Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Igor Grivennikov and Version 2 by Camila Xu.
Astrocytes play a key role in the functioning of neurons in norm and pathology, being a target for stress and glucocorticoids, are a promising target for the treatment of stress-dependent depression and Alzheimer’s disease (AD). Depression, as a mental disorder, is characterized by two core symptoms, depressed mood and loss of interest or pleasure in nearly all activities, and may be accompanied by other symptoms such as cognitive impairments, sleep disturbance, psychomotor retardation or agitation, feelings of worthlessness or excessive or inappropriate guilt.
- depression
- neurodegeneration
- Alzheimer’s disease
- astrocytes
1. Introduction
According to Hans Selye, “stress is the nonspecific response of the body to any demand” [1]. Stress is also defined as a state of threatened (or perceived as threatened) internal dynamic balance (“homeostasis”) caused by external or internal stimuli (“stressors”) [2]. To achieve homeostasis, the highly conservative regulatory neuroendocrine system, the “stress system”, is activated through synchronized interaction between the hypothalamic–pituitary–adrenal axis (HPAA) and the autonomic nervous system [2]. In principle, stress is necessary to adapt to changing environmental or internal conditions and increase the chance of survival. It is known that moderate stress is able to activate mental and behavioral processes to find solutions to the challenges facing an individual. However, excessive and/or prolonged stressors and the consequent chronic deregulation of the stress system can lead to a wide range of chronic pathological conditions, including pathologies of the cardiovascular, endocrine, immune and nervous systems. One of the stress-related pathologies of the nervous system is depression (major depressive disorder). Currently, depression is a most widespread mental disorder worldwide, which, according to World Health Organization, affects approximately 280 million people in the world, or about 4.0% of the population, including at least 5% of adults [3]. The incidence of depression increases with age, so it is about 27% in the age group of 75–80 years, 33% in the age group of 81–85 years and reaches 46% in the age group of 91 years and older [4]. Considering that the frequency of neurodegenerative diseases (in particular, dementia) also increases with age and that depression often manifests itself in dementia, it is possible that pathological changes in the course of dementia are associated with the development of depression.
Depression, as a mental disorder, is characterized by two core symptoms, depressed mood and loss of interest or pleasure in nearly all activities, and may be accompanied by other symptoms such as cognitive impairments, sleep disturbance, psychomotor retardation or agitation, feelings of worthlessness or excessive or inappropriate guilt [5]. Depression significantly worsens the quality of life. A large percentage of suicides, especially among young people, is associated with depression.
Figure 1 schematically shows the general sequence of events leading to the development of chronic stress, depression and, ultimately, to the degeneration of nerve cells.
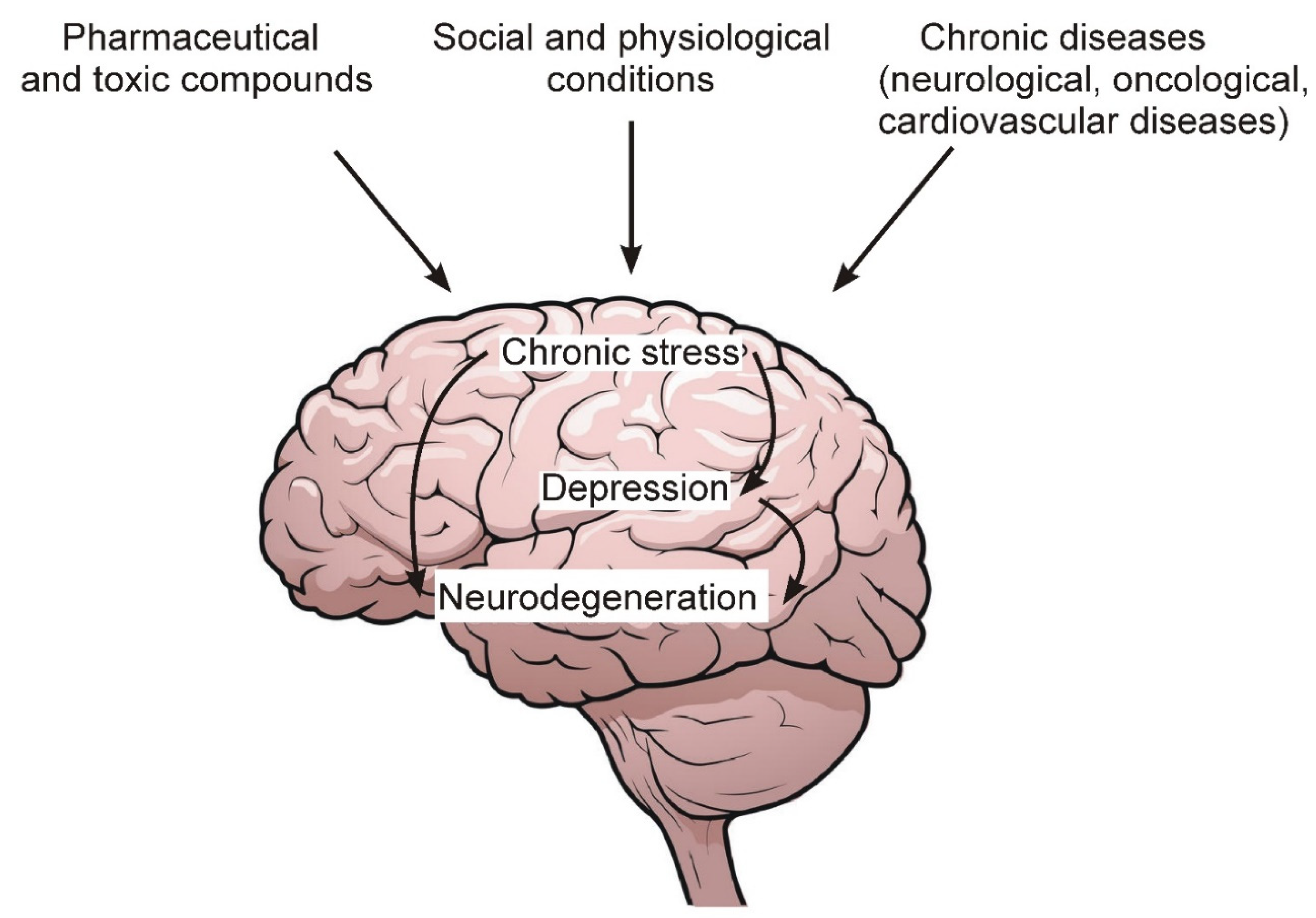
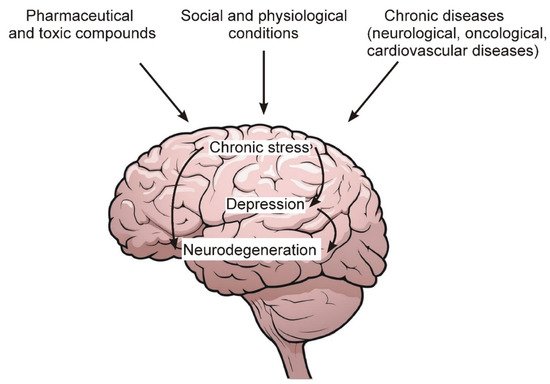
Figure 1. Some environmental factors, as well as chronic diseases that lead to the development of chronic stress and depression.
Among the main factors leading to the development of chronic stress and depression, the following should be noted:
-
Chronic pathologies of the nervous and cardiovascular systems, as well as oncological diseases.
-
Social and psychological factors related to human living conditions and contacts with surrounding members of society and the external environment.
-
Excessive and prolonged intake of various pharmaceutical preparations, as well as toxic compounds from the external environment, the number of which increases with the deterioration of the overall environmental situation in the world.
Alzheimer’s disease (AD) is the most common cause of dementia, which is estimated to account for 60% to 80% of cases [6]. AD is considered one of the main causes of morbidity and mortality among the elderly [7]. The prevalence of AD in Europe is estimated at 5.0%, which is 3.3% in men and 7.1% in women [8]. AD is a slowly progressive brain pathology that begins many years before the onset of symptoms. Clinical symptoms in early stages of AD include difficulty remembering recent conversations, names, or events, which are often accompanied by apathy and depression. In later stages of AD, symptoms include impaired communication, disorientation, behavioral changes, and ultimately difficulty speaking, swallowing, and walking [6]. AD is characterized by the accumulation of beta-amyloid peptide (Aß) (amyloid plaques) in brain tissues and a destabilization of the cytoskeleton of neurons caused by hyperphosphorylation of microtubule-associated Tau-protein. However, the poor correlation between cognitive decline and amyloid plaques raises the question of whether Aß accumulation actually causes neurodegeneration in AD. The formation of neurofibrillary tangles of Tau correlates better with neurodegeneration and clinical symptoms, and although Aß can initiate a cascade of events leading to neurodegeneration, Tau hyperphosphorylation is assumed to be key in neurodegeneration in AD [9]. The vast majority of cases of AD belong to a sporadic form (usually, late onset of symptoms). The sporadic form of AD is associated with the interaction of genetic and environmental factors, and aging is the main risk factor. Two main genetic risk factors for sporadic AD have been identified. Firstly, the presence of the APOE4 allele encoding one of the three isoforms of Apolipoprotein E (apoE2, apoE3 and apoE4), the main transporter of cholesterol in the brain, which is synthesized and secreted by astrocytes [10], is the most significant risk factor for sporadic AD [11]. Other genetic risk factors for sporadic AD are polymorphisms and mutations in a number of genes expressed in microglia, in particular, polymorphism in the TREM2 gene encoding transmembrane glycoprotein, which acts as a receptor on the surface of microglia and perceives lipids that are exposed after cell damage [12]. The familial form of AD (early onset) accounts for about 5% of cases of AD and is associated with mutations in the genes encoding the precursor protein for Aß and presenilins 1 and 2, which leads to increased aggregation of Aß, but a small part of mutations in the gene encoding presenilin 1 is not familial and occurs de novo [13].
The development of depression is accompanied by changes in the metabolism of nerve and glial cells and an impairment of synaptic transmission between neurons. Prolonged action of harmful factors can eventually lead to the degeneration of nerve cells in the brain (Figure 1). Recent ideas about the functions of astrocytes assign them an extremely important role both in the normal functioning of the brain and in the development of brain pathologies [14][15][14,15]. In particular, astrocytes are a source of neurotrophic factors, regulate synaptic transmission and neurotransmitter levels in the synaptic cleft [15][16][17][15,16,17], and regulate neurogenesis in the adult hippocampus [17][18][17,18]. Thus, astrocytes are key actors in the processes, the deregulation of which is considered as an important component of the pathogenesis of depression. Depression is a frequent symptom of AD and may precede the manifestation of AD symptoms. Stress is an important risk factor for depression [19][20][19,20], while there are no direct data on whether stress is a risk factor for AD. The etiology and mechanisms of development of both pathologies are obviously complex and unclear, but the question arises whether there are common features in relation to astrocytes and stress response.
2. The Role of Astrocytes in the Functioning of Neurons
For a long time, it was believed that the human brain contains about 100 billion neurons and about one trillion glial cells (a ratio of 1:10). However, recent studies using more advanced cell counting methods have shown that the number of glial cells in the human brain is approximately equal to the number of neurons and ranges from 40 to 130 billion [21]. A characteristic feature of glial cells, both in the brain and on the periphery, is the lack of the ability to generate and conduct nerve impulses [22]. There are three main types of glial cells: astrocytes, microglia and myelin-producing cells (oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system). Astrocytes and oligodendrocytes are the most common type of glial cells in the CNS. For quite a long period of time, the main function of astrocytes was considered to be the passive support of neurons that transmit nerve impulses, process and store information in the brain. Currently, such views have been revised, and astrocytes are assigned an important role both in the normal functioning of the CNS and in the development of various pathologies [23][24][25][23,24,25]. In the brain, astrocytes are a structural component of the so-called tripartite synapse, which includes, in addition to astrocytes, pre- and postsynaptic endings of neurons [26][27][28][26,27,28]. The morphological complexity of these cells should be particularly noted. Recent data show that one mature rodent astrocyte covers from 20,000 to 80,000 μ3 of domain space in the brain, and at the same time can interact with 300–600 neuronal dendrites [29]. Moreover, morphological studies found that mature astrocytes are able to interact with many thousands of synapses and at the same time are able to unite with other astrocytes, occupying unique spatial regions in the brain [30]. Figure 2 illustrates some functions of astrocytes in the tripartite synapse.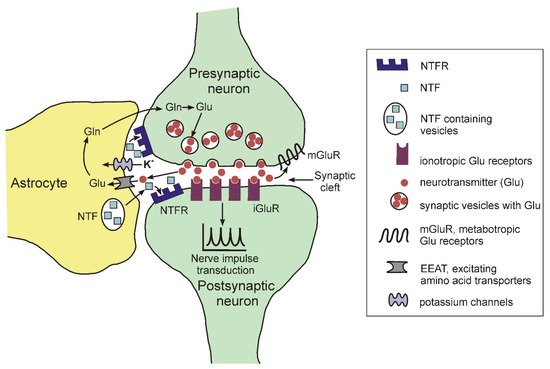
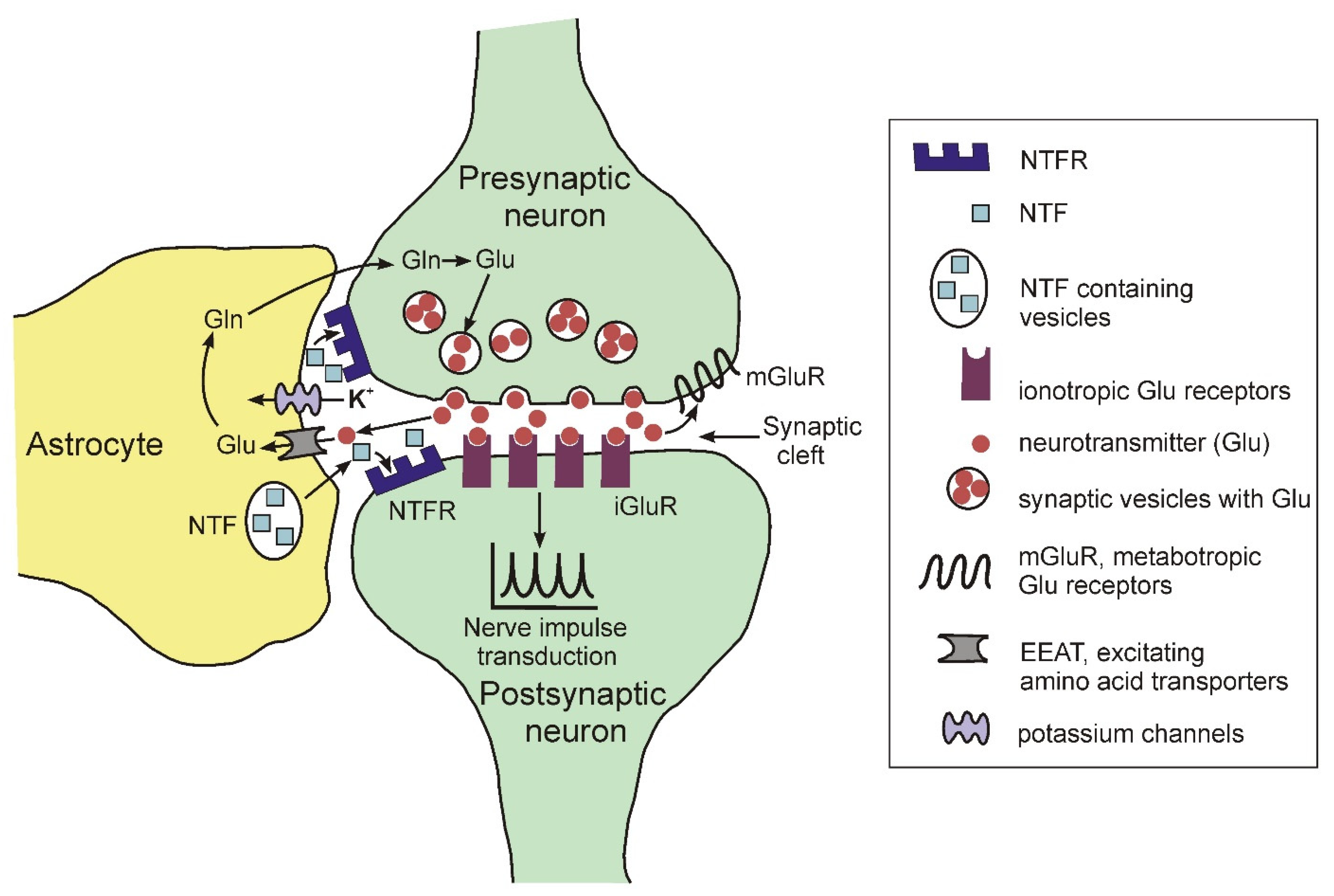
Figure 2. Schematic representation of the structure of the tripartite synapse.
-
The elimination of neurotransmitters such as glutamate, gamma-aminobutyric acid, dopamine, norepinephrine from the synaptic cleft during the transmission of a nerve impulse using specific transporters. For glutamate, EEAT serves as a transporter [31].
-
The regulation of the concentration of potassium ions in the synaptic cleft using specific inward K+ channels Kir 4.1. (for review see: [32]).
-
The expression and secretion of neurotrophic factors regulated the functioning and the viability of neurons, such as BDNF (brain-derived neurotrophic factor), FGF (fibroblast growth factor), NGF (nerve growth factor), GDNF (glial cell-derived neurotrophic factor), etc. (for review, see [33]).
