector armus, which inhibits Rab7a, blocking E-cadherin degradation in lysosomes [17][2]. Modulation of E-cadherin surface levels is fundamental for cell adhesion of epithelia both in health and disease, affecting epithelial morphogenesis and differentiation, but also acquisition of mesenchymal characteristics. Epithelial cancer cells can detach from the close layer of cells, undergo EMT, and spread in other tissues, becoming a metastatic cell. Interestingly, upregulation of Rab7a and armus-related proteins has been reported in several epithelial tumors [17,86,87][2][34][35]. In line with the effect of active Rac1 on the activation state of Rab7a, less Rab7a is pulled down by RILP, a GTP-Rab7a interactor [88][36], when Rac1 is activated [17][2]. WResearchers recently proved that also RILP is able to regulate cell adhesion and migration [89][37]. Although Rab5a, Rab5c, or Rab7a silencing showed a reduction in cell migration [56[3][21][22],73,74], RILP depletion determined an increase in cell motility and speed [89][37]. Interestingly, RILP-depleted cells showed the ability to migrate as single cells [89][37], prompting the evaluation of E-cadherin turnover in these cells, in order to elucidate the mechanisms behind the regulation of cell migration and adhesion mediated by Rab7a and RILP.
Another connection between Rac1 and Rab GTPases in the regulation of cell migration is the one discovered between Rab8 and Rac1 [75][23]. Rab8 is important in membrane transport to the plasma membrane, but it also regulates the organization of the actin cytoskeleton and the size and distribution of focal adhesions during cell migration [75,90,91,92][23][38][39][40]. Interestingly, the Rab8-dependent regulation of the actin cytoskeleton organization is mediated by Rac1. In fact, expression of a constitutively-active mutant of Rab8 induced an increase of Rac1 activity, probably due to the redistribution of Tiam1, a Rac1 GEF, to the plasma membrane (Figure 1e). This coordination regulates cell protrusions and contributes to the loss of focal adhesions [75][23]. Surprisingly, Tiam1-dependent activation has been observed also in Rab5-positive endosomes for the regulation of actin polymerization in dorsal ruffles [93][41]. However, specific recruitment of Rab8 to the protrusive edge is necessary to ensure persistent migration, and it could be mediated by the Rab8 GEF Rabin8, a Rab11 effector responsible for the apical exocytosis during lumen formation mediated by Rab8 [75,94][23][42]. Similar to Rab8, also Rab11 is able to interplay with Rac1 and regulate cell migration [76][24]. Rab11 regulates transport from recycling endosomes to the plasma membrane, and it has been associated, together with Rac1, to colorectal carcinoma and cervical cancer, where it regulates tumor progression and metastasis through two different processes, collective cell migration and hypoxia [76,77,95][24][25][43]. Collective cell migration has been reported to occur during invasion in malignancy. Indeed, expression of Rab11 and its interactor E-cadherin have been associated with poor survival in patients affected by colorectal cancer [76][24]. Moreover, by interacting with the adhesion molecule E-cadherin, Rab11 is able to promote cell-cell contacts and increase Rac1 activity and expression of the matrix metalloproteinase-2. All these effects contribute to the increased collective cell migration, Rab11-dependent collective cell invasion and anchorage-independent cell growth [76][24]. Therefore, Rab11 activity could be important in the early stages of colorectal cancer.
A condition that stimulates cancer invasion and migration is hypoxia. Hypoxia can occur in many solid tumors where there is poor vascularization. Therefore, hypoxia functions as selective pressure for the survival of the most aggressive and metastatic cells and could promote tumor cell invasion and lead to poor prognosis and low survival rate for the patient. Rab11 has been recently associated with hypoxia-stimulated invasion and migration of cervical cancer cells [77][25]. In fact, Rab11 stimulates αvβ3 integrin and the activation of FAK and PI3K through their phosphorylation under hypoxia, which then affect the expression and localization of Rac1. Indeed, when Rab11 is present, Rac1 is more expressed in hypoxia, and it is distributed also in plasma membrane and cytoplasm, besides the nucleus, where it normally localizes during normoxia [77][25]. Therefore, Rab11 plays an important role in coordination with Rac1 for the regulation of cell migration and development of metastasis through its action in hypoxia and collective cell migration [76,77][24][25] (Figure 1b).
Another Rab GTPase, called Rab23, is important during mouse development, and it has been associated with several types of cancer. Interestingly, Rab23 was found to be over-expressed in squamous cell carcinoma cells, and it was demonstrated that active Rab23 interacts with β1 integrin and, through it, with Tiam1, which mediates Rac1 activation and cell migration and invasion [58][5] (Figure 1e). Moreover, Rab23 was found absent in normal astrocytes, while it was expressed in almost 50% of astrocytoma-affected patients, showing a correlation with a higher stage of cancer progression [78][26]. Thus, it was demonstrated that Rab23 modulates Rac1 activity and, in turn, cell proliferation, colony formation, migration, and invasion [78][26].
A strong connection between Rab35 protein and Rac1 has been proven [79][27]. In fact, Wnt5a, which is normally involved in cell growth, proliferation, differentiation, motility, and survival, activates Disheveled 2 (Dvl2) by phosphorylating it, and Dvl2 then interacts with Rab35. Subsequently, Rab35 activates Rac1, which, in turn, promotes cell migration of breast cancer cells [79][27] (Figure 1d).
All together, the studies conducted on the coordination of Rac1 and Rab proteins in several processes occurring during cancer development, progression, and metastasis show how interconnected the roles of these proteins are (Figure 1). Interestingly, research on the use of Rac1 inhibitors in the case of common and/or aggressive tumors showed that Rac1 could be a good target for counteracting cancer progression [58,[5]78
References
- Sun, Y.; Buki, K.G.; Ettala, O.; Vaaraniemi, J.P.; Vaananen, H.K. Possible role of direct rac1-rab7 interaction in ruffled border formation of osteoclasts. J. Biol. Chem. 2005, 280, 32356–32361.
- Frasa, M.A.; Maximiano, F.C.; Smolarczyk, K.; Francis, R.E.; Betson, M.E.; Lozano, E.; Goldenring, J.; Seabra, M.C.; Rak, A.; Ahmadian, M.R.; et al. Armus is a rac1 effector that inactivates rab7 and regulates e-cadherin degradation. Curr. Biol. 2010, 20, 198–208.
- Margiotta, A.; Progida, C.; Bakke, O.; Bucci, C. Rab7a regulates cell migration through rac1 and vimentin. Biochim. Biophys. Acta 2017, 1864, 367–381.
- Mascia, A.; Gentile, F.; Izzo, A.; Mollo, N.; De Luca, M.; Bucci, C.; Nitsch, L.; Calì, G. Rab7 regulates cdh1 endocytosis, circular dorsal ruffles genesis and thyroglobulin internalization in a thyroid cell line. J. Cell Physiol. 2016, 231, 1695–1708.
- Jian, Q.; Miao, Y.; Tang, L.; Huang, M.; Yang, Y.; Ba, W.; Liu, Y.; Chi, S.; Li, C. Rab23 promotes squamous cell carcinoma cell migration and invasion via integrin beta1/rac1 pathway. Oncotarget 2016, 7, 5342–5352.
- Kunita, R.; Otomo, A.; Mizumura, H.; Suzuki-Utsunomiya, K.; Hadano, S.; Ikeda, J.E. The rab5 activator als2/alsin acts as a novel rac1 effector through rac1-activated endocytosis. J. Biol. Chem. 2007, 282, 16599–16611.
- Morrison Joly, M.; Williams, M.M.; Hicks, D.J.; Jones, B.; Sanchez, V.; Young, C.D.; Sarbassov, D.D.; Muller, W.J.; Brantley-Sieders, D.; Cook, R.S. Two distinct mtorc2-dependent pathways converge on rac1 to drive breast cancer metastasis. Breast Cancer Res. 2017, 19, 74.
- Hong, M.; Zhang, Z.; Chen, Q.; Lu, Y.; Zhang, J.; Lin, C.; Zhang, F.; Zhang, W.; Li, X.; Zhang, W.; et al. Irf1 inhibits the proliferation and metastasis of colorectal cancer by suppressing the ras-rac1 pathway. Cancer Manag. Res. 2019, 11, 369–378.
- Peng, J.X.; Liang, S.Y.; Li, L. Sfrp1 exerts effects on gastric cancer cells through gsk3beta/rac1mediated restraint of tgfbeta/smad3 signaling. Oncol. Rep. 2019, 41, 224–234.
- Caggia, S.; Chunduri, H.; Millena, A.C.; Perkins, J.N.; Venugopal, S.V.; Vo, B.T.; Li, C.; Tu, Y.; Khan, S.A. Novel role of gialpha2 in cell migration: Downstream of pi3-kinase-akt and rac1 in prostate cancer cells. J. Cell Physiol. 2018, 234, 802–815.
- Hu, J.; Meng, Y.; Zeng, J.; Zeng, B.; Jiang, X. Ubiquitin e3 ligase march7 promotes proliferation and invasion of cervical cancer cells through vav2-rac1-cdc42 pathway. Oncol. Lett. 2018, 16, 2312–2318.
- Zhou, K.; Rao, J.; Zhou, Z.H.; Yao, X.H.; Wu, F.; Yang, J.; Yang, L.; Zhang, X.; Cui, Y.H.; Bian, X.W.; et al. Rac1-gtp promotes epithelial-mesenchymal transition and invasion of colorectal cancer by activation of stat3. Lab. Invest. 2018, 98, 989–998.
- Jamieson, C.; Lui, C.; Brocardo, M.G.; Martino-Echarri, E.; Henderson, B.R. Rac1 augments wnt signaling by stimulating beta-catenin-lymphoid enhancer factor-1 complex assembly independent of beta-catenin nuclear import. J. Cell Sci. 2015, 128, 3933–3946.
- Bopp, A.; Wartlick, F.; Henninger, C.; Schwarz, M.; Kaina, B.; Fritz, G. Rac1 promotes diethylnitrosamine (den)-induced formation of liver tumors. Carcinogenesis 2015, 36, 378–389.
- Ma, J.; Xue, Y.; Liu, W.; Yue, C.; Bi, F.; Xu, J.; Zhang, J.; Li, Y.; Zhong, C.; Chen, Y. Role of activated rac1/cdc42 in mediating endothelial cell proliferation and tumor angiogenesis in breast cancer. PLoS ONE 2013, 8, e66275.
- Liang, Y.; Wang, S.; Zhang, Y. Downregulation of dock1 and elmo1 suppresses the migration and invasion of triple-negative breast cancer epithelial cells through the rhoa/rac1 pathway. Oncol. Lett. 2018, 16, 3481–3488.
- Ridley, A.J.; Paterson, H.F.; Johnston, C.L.; Diekmann, D.; Hall, A. The small gtp-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992, 70, 401–410.
- Recouvreux, M.V.; Commisso, C. Macropinocytosis: A metabolic adaptation to nutrient stress in cancer. Front Endocrinol. (Lausanne) 2017, 8, 261.
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73.
- Vicente-Manzanares, M.; Webb, D.J.; Horwitz, A.R. Cell migration at a glance. J. Cell Sci 2005, 118, 4917–4919.
- Liu, S.S.; Chen, X.M.; Zheng, H.X.; Shi, S.L.; Li, Y. Knockdown of rab5a expression decreases cancer cell motility and invasion through integrin-mediated signaling pathway. J. Biomed. Sci. 2011, 18, 58.
- Chen, P.I.; Schauer, K.; Kong, C.; Harding, A.R.; Goud, B.; Stahl, P.D. Rab5 isoforms orchestrate a "division of labor" in the endocytic network; rab5c modulates rac-mediated cell motility. PLoS ONE 2014, 9, e90384.
- Bravo-Cordero, J.J.; Cordani, M.; Soriano, S.F.; Diez, B.; Munoz-Agudo, C.; Casanova-Acebes, M.; Boullosa, C.; Guadamillas, M.C.; Ezkurdia, I.; Gonzalez-Pisano, D.; et al. A novel high-content analysis tool reveals rab8-driven cytoskeletal reorganization through rho gtpases, calpain and mt1-mmp. J. Cell Sci. 2016, 129, 1734–1749.
- Chung, Y.C.; Wei, W.C.; Hung, C.N.; Kuo, J.F.; Hsu, C.P.; Chang, K.J.; Chao, W.T. Rab11 collaborates e-cadherin to promote collective cell migration and indicates a poor prognosis in colorectal carcinoma. Eur J. Clin. Invest. 2016, 46, 1002–1011.
- Xu, H.; Yuan, Y.; Wu, W.; Zhou, M.; Jiang, Q.; Niu, L.; Ji, J.; Liu, N.; Zhang, L.; Wang, X. Hypoxia stimulates invasion and migration of human cervical cancer cell lines hela/siha through the rab11 trafficking of integrin alphavbeta3/fak/pi3k pathway-mediated rac1 activation. J. Biosci. 2017, 42, 491–499.
- Wang, M.; Dong, Q.; Wang, Y. Rab23 is overexpressed in human astrocytoma and promotes cell migration and invasion through regulation of rac1. Tumour Biol. 2016, 37, 11049–11055.
- Zhu, Y.; Shen, T.; Liu, J.; Zheng, J.; Zhang, Y.; Xu, R.; Sun, C.; Du, J.; Chen, Y.; Gu, L. Rab35 is required for wnt5a/dvl2-induced rac1 activation and cell migration in mcf-7 breast cancer cells. Cell Signal 2013, 25, 1075–1085.
- Bucci, C.; Parton, R.G.; Mather, I.H.; Stunnenberg, H.; Simons, K.; Hoflack, B.; Zerial, M. The small gtpase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992, 70, 715–728.
- Bucci, C.; Wandinger-Ness, A.; Lutcke, A.; Chiariello, M.; Bruni, C.; Zerial, M. Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells. Proc. Natl. Acad. Sci. USA. 1994, 91, 5061–5065.
- Sandri, C.; Caccavari, F.; Valdembri, D.; Camillo, C.; Veltel, S.; Santambrogio, M.; Lanzetti, L.; Bussolino, F.; Ivaska, J.; Serini, G. The r-ras/rin2/rab5 complex controls endothelial cell adhesion and morphogenesis via active integrin endocytosis and rac signaling. Cell Res. 2012, 22, 1479–1501.
- Pfeffer, S.R. Rab gtpase regulation of membrane identity. Curr. Opin. Cell Biol. 2013, 25, 414–419.
- Zhang, M.; Chen, L.; Wang, S.; Wang, T. Rab7: Roles in membrane trafficking and disease. Biosci. Rep. 2009, 29, 193–209.
- Guerra, F.; Bucci, C. Multiple roles of the small gtpase rab7. Cells 2016, 5, 34.
- Zhou, Y.; Toth, M.; Hamman, M.S.; Monahan, S.J.; Lodge, P.A.; Boynton, A.L.; Salgaller, M.L. Serological cloning of paris-1: A new tbc domain-containing, immunogenic tumor antigen from a prostate cancer cell line. Biochem. Biophys. Res. Commun. 2002, 290, 830–838.
- Croizet-Berger, K.; Daumerie, C.; Couvreur, M.; Courtoy, P.J.; van den Hove, M.F. The endocytic catalysts, rab5a and rab7, are tandem regulators of thyroid hormone production. Proc. Natl. Acad. Sci. USA 2002, 99, 8277–8282.
- Cantalupo, G.; Alifano, P.; Roberti, V.; Bruni, C.B.; Bucci, C. Rab-interacting lysosomal protein (rilp): The rab7 effector required for transport to lysosomes. EMBO J. 2001, 20, 683–693.
- Margiotta, A.; Progida, C.; Bakke, O.; Bucci, C. Characterization of the role of rilp in cell migration. Eur. J. Histochem. 2017, 61, 2783.
- Huber, L.A.; Pimplikar, S.; Parton, R.G.; Virta, H.; Zerial, M.; Simons, K. Rab8, a small gtpase involved in vesicular traffic between the tgn and the basolateral plasma membrane. J. Cell Biol. 1993, 123, 35–45.
- Hattula, K.; Furuhjelm, J.; Arffman, A.; Peranen, J. A rab8-specific gdp/gtp exchange factor is involved in actin remodeling and polarized membrane transport. Mol. Biol. Cell 2002, 13, 3268–3280.
- Peranen, J.; Auvinen, P.; Virta, H.; Wepf, R.; Simons, K. Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J. Cell Biol. 1996, 135, 153–167.
- Palamidessi, A.; Frittoli, E.; Garré, M.; Faretta, M.; Mione, M.; Testa, I.; Diaspro, A.; Lanzetti, L.; Scita, G.; Di Fiore, P.P. Endocytic trafficking of rac is required for the spatial restriction of signaling in cell migration. Cell 2008, 134, 135–147.
- Bryant, D.M.; Datta, A.; Rodriguez-Fraticelli, A.E.; Peranen, J.; Martin-Belmonte, F.; Mostov, K.E. A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 2010, 12, 1035–1045.
- Ramel, D.; Wang, X.; Laflamme, C.; Montell, D.J.; Emery, G. Rab11 regulates cell-cell communication during collective cell movements. Nat. Cell Biol. 2013, 15, 317–324.
More
