You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Maria De la Fuente.
Zebrafish (Danio rerio) is a vertebrate model species traditionally used for studying developmental biology and vertebrate genetics, and more recently, to model human diseases such as cancer. The role of zebrafish as a platform for anticancer therapy studies has been highly evidenced, allowing researchers not only to perform drug screenings but also to evaluate novel therapies such as immunotherapies and nanotherapies.
- zebrafish
- nanomedicine
- cancer
- personalized medicine
1. The Potential of Zebrafish for Preclinical Evaluation of Novel Cancer Therapeutics
Zebrafish (Danio rerio) is a vertebrate model species traditionally used for studying developmental biology and vertebrate genetics, and more recently, to model human diseases such as cancer, thus playing a key role in the discovery of new drugs for treating these illnesses [45,46,47][1][2][3]. Zebrafish characteristics define it as a model species between invertebrate models and murine models because it collects the vertebrate traits and allows large experiments [45,46][1][2]. One of the features that make zebrafish an appropriate human disease model is its homology with the human genome, around 70%, which increases to 82% in the case of human disease-related genes [48][4]. Furthermore, there are multiple advantages associated with the use of zebrafish, such as high fecundity and fertilization rate, producing a large offspring [49][5]. In addition, the external fertilization and optical transparency of embryos and larvae allow direct visualization of the overall development and enable the imaging of cells without the use of invasive techniques [50][6].
In terms of cancer research, aside from the robustness of zebrafish embryos to be easily manipulated, the adaptive immune system is not active until 2–4 weeks post-fertilization (wpf), and complete immunocompetence is only achieved at 4–6 wpf [51][7]. This feature, together with the previously mentioned transparency, enables the transplantation of fluorescent cancer cells (xenotransplantation or xenograft) and the visualization and tracking of their growth, biodistribution, metastasis, and neovascularization processes, as well as the evaluation of drug responses [50,52][6][8]. The main advantages and disadvantages of zebrafish as a model for human diseases are summarized in Table 1.
Table 1.
Benefits and drawbacks of using zebrafish for modeling human diseases in comparison with other animal models.
| Advantages | Disadvantages |
|---|---|
| Simple anatomy | Some mammalian organs are missing |
| External fertilization | Optimal temperature at 28 °C, compromising human cell viability |
| Embryo and larvae optical transparency | Lack of sexual chromosomes |
| Rapid development and sexual maturation | Pooling individuals prevent the observation of interindividual differences |
| High fertility rates | Mice genetic homology is higher |
| Large number of individuals and statistical power | Low amount of certain tissues for biological assays |
| Robust embryos | Genetic duplication |
| High homology in human disease-related genes | Protocol variability, limiting the comparison among studies |
| Late activation of the adaptive immune system | Need of mammal models for further preclinical studies |
| Cost-effective and easy maintenance | Low antibodies availability for molecular assays |
| Easy genetic manipulation | |
| Low number of cells for xenograft assays | |
| Availability of reporter lines | |
| Many existing zebrafish resources and repositories |
The set of these characteristics have allowed researchers to develop several genetic and xenotransplantation zebrafish models and thus unravel the cellular, molecular, and physiological basis of different types of cancer, as well as drug response/resistance processes.
1.1. Genetic Models
1.1.1. Forward Genetics
Several carcinogens are able to induce human-like tumors in different zebrafish organs (Figure 1) [53][9]. Thus, studies have been performed, allowing a better understanding of the carcinogenesis process, main target tissues, type of tumor, signaling pathways, and chemoprevention measures. For instance, exposure to N1-nitro-N-nitrosoguanidine (MNNG) in 86 h post-fertilization (hpf) embryos and 3 wpf fry (immersion), 72 hpf embryos (microinjection), and 6 wpf juveniles (diet) showed that embryos and fry are responsive to carcinogenic effects, whereas juveniles are remarkably resistant to neoplasia [54][10]. Embryos developed mainly hepatic and mesenchymal neoplasms, including chondroma, hemangioma, hemangiosarcoma, leiomyosarcoma, and rhabdomyosarcoma. The blood vessels and testis were the main target organs in fry, developing seminoma, hemangioma, hemangiosarcoma, and various other epithelial and mesenchymal neoplasms. Similarly, it has been shown that exposing zebrafish to Dimethylbenzanthracene (DMBA) at 3 wpf led, principally, to hepatic neoplasia in adults [55][11], with conservation of human transcriptome profiles, highlighting the potential of zebrafish for modeling human liver cancer [56][12]. Maid, a protein involved in cell proliferation, is abundantly expressed in the liver hepatocytes’ cytoplasm of zebrafish; its role as a regulator of hepatocarcinogenesis was explored by treating adult zebrafish with Diethylnitrosamine (DEN) for 8 weeks. After treatment, these fish presented distended abdomens, extremely swollen livers, and different types of liver tumors. However, Maid appeared to translocate from the cytoplasm to the hepatocyte nucleus, presumably to participate in growth-inhibitory signaling and display its tumor-suppressor activity [57][13]. It has been stated that polyploidy in lower vertebrates decreases the probability of inactivation of all alleles of tumor suppressor genes, so the incidence of tumors might be lower [58][14]. In this regard, the relationship between polyploidy and tumor formation has been investigated through N-nitrosodimethylamine (NDMA)-induced hepatocarcinogenesis [59][15]. Diploid and triploid 6 wpf zebrafish exposed to this chemical for 8 weeks developed hepatocellular adenomas and trabecular hepatocellular carcinomas after 24 weeks from the beginning of the treatment, although cholangiolar tumors were not detected in triploid fish until 36 weeks, serving as evidence that polyploidy is a protective factor in pathogenesis of this type of tumor, probably indicating a lower probability for putative tumor suppressor genes to be inactivated in polyploid cholangiolar cells.
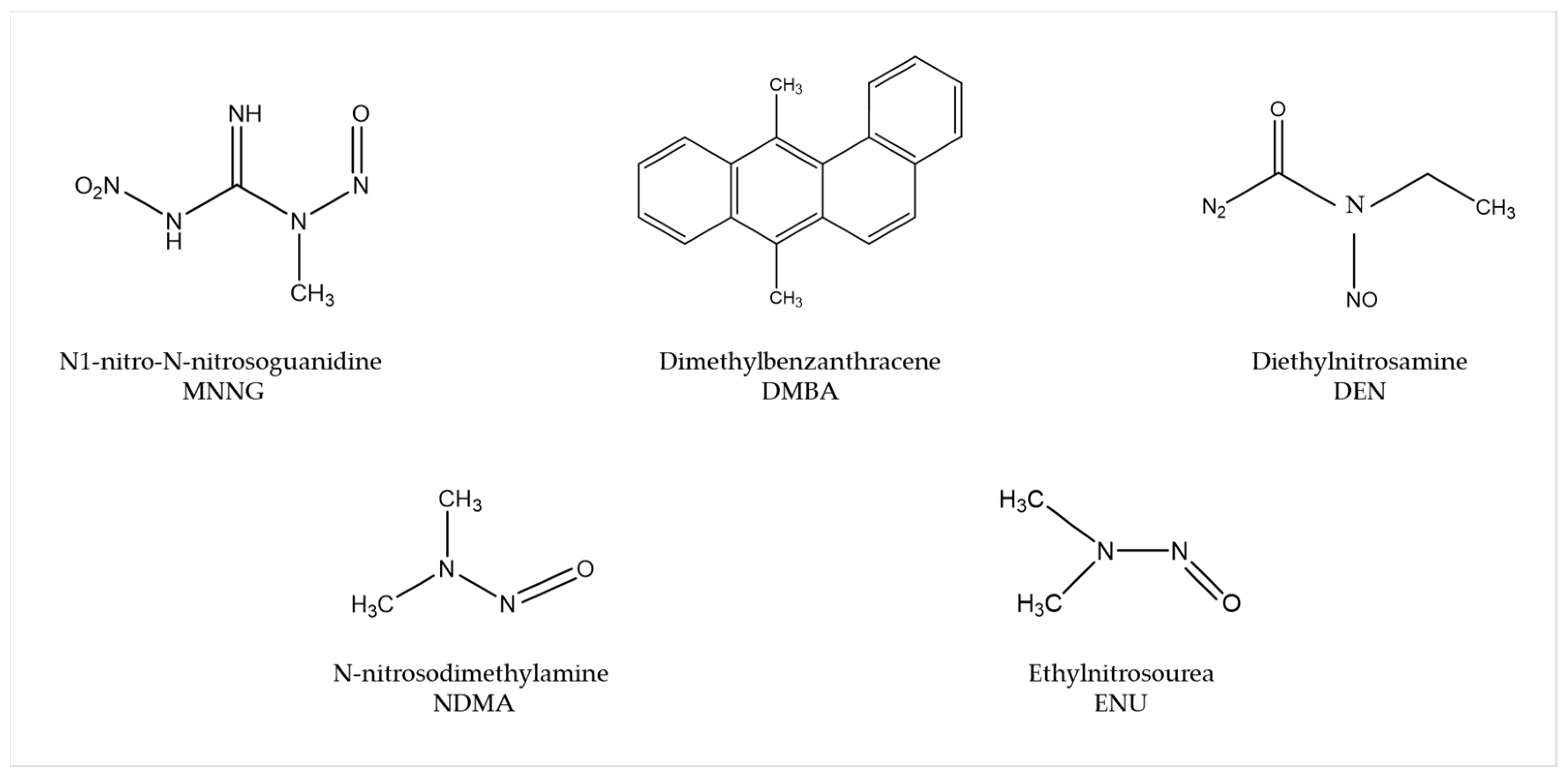
Figure 1.
Most common carcinogenic substances used for tumor induction in zebrafish.
The mutagen ethylnitrosourea (ENU) has been used to generate point mutations, leading to the identification of several mutant zebrafish lines with an increased incidence of spontaneous neoplasia or higher sensitivity to chemical exposure [53][9]. For instance, Basten et al., in an attempt to study ciliary motility defects in the lrrc50 mutant zebrafish line, unexpectedly found development of seminomas in 2-year-old adults, with a penetrance of >90%. This observation allowed establishment of a correlation between the gen and such testicular germ cell tumors (GCTs) and proposes lrrc50 as a novel tumor suppressor [60][16]. Similarly, Neumann et al., while screening for cancer susceptibility genes, isolated a zebrafish mutant line with highly penetrant, heritable testicular GCTs in which testicular tumors spontaneously developed. Indeed, DMBA or MNNG exposure resulted in enhanced germ cell tumorigenesis [61][17].
1.1.2. Transgenic Zebrafish Lines
Several zebrafish cell and tissue-specific reporter lines have been developed over the last years to improve the comprehension and characterization of different cancer traits, such as tumor cell growth, migration, invasion, angiogenesis, drug responses, or interactions with immune cells. Some examples are Tg(mpx:GFP) and Tg(mpeg1:eGFP) [62[18][19],63], which fluorescently label neutrophils and macrophages, respectively, or Tg(fli1:eGFP) [64][20], which labels the vasculature. Furthermore, human or murine oncogene transgenic expression in zebrafish has also helped to understand their role in tumor development; for example, Tg(ptf1a:eGFP-KRASG12V) in pancreatic cancer [65][21] and Tg(mitfa:HRASG12V; mitfa:GFP) or Tg(mitfa:BRAFV600E); tp53−/− for melanoma [66,67][22][23]. The binary transgenic system Gal4/UAS has also been extensively used. Gal4 is a transcriptional activator that, when expressed under the control specific tissue-specific promoters, binds to UAS enhancer sequences in the DNA, recruiting transcription machinery to induce gene expression, so genes under the control of UAS sites are expressed when Gal4 is present [68][24]. With this methodology, reseauthorchers have shown, for instance, that crossing Gal4-expressing lines with Tg(UAS:HRASG12V) transgenic line resulted in the development of different types of tumors, such as leukemia, glioma, or chordoma [69,70,71][25][26][27]. As transgenic fish with overexpression of some oncogenes might not survive to adulthood, transgenic inducible lines can also be generated, for instance, TetOn system-based transgenic lines, in which the oncogene expression is induced by doxycycline. Doxycycline-inducible expression of oncogenic KRAS in brain cells under the control of the krt5 and gfap gene promoters using the TetOn system (Tg(TRE:mCherry-KRASG12V; krt5/gfap:rtTa)) led to the development of malignant tumors in the cranial cavity and parenchymal brain tumors, respectively [72][28].
1.1.3. Reverse Genetics
Morpholinos (MO) are commonly used in zebrafish to achieve transient expression silencing without modifying the genome sequence [73][29] and thus to determine certain cancer invading mechanisms, such as angiogenesis. For instance, Jacob et al. reported that Plexin-A1 (PlexA1) could be a potential prognostic marker for glioma patients’ survival, as quantitative analysis correlates tumor grade and the level of PlexA1 expression in brain blood vessels [74][30]. They knocked down PlexA1 in Tg(kdrl:eGFP) zebrafish and observed a significant number of abnormal angiogenic sprouts in intersegmental vessels (ISVs) at 28 hpf, confirming the relevance of PlexA1 in blood vessel development. Royet et al. observed that high expression of Ephrin-B3 in human glioblastoma biopsies promotes tumor growth and angiogenesis by inhibition of EphA4-induced apoptosis [75][31]. They knocked down Ephrin-B3 in Tg(fli:EGFP) embryos and observed an impaired ISVs formation associated with an increase in apoptosis. Co-silencing of EphA4 resulted in the rescue of the angiogenic defects, suggesting that enhancing EphA4-induced cell death could be envisaged as a relevant strategy to slow glioblastoma (GBM) growth.
In order to generate stable mutant models, Targeted Induced Local Lesions in Genomes (TILLING), based on the exposure to ENU and further sequencing [76][32], has been extensively used. In this sense, mutations in tumor suppressor genes, such as tp53, pten, and apc, have been identified in ENU mutagenesis libraries, and fish were found to develop malignant peripheral nerve sheath tumors (MPNSTs), ocular hemangiosarcomas, and intestinal adenomas, hepatomas, and pancreatic adenomas, respectively [77,78,79,80][33][34][35][36]. Interestingly, ENU homozygous brca2 mutants were shown to be unable to develop ovaries during sexual differentiation, developing as infertile males that were prone to develop testicular neoplasias during adulthood [81][37]. By combining the use of vhl zebrafish ENU heterozygous mutants and the exposure to DMBA, Santhakumar et al. established the von Hippel-Lindau protein (pVHL) as a genuine tumor suppressor in zebrafish, due to the increase in the occurrence of hepatic and intestinal tumors in mutants [82][38]. Although TILLING has proven to be useful to correlate genotypes with phenotypes, the difficulty involved in the screening process, together with the possibility of having further mutations than the one desired, led researchers to introduce other methodologies, such as nuclease-based techniques, which include Zinc Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs).
ZFNs were used to generate tet2 mutants, which developed progressive clonal myelodysplasia, culminating in myelodysplastic syndrome, with dysplasia of myeloid progenitor cells and abnormal circulating erythrocytes [83][39]. As it recapitulates human TET2 loss-of-function phenotypes, this model was proposed as a platform for small-molecule screenings to identify compounds with specific activity against tet2 mutant cells. The function of the neurofibromin 1 (NF1) gene in brain tumorigenesis was explored by Shin et al. through the generation of stable mutant lines for the zebrafish orthologs (nf1a and nf1b) by ENU and ZFNs [84][40]. nf1a+/−; nf1b−/− mutants in a p53 mutant background presented an increased penetrance of high-grade gliomas MPNSTs as well as hyperactivation of ERK and mTOR pathways, consistent with mouse and human NF1-derived MPNSTs and gliomas. Similarly, bi-allelic cdkn2a/b and rb1 mutants generated by TALENs developed MPNSTs and medulloblastoma-like primitive neuroectodermal tumors, respectively, in a p53 mutant background [85][41]. Nevertheless, the design of nuclease-based systems is challenging, and there is still a high rate of off-targets. Thus, the introduction of the CRISPR/Cas9 system has allowed the optimization of the genome editing protocols and the improvement of the efficiency and accuracy of zebrafish lines.
For instance, p53/nf1-deficient fish were used by Oppel et al. to knock out by CRISPR/cas9 the atrx gene, a known tumor suppressor in gliomas or sarcomas, confirming that its loss facilitates the development of various malignancies, together with the downregulation of telomerase, which causes the alternative lengthening of telomeres [86][42]. Loss-of-function mutations in SUZ12, a subunit of the Polycomb repressive complex 2, have been identified in a variety of tumors, including MPNSTs. The knockout of suz12a and suz12b in a p53/nf1-deficient model significantly accelerated the onset and the penetrance of MPNSTs, and additional types of tumors were detected, including leukemia with histological characteristics of lymphoid malignancies, soft tissue sarcoma, and pancreatic adenocarcinoma [87][43].
These are examples of studies in which researchers developed zebrafish models harboring mutations in tumor suppressor genes and novel candidate genes, among others, to investigate their roles and unravel the relationship among mutations and the tumorigenesis and progression of different types of cancer (Figure 2).
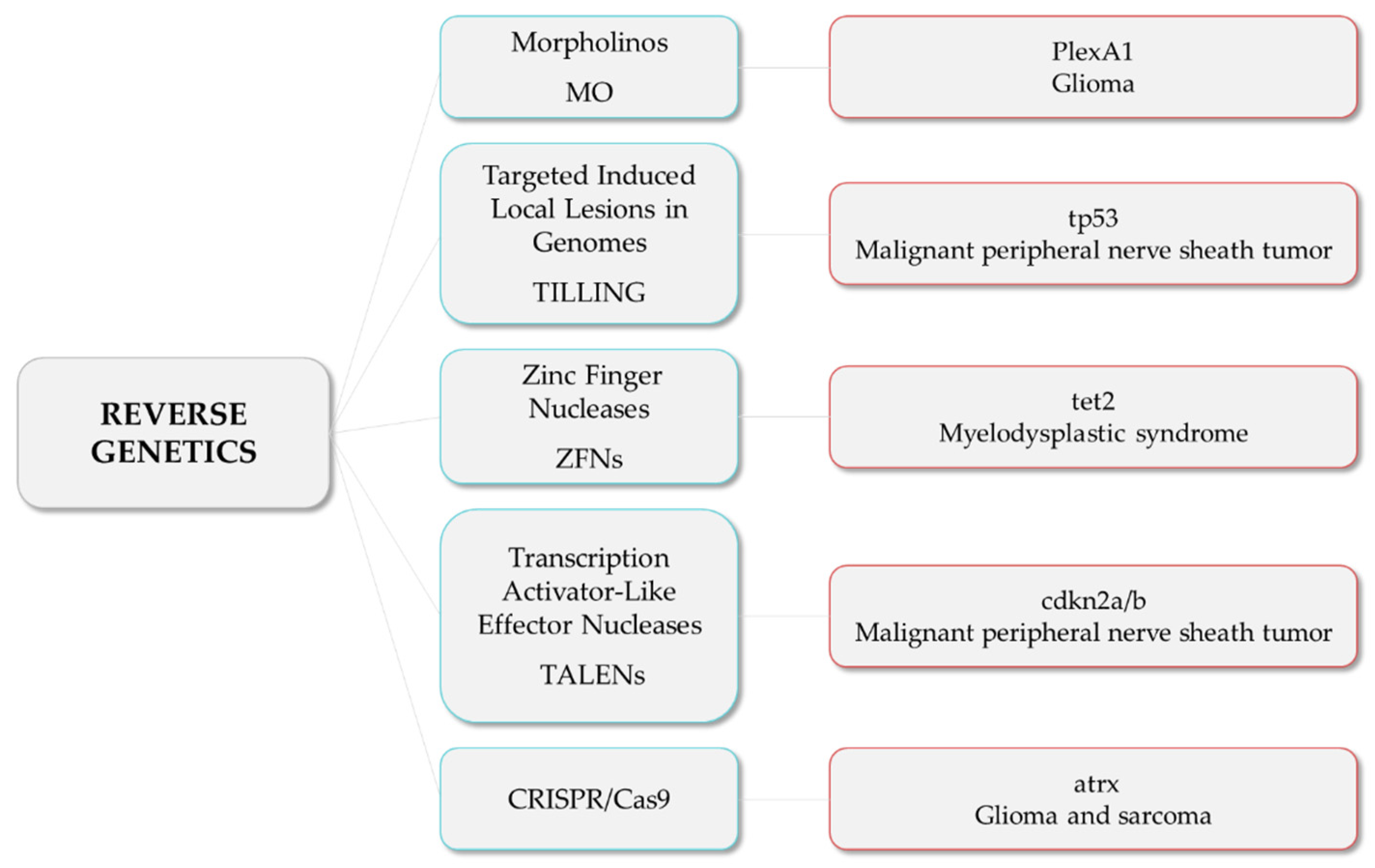
Figure 2.
Reverse genetics strategies (in blue) and their respective examples of altered genes and the associated tumor types.
1.2. Transplantation Models
Transplantation in zebrafish is based on the injection of fluorescently labeled cancer cells into zebrafish embryos. The main transplantation techniques include allotransplantation and xenotransplantation, and both can be orthotopic or heterotopic, depending on whether the cells are injected in an equivalent anatomical site to tumor origin or into a different anatomical site, respectively.
Allograft consists of tumor cells being transferred from one individual to another of the same species [88][44], while xenograft is based on the injection of labeled human, murine, or patient-derived cancer cells into zebrafish, to track their survival, engraftment, growth, behavior, and interaction with the microenvironment [89][45]. In order to avoid immune rejection in adults, transplantations require immunosuppression with sublethal γ-irradiation or dexamethasone [92,93][46][47] or the use of immunocompromised lines, such as the Rag2, lacking mature T-cells and having a reduced number of B cells, or the compound mutant prkdc−/−, il2rga−/−, lacking T, B, and natural killer (NK) cells [94,95][48][49]. In the particular case of allografts, engraftment can also be achieved without the need for immunosuppression by the transplantation from a donor fish to a genetically identical recipient (syngeneic or clonal models) [96][50], serving as a model for long-term engraftment and self-renewal potential [97,98,99][51][52][53]. An interesting approach combining different strategies allowed Ignatius et al. to confirm the role of tp53 in the development of a wide spectrum of tumors [100][54]. By using TALENs, they created tp53 mutants in which MPNSTs, angiosarcoma, germ cell tumors, and leukemia spontaneously developed during adulthood, and such tumor cells were transplantable to syngeneic fish, so engraftment of fluorescent-labeled tumors could be dynamically visualized over time. Additionally, White et al. proposed a mutant transparent recipient, known as casper zebrafish (roy−/−; nacre−/−), as a platform to study cancer cell engraftment, proliferation, and distant metastases in vivo [101][55]. Nevertheless, the prompt recovery from chemical immune ablation, the vulnerability of mutant immunocompromised fish, and the limited number of syngeneic zebrafish lines made embryo xenograft the most cost-effective technique, together with the higher number of individuals used, which increased statistical power and the reduced ethical issues in comparison with adults.
The first xenograft assay was performed by injecting melanoma cells in zebrafish blastula, which showed the ability of melanoma cells to survive, proliferate, and specifically migrate to the skin, highlighting the validity of the zebrafish for cancer research [102][56]. Since then, this technique has been improved and extended for studying several cancer features, including not only survival, proliferation, or migration, but also the ability to invade, form new blood vessels (angiogenesis), metastasize, and respond or resist different treatments. Additionally, researchers have made efforts to mimic human tumor conditions and microenvironments as much as possible, as reviewed by Cabezas-Sáinz et al. [103][57]. For instance, the use of transgenic zebrafish lines, such as the previously mentioned ones, labeling immune cells or vasculature, has led several researchers to better understand the interaction among tumor cells and macrophages or neutrophils, and their involvement in tumor growth, vascularization, invasion, and metastasis [104,105,106,107][58][59][60][61]. In this line, Allen et al. recently presented a new model for tumor cell extravasation of both individual and multicellular circulating tumor cells, known as angiopellosis, and their ability to form tumors at distant sites [108][62].
With the aim of recapitulating, not only the cellular but also the non-cellular environment provided by the specific site and/or organ orthotopic xenografts have been developed, mainly with brain tumor cells. A pioneering study was performed by Lal et al., in which GBM cells behaved differently when injected into the yolk sac or in the brain. While cells in the yolk were unable to proliferate or invade, cells injected orthotopically showed the ability to invade the brain and disperse along the vessels [109][63]. By combining MOs, orthotopic xenograft, and 4D individual tracking technology, Gamble et al. showed that laminin subunit alpha 5, an important component of blood vessels, increases the attachment of GBM cells to blood vessels, suppressing tumor invasion but promoting tumor formation [110][64]. Additionally, orthotopic brain xenografts have proven to be unique models to study the ability of different drugs to penetrate the blood–brain barrier [111][65]. Retinoblastoma has also been studied by orthotopic xenografts. The inhibition of Nodal using short hairpin (shRNA) reduced the ability of retinoblastoma cells to disseminate outside the eye, highlighting the importance of Nodal in promoting growth, proliferation, and invasion [112][66].
Although the above-mentioned techniques have helped to improve the knowledge of several cancer processes, tumors present high interindividual heterogeneity. In addition, established cancer cell lines often differ significantly from patients’ tumor cells. Thus, to preserve the patients’ tumor biological and genetic profile and improve the accuracy of tumor drug-response studies, zebrafish patient-derived xenografts (zPDXs) have arisen as a potential solution [106][60]. zPDXs are established from tumor cells or masses isolated from patients during biopsy or excision, which are subsequently hetero- or orthotopically implanted into zebrafish. The pioneers of this technique were Marques et al., who observed cell invasion and metastasis formation after injection of colon, pancreas, and stomach primary tumor samples into the yolk sac [113][67]. Since then, the survival, proliferation, angiogenesis, or invasion ability of different patient-derived tumor cells have been studied in zebrafish models, from pancreatic, colon, gastric, head and neck, or pituitary cancer, to abdominal liposarcoma or T-cell acute lymphoblastic leukemia [114,115,116,117,118][68][69][70][71][72]. Furthermore, in order to improve patients’ treatments, zPDXs have served as a platform to develop drug response/resistance assays and thus move towards personalized medicine. In this sense, several strategies are reviewed below.
2. Zebrafish as a Platform for Drug Screening
Zebrafish are used as a screening platform to adjust drug concentrations, to improve combinatorial treatments for a less toxic effect on the patient, or to overcome resistances, as well as a tool to study the mechanism of action of drugs in the organism and to alter the function of a biological pathway without previously knowing the components. Small molecule screening in zebrafish started in 2000 with a work by Peterson et al., who tested the effect of a variety of molecules in the development of vertebrate animals to understand how these molecules can be used to determine the timing of critical developmental events [119][73].
In the context of cancer, zebrafish xenotransplants have been useful as in vivo preclinical tools for drug testing. This approach has been validated by different works, showing its complementarity with other in vivo models such as the mouse [120][74]. However, xenotransplantation of human cancer cells into the zebrafish is not without difficulties. For instance, the normal growth of human cells is at 37 °C, and the temperature of development of the zebrafish is 28 °C. To overcome this issue, the field has established 31–34 °C as a consensus temperature for xenograft assays. However, these temperatures could cast some doubts about the efficiency of xenograft models for drug screening and the subsequent translation to the patient. In this sense, Cabezas-Sáinz et al. demonstrated that zebrafish larvae can live until 36 °C, allowing them to test drugs in a cancer model with characteristics close to humans [121][75]. In addition, Cornet et al. developed the ZeOnco Test, an optimized and standardized (regarding cell labeling, injection site, image acquisition, etc.) xenograft assay, aiming at reducing attrition rate [122,123][76][77]. In the same way, xenografted Tg(fli1:EGFP) transgenic models with human lung cancer cell lines were used to compare the effects of different known drugs, promoting these models as a real-time drug screening platform for clinical lung cancer patients [124][78].
Zebrafish have been used for the development of combined treatment approaches to improve treatment efficacy. An example of this goal was the investigation of Precazzini et al., where the melanoma kita:ras transgenic zebrafish line was used to test the antifungal Clotrimazol in combination with antitumoral drugs, showing a synergistic anti-melanoma effect with limited toxicity [125][79]. In addition, other reseauthorchers used the casper transgenic line to evaluate individually and in combination the antitumor activity of chemotherapy drugs used in the clinic [126][80].
In addition to pharmacological cancer treatments, there are other treatment options, such as radiotherapy, based on the use of ionizing radiation. An example is the work by Costa et al., who combined ionizing radiation and chemotherapy in colorectal cancer tumors xenografted into the nacre (casper and [Tg(fli1:EGFP)]) zebrafish line and observed that the responses achieved in the zebrafish matched the clinical responses of patients [127][81].
Transgenic Tg(fli1:EGFP) zebrafish with fluorescent vasculature has been used in a wide range of screenings in studies related to the angiogenesis process, such as new synthetic compounds [128[82][83],129], analogous molecules for drugs in use [130,131][84][85], natural compounds used in traditional medicine [132[86][87],133], and natural analogous compounds [134][88]. The combination between fluorescent vasculature and xenograft transplantation offers a potent cancer research tool to study the action of compounds in vivo, in which to test the potential of natural products in anticancer therapy [135[89][90],136], modification of natural compounds [137][91], and new chemical compound structures that are already utilized in the clinic [138][92]. Lin et al. combined the fluorescent vasculature of zebrafish with other genetic modifications and cancer cell xenotransplantation to screen and identify new anticancer molecules [139][93].
Even so, wild-type zebrafish is also used for screening of new molecules obtained from marine organisms [140,141,142][94][95][96] as well as the restudyearch of the potential of some fungicides against cancer cells [143][97].
Furthermore, other specific transgenic zebrafish were used in the restudyearch of different tumor drugs. For instance, the vhlhu2117 mutant transgenic zebrafish, which shows an excess of vascularization, was used to evaluate the antiangiogenic effect of the compound largazole [144][98]. In addition, a transgenic zebrafish for liver cancer overexpressing the oncogene KRAS was used to study the effects of environmental toxicants on tumor development and inflammatory response [145][99].
2.1. Peptides
Among the different types of biomolecules, zebrafish have proved their potential for evaluating the activity of novel peptide therapies. Cancer peptide-based therapy might play a role in the treatment of patients, and peptides can be obtained from different sources, such as natural organisms, peptide libraries, and de novo synthesis [146,147][100][101]. Some peptides produced by bacteria are also used to treat some types of cancer due to their antitumor effect. A shining example is the microcin E492, which is a peptide produced by the bacteria Klebsiella pneumoniae, which has shown antineoplasic properties in zebrafish embryos xenografted with colorectal cancer cells [148][102]. Another example, provided by Hsieh et al., studied the effect of TAT-NLS-BLBD-6, a synthesized peptide able to suppress breast cancer growth. This in vivo assay was carried out through a co-microinjection of peptide and labelled breast cells into the zebrafish yolk [149][103]. Furthermore, the evaluation of anticancer peptides in zebrafish embryos can be carried out by xenotransplantation of treated cells. An example of this assay was performed with NuBCP-9, a growth factor Nur77 derived peptide, which demonstrated apoptotic effect in paclitaxel-resistant lung tumor cells [150][104].
2.2. Gene Therapies
Zebrafish have also been used in the research of gene therapies based on the introduction of exogenous genomic materials on the organism to study or silence the expression of genes. A significant example is the research performed by Cordeiro et al., who used a specific ssDNA in the fli-EGFP transgenic zebrafish to measure the capacity of this material to reduce the GFP signal; the reduction of the EGFP emission indicates the downregulation of EGFP expression [151][105].
Transgenic zebrafish line Tg(Kdrl:eGFP)s843 has been used as an in vivo model to study the antiangiogenic effects of miRNA-based therapies. This assay was carried out with xenografts based on miRNA transfected prostate cancer cells, which allowed the evaluation of new vessel formation [152][106]. In the same vein, Kiener et al. studied the antitumor effect of a miRNA in the same type of cancer by microinjecting transgenic Tg(mpo:GFP)i114 zebrafish with miRNA transfected cells, proving the reduction of the tumor due to the miRNA effect [153][107].
2.3. Immunotherapeutics: Monoclonal Antibodies and CAR-T
Monoclonal antibodies can target cancer cells by binding to their specific surface antigens [154][108]. Zebrafish embryos play a meaningful role in the restudyearch of the anti-cancer efficacy of monoclonal antibodies, their toxicity, and the comparison between different therapies.
Zebrafish were used to study cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor (EGFR), for the treatment of colorectal and head and neck cancer [154][108]. The response of cetuximab treatment was evaluated using colorectal cancer zebrafish patient-derived xenografts (zPDX), which included the drug in the injected cell suspension, and the results showed that the zebrafish model allows the detection of differential responses to the antibody according to the KRAS mutational status of the tumor [155][109].
Furthermore, the zebrafish transgenic line Tg(fli1:EGFP) is commonly used to study the antiangiogenic capacity of drugs [156[110][111][112],157,158], such as Bevacizumab, a humanized anti-vascular endothelial growth factor (VEGF) antibody for the treatment of some solid cancers (such as breast and lung cancer). Bevacizumab was studied in this zebrafish transgenic line to define its antiangiogenic effect and antitumor capacity, in contrast to toxicity assay, which was performed in wild-type embryos [154,157,158][108][111][112]. Another monoclonal antibody studied in zebrafish models is ramucirumab, used to treat lung, gastric, and colorectal cancer. Its toxicity was assessed in wild-type zebrafish embryos, and the antiangiogenic and anticancer capacity was tested in the Tg(fli1:EGFP) line, in the same way as Bevacizumab [154,156][108][110].
Moreover, chimeric antigen receptor T cell (CAR-T cell) therapy has achieved clinical success in specific tumor types, such as several types of leukemia [159,160][113][114]. Recent studies by Pascoal et al., for the first time, evaluated the capacity of CAR-T cells to kill cancer cells in vivo in zebrafish. To carry out this assay, labelled Nalm-6 leukemia cells and CAR-T cells were injected into zebrafish vasculature. The results show that zebrafish embryos are a potential model for in vivo studies of the efficacy of CAR-T cell therapy against cancer [161][115].
2.4. Nanomedicines
2.4.1. Toxicity
Zebrafish embryos are currently used for nanomedicine toxicity testing due to advantages such as their high fertilization rate. The most common method to perform toxicity assays is the incubation of nanomedicines into zebrafish medium, usually with dechorionated zebrafish. As well as the incubation, microinjection of test drugs into zebrafish circulation ensures that the concentration is absorbed by the embryos [49][5].
Table 2.
Zebrafish-based toxicity studies of different nanoparticles for cancer therapies.
| Nanoparticles | Conditions | Higher Mortality Rate | Morphological Effects | Ref. |
|---|---|---|---|---|
| AgNPs | 3 hpf embryos 72 h incubation 28.5 °C |
100% (3 μg/mL) | Yolk sac edema Tail malformation |
[162][116] |
| AuNPs | 3 hpf embryos 72 h incubation 28.5 °C |
100% (300 mg/mL) | Yolk sac edema | [162][116] |
| MMDOX | 4 dpf embryos 72 h incubation 28 ± 1 °C |
100% (100 μg/mL) | Uninflated swim bladder Arched body Alteration of the spontaneous swimming activity |
[163][117] |
| MSNs-FA | 48 hpf embryos 72 h incubation 27 ± 1 °C |
~30% (200 μg/mL) | Hatching rate | [164][118] |
Several aspects of zebrafish embryos can be analyzed to determine the toxicity of a specific nanomedicine; some examples are compiled in Table 2. The correct hatching process, malformation appearance, the response of the immune system, and mortality are some of the guidelines to evaluate the toxicity effect of nanoparticles [165,166][119][120]. As a result, toxicity tests based on zebrafish have become an indispensable step to assess the effect of several therapies based on nanosystems, from metal-based nanoparticles to lipidic nanosystems. For instance, golden (AuNPs) and silver (AgNPs) nanoparticles for anticancer application were tested to evaluate their toxicity using zebrafish embryos. Mortality rate and morphological anomalies showed differences between nanoparticle types and concentration [162][116]. However, metal nanoparticles are not the only kind of nanomedicines evaluated by the zebrafish toxicity test. In fact, micelles loaded with doxorubicin hydrochloride (DOX-loaded mixed micelles (MMDOX)), commonly used to treat metastatic breast cancer, were tested by Calienni et al. in zebrafish embryos to rate their toxicity in vivo [163][117].
Another important example is the research of Wu et al., who used zebrafish embryos to evaluate the biosafety of mesoporous silica nanoparticles coated with folic acid (MSNs-FA) as carriers of therapeutic peptides, evaluating the embryo mortality and hatching [164][118].
Zebrafish have demonstrated their huge capacity to be a platform for testing different types of nanomaterials, not only for cancer treatment but also for other applications such as antibacterial and heart-associated disease treatment. The review of Jia et al. compiled information about different nanoparticles and their toxicity evaluation using zebrafish [167][121].
2.4.2. Biodistribution and Average Life in Circulation
In vivo behavior, distribution along the body, and interaction with tumor cells are key qualities to develop new anticancer nanomedicines; therefore, analyzing these aspects is essential to achieve a translation to the clinic of nano-based therapies. Due to this, zebrafish embryos play an important role as a platform to evaluate these properties in vivo.
Chang et al. performed an assay to evaluate differences in the distribution of polystyrene nanoparticles and glycol chitosan nanoparticles for cancer treatment along blood circulation. Adult wild-type zebrafish were retro-orbitally injected with nanoparticles to observe their capacity to circulate along the vasculature; this allowed the reseauthorchers to predict in vivo nanoparticle behavior [168][122]. In the same way, Gundersen et al. also used wild-type zebrafish to evaluate the biodistribution of chlorpromazine-loaded PEGylated PLGA nanoparticles for leukemia treatment [169][123].
In another fashion, transgenic Tg(FLK-1: mCherry) zebrafish embryos, in which endothelial cell membranes are fluorescently labeled, were used to evaluate the distribution of nanoparticles throughout the vasculature, showing the interaction of nanoparticles with the blood vessels and the ability to extravasate [170][124]. Along this transgenic line, other lines with fluorescent endothelial cells have been utilized for these types of assays, such as the Tg(kdrl:GFP)la116tg line, which allowed the observation of the endocytosis of nanoparticles by endothelial cells and their behavior inside them [171][125].
Another important trait to evaluate is the time that nanoparticles can remain in the organism. This fact depends on the composition of the nanosystem and the response of the body’s immune system, such as macrophage uptake. Leveraging the zebrafish embryo transparency, microinjected fluorescent nanoparticles can be observed over time to evaluate their capacity to stay in the organism. An example that illustrates this usage is the study performed by Wang et al., involving nanosystems that can be used to carry anticancer drugs. In this restudyearch, FITC-labelled nanospheres were microinjected and evaluated during 72 h post-injection to study the biodistribution and their elimination progress of by the organism [172][126].
As the uptake of nanoparticles by macrophages decreases their half-life in circulation, one of the main objectives is testing the capacity of avoiding this nanoparticle uptake. To carry out this type of procedure, transgenic zebrafish reporter lines for fluorescently labelled macrophages, such as Tg(mpeg1:mCherry)UMSF001 and Tg(mpeg1:EGFP), have been used [173,174][127][128]. Making use of the Tg(mpeg1:mCherry)UMSF001 line, Evensen et al. studied the differences of anticancer nanoparticles with and without polyethylene glycol, observing a decrease in the uptake of the former by macrophages [173][127].
Though the evaluation of the behavior of microinjected nanoparticles is key, it is also important to study the effect of nanoparticles that are specifically developed for external treatments. In this field, Jia et al. developed a fluorescence probe, composed of cholesterol, poly(ethylene glycol)2k, and Cy5, for imaging zebrafish cell surfaces and demonstrated their utility for the assessment of nanoparticle toxicity in zebrafish upon observation of epidermal abnormalities related to damage [175][129].
2.4.3. Anticancer Drug Delivery in Targeted Medicine
The transgenic Tg(FLK-1:EGFP) zebrafish line, which has green fluorescent endothelial cells, is one of the most common lines used to evaluate the antiangiogenesis capacity of drugs, including nanomedicines. The antiangiogenic effect of curcumin polymeric micelles was evaluated in this transgenic line, resulting in an effective inhibition of embryonic angiogenesis as well as tumor-derived angiogenesis owing to tumor cell xenotransplantation [170][124].
The microinjection of cancer cells allows assessing the capacity of nanoparticles to interact with xenotransplanted cells as well as their antitumoral efficacy [176,177,178,179][130][131][132][133]. A recent example is the work of Saraiva et al., who evaluated tumor reduction in xenografted zebrafish embryos treated with nanoemulsions comprising edelfosine, as a triple negative breast cancer treatment [180][134]. In a similar way, Moret et al. used zebrafish embryos that were the offspring of Casper mutants and fli1a:EGFP transgenic zebrafish to evaluate the efficacy of biodegradable poly(ethylene glycol)-poly(ε-caprolactone) nanoparticles, loaded with docetaxel, for epithelial cancer treatment, by measuring mass tumor reduction and antiangiogenic effect [181][135].
In addition, metastasis modelling in zebrafish embryos can be performed as a result of spreading across the circulation of xenotransplanted tumor cells. The microinjection or incubation of different types of antitumoral drugs allows the evaluation of their capacity to inhibit these metastatic processes [182,183][136][137].
References
- Delvecchio, C.; Tiefenbach, J.; Krause, H.M. The Zebrafish: A powerful platform for in vivo, HTS drug discovery. Assay Drug Dev. Technol. 2011, 9, 354–361.
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367.
- Zon, L.I. Zebrafish: A new model for human disease. Genome Res. 1999, 9, 99–100.
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503.
- Sieber, S.; Grossen, P.; Bussmann, J.; Campbell, F.; Kros, A.; Witzigmann, D.; Huwyler, J. Zebrafish as a preclinical in vivo screening model for nanomedicines. Adv. Drug Deliv. Rev. 2019, 151–152, 152–168.
- Zhao, S.; Huang, J.; Ye, J. A fresh look at zebrafish from the perspective of cancer research. J. Exp. Clin. Cancer Res. 2015, 34, 80.
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28.
- Zon, L.I.; Peterson, R. The new age of chemical screening in Zebrafish. Zebrafish 2010, 7, 1.
- Raby, L.; Völkel, P.; Le Bourhis, X.; Angrand, P.O. Genetic engineering of zebrafish in cancer research. Cancers 2020, 12, 2168.
- Spitsbergen, J.M.; Tsai, H.W.; Reddy, A.; Miller, T.; Arbogast, D.; Hendricks, J.D.; Bailey, G.S. Neoplasia in zebrafish (Danio rerio) treated with N-methyl-N’-nitro-N-nitrosoguanidine by three exposure routes at different developmental stages. Toxicol. Pathol. 2000, 28, 716–725.
- Spitsbergen, J.M.; Tsai, H.W.; Reddy, A.; Miller, T.; Arbogast, D.; Hendricks, J.D.; Bailey, G.S. Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenzanthracene by two exposure routes at different developmental stages. Toxicol. Pathol. 2000, 28, 705–715.
- Siew, H.L.; Yi, L.W.; Vega, V.B.; Miller, L.D.; Spitsbergen, J.; Tong, Y.; Zhan, H.; Govindarajan, K.R.; Lee, S.; Mathavan, S.; et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat. Biotechnol. 2006, 24, 73–75.
- Fujisawa, K.; Terai, S.; Matsumoto, T.; Takami, T.; Yamamoto, N.; Nishina, H.; Furutani-Seiki, M.; Sakaida, I. Evidence for a role of the transcriptional regulator Maid in tumorigenesis and aging. PLoS ONE 2015, 10, e0129950.
- Thorgaard, G.H.; Arbogast, D.N.; Hendricks, J.D.; Pereira, C.B.; Bailey, G.S. Tumor suppression in triploid trout. Aquat. Toxicol. 1999, 46, 121–126.
- Mizgireuv, I.V.; Majorova, I.G.; Gorodinskaya, V.M.; Khudoley, V.V.; Revskoy, S.Y. Carcinogenic Effect of N-Nitrosodimethylamine on Diploid and Triploid Zebrafish (Danio rerio). Toxicol. Pathol. 2016, 32, 514–518.
- Basten, S.G.; Davis, E.E.; Gillis, A.J.M.; van Rooijen, E.; Stoop, H.; Babala, N.; Logister, I.; Heath, Z.G.; Jonges, T.N.; Katsanis, N.; et al. Mutations in LRRC50 Predispose Zebrafish and Humans to Seminomas. PLoS Genet. 2013, 9, e1003384.
- Neumann, J.C.; Dovey, J.S.; Chandler, G.L.; Carbajal, L.; Amatruda, J.F. Identification of a heritable model of testicular germ cell tumor in the zebrafish. Zebrafish 2009, 6, 319–327.
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.I.; Elworthy, S.; Ingham, P.W.; Whyte, M.K.B. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–3978.
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49.
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318.
- Park, S.W.; Davison, J.M.; Rhee, J.; Hruban, R.H.; Maitra, A.; Leach, S.D. Oncogenic KRAS Induces Progenitor Cell Expansion and Malignant Transformation in Zebrafish Exocrine Pancreas. Gastroenterology 2008, 134, 2080–2090.
- Michailidou, C.; Jones, M.; Walker, P.; Kamarashev, J.; Kelly, A.; Hurlstone, A.F.L. Dissecting the roles of Raf- and PI3K-signalling pathways in melanoma formation and progression in a zebrafish model. DMM Dis. Model. Mech. 2009, 2, 399–411.
- Patton, E.E.; Widlund, H.R.; Kutok, J.L.; Kopani, K.R.; Amatruda, J.F.; Murphey, R.D.; Berghmans, S.; Mayhall, E.A.; Traver, D.; Fletcher, C.D.M.; et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 2005, 15, 249–254.
- Busson, D.; Pret, A.-M. GAL4/UAS Targeted Gene Expression for Studying Drosophila Hedgehog Signaling. In Hedgehog Signaling Protocols; Humana Press: Totowa, NJ, USA, 2007; Volume 397, pp. 161–201.
- Alghisi, E.; Distel, M.; Malagola, M.; Anelli, V.; Santoriello, C.; Herwig, L.; Krudewig, A.; Henkel, C.V.; Russo, D.; Mione, M.C. Targeting oncogene expression to endothelial cells induces proliferation of the myelo-erythroid lineage by repressing the notch pathway. Leukemia 2013, 27, 2229–2241.
- Mayrhofer, M.; Gourain, V.; Reischl, M.; Affaticati, P.; Jenett, A.; Joly, J.S.; Benelli, M.; Demichelis, F.; Poliani, P.L.; Sieger, D.; et al. A novel brain tumour model in zebrafish reveals the role of YAP activation in MAPK- and PI3K-induced malignant growth. DMM Dis. Model. Mech. 2017, 10, 15–28.
- Burger, A.; Vasilyev, A.; Tomar, R.; Selig, M.K.; Nielsen, G.P.; Peterson, R.T.; Drummond, I.A.; Haber, D.A. A zebrafish model of chordoma initiated by notochord-driven expression of HRASV12. DMM Dis. Model. Mech. 2014, 7, 907–913.
- Ju, B.; Chen, W.; Orr, B.A.; Spitsbergen, J.M.; Jia, S.; Eden, C.J.; Henson, H.E.; Taylor, M.R. Oncogenic KRAS promotes malignant brain tumors in zebrafish. Mol. Cancer 2015, 14, 18.
- Nasevicius, A.; Ekker, S.C. Effective targeted gene “knockdown” in zebrafish. Nat. Genet. 2000, 26, 216–220.
- Jacob, L.; Sawma, P.; Garnier, N.; Meyer, L.A.T.; Fritz, J.; Hussenet, T.; Spenlé, C.; Goetz, J.; Vermot, J.; Fernandez, A.; et al. Inhibition of PlexA1-mediated brain tumor growth and tumor-associated angiogenesis using a transmembrane domain targeting peptide. Oncotarget 2016, 7, 57851–57865.
- Royet, A.; Broutier, L.; Coissieux, M.M.; Malleval, C.; Gadot, N.; Maillet, D.; Gratadou-Hupon, L.; Bernet, A.; Nony, P.; Treilleux, I.; et al. Ephrin-B3 supports glioblastoma growth by inhibiting apoptosis induced by the dependence receptor EphA4. Oncotarget 2017, 8, 23750–23759.
- Wienholds, E.; van Eeden, F.; Kosters, M.; Mudde, J.; Plasterk, R.H.A.; Cuppen, E. Efficient target-selected mutagenesis in zebrafish. Genome Res. 2003, 13, 2700–2707.
- Berghmans, S.; Murphey, R.D.; Wienholds, E.; Neuberg, D.; Kutok, J.L.; Fletcher, C.D.M.; Morris, J.P.; Liu, T.X.; Schulte-Merker, S.; Kanki, J.P.; et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 407–412.
- Faucherre, A.; Taylor, G.S.; Overvoorde, J.; Dixon, J.E.; Den Hertog, J. Zebrafish pten genes have overlapping and non-redundant functions in tumorigenesis and embryonic development. Oncogene 2008, 27, 1079–1086.
- Choorapoikayil, S.; Kuiper, R.V.; De Bruin, A.; Den Hertog, J. Haploinsufficiency of the genes encoding the tumor suppressor Pten predisposes zebrafish to hemangiosarcoma. DMM Dis. Model. Mech. 2012, 5, 241–247.
- Haramis, A.P.G.; Hurlstone, A.; van der Velden, Y.; Begthel, H.; van den Born, M.; Offerhaus, G.J.A.; Clevers, H.C. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006, 7, 444–449.
- Shive, H.R.; West, R.R.; Embree, L.J.; Azuma, M.; Sood, R.; Liu, P.; Hicksteina, D.D. Brca2 in zebrafish ovarian development, spermatogenesis, and tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 19350–19355.
- Santhakumar, K.; Judson, E.C.; Elks, P.M.; McKee, S.; Elworthy, S.; Van Rooijen, E.; Walmsley, S.S.; Renshaw, S.A.; Cross, S.S.; Van Eeden, F.J.M. A zebrafish model to study and therapeutically manipulate hypoxia signaling in tumorigenesis. Cancer Res. 2012, 72, 4017–4027.
- Gjini, E.; Mansour, M.R.; Sander, J.D.; Moritz, N.; Nguyen, A.T.; Kesarsing, M.; Gans, E.; He, S.; Chen, S.; Ko, M.; et al. A Zebrafish Model of Myelodysplastic Syndrome Produced through tet2 Genomic Editing. Mol. Cell. Biol. 2015, 35, 789–804.
- Shin, J.; Padmanabhan, A.; De Groh, E.D.; Lee, J.S.; Haidar, S.; Dahlberg, S.; Guo, F.; He, S.; Wolman, M.A.; Granato, M.; et al. Zebrafish neurofibromatosis type 1 genes have redundant functions in tumorigenesis and embryonic development. DMM Dis. Model. Mech. 2012, 5, 881–894.
- Shim, J.; Choi, J.H.; Park, M.H.; Kim, H.; Kim, J.H.; Kim, S.Y.; Hong, D.; Kim, S.; Lee, J.E.; Kim, C.H.; et al. Development of zebrafish medulloblastoma-like PNET model by TALEN-mediated somatic gene inactivation. Oncotarget 2017, 8, 55280–55297.
- Oppel, F.; Tao, T.; Shi, H.; Ross, K.N.; Zimmerman, M.W.; He, S.; Tong, G.; Aster, J.C.; Thomas Look, A. Loss of atrx cooperates with p53-deficiency to promote the development of sarcomas and other malignancies. PLoS Genet. 2019, 15, e1008039.
- Oppel, F.; Ki, D.H.; Zimmerman, M.W.; Ross, K.N.; Tao, T.; Shi, H.; He, S.; Aster, J.C.; Look, A.T. suz12 inactivation in p53- And nf1-deficient zebrafish accelerates the onset of malignant peripheral nerve sheath tumors and expands the spectrum of tumor types. DMM Dis. Model. Mech. 2020, 13, dmm042341.
- Moore, J.C.; Langenau, D.M. Allograft cancer cell transplantation in zebrafish. In Advances in Experimental Medicine and Biology; Springer: Cham, Germany, 2016; Volume 916, pp. 265–287.
- Nicoli, S.; Presta, M. The zebrafish/tumor xenograft angiogenesis assay. Nat. Protoc. 2007, 2, 2918–2923.
- Khan, N.; Mahajan, N.K.; Sinha, P.; Jayandharan, G.R. An efficient method to generate xenograft tumor models of acute myeloid leukemia and hepatocellular carcinoma in adult zebrafish. Blood Cells, Mol. Dis. 2019, 75, 48–55.
- Gómez-Abenza, E.; Ibáñez-Molero, S.; García-Moreno, D.; Fuentes, I.; Zon, L.I.; Mione, M.C.; Cayuela, M.L.; Gabellini, C.; Mulero, V. Zebrafish modeling reveals that SPINT1 regulates the aggressiveness of skin cutaneous melanoma and its crosstalk with tumor immune microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 405.
- Tang, Q.; Abdelfattah, N.S.; Blackburn, J.S.; Moore, J.C.; Martinez, S.A.; Moore, F.E.; Lobbardi, R.; Tenente, I.M.; Ignatius, M.S.; Berman, J.N.; et al. Optimized cell transplantation using adult rag2 mutant zebrafsh. Nat. Methods 2014, 11, 821–824.
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D.; et al. Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 2019, 177, 1903–1914.
- Letrado, P.; De Miguel, I.; Lamberto, I.; Díez-Martínez, R.; Oyarzabal, J. Zebrafish: Speeding up the cancer drug discovery process. Cancer Res. 2018, 78, 6048–6058.
- Blackburn, J.S.; Liu, S.; Langenau, D.M. Quantifying the frequency of tumor-propagating cells using limiting dilution cell transplantation in syngeneic zebrafish. J. Vis. Exp. 2011, 14, e2790.
- Smith, A.C.H.; Raimondi, A.R.; Salthouse, C.D.; Ignatius, M.S.; Blackburn, J.S.; Mizgirev, I.V.; Storer, N.Y.; De Jong, J.L.O.; Chen, A.T.; Zhou, Y.; et al. High-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell acute lymphoblastic leukemia. Blood 2010, 115, 3296–3303.
- Blackburn, J.S.; Liu, S.; Wilder, J.L.; Dobrinski, K.P.; Lobbardi, R.; Moore, F.E.; Martinez, S.A.; Chen, E.A.; Lee, C.; Langenau, D.M. Clonal evolution enhances leukemia-propagating cell frequency in T cell acute lymphoblastic leukemia through Akt/mTORC1 pathway activation. Cancer Cell 2014, 25, 366–378.
- Ignatius, M.S.; Hayes, M.N.; Moore, F.E.; Tang, Q.; Garcia, S.P.; Blackburn, P.R.; Baxi, K.; Wang, L.; Jin, A.; Ramakrishnan, A.; et al. Tp53 deficiency causes a wide tumor spectrum and increases embryonal rhabdomyosarcoma metastasis in zebrafish. eLife 2018, 7, e37202.
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent Adult Zebrafish as a Tool for In Vivo Transplantation Analysis. Cell Stem Cell 2008, 2, 183–189.
- Lee, L.M.J.; Seftor, E.A.; Bonde, G.; Cornell, R.A.; Hendrix, M.J.C. The fate of human malignant melanoma cells transplanted into zebrafish embryos: Assessment of migration and cell division in the absence of tumor formation. Dev. Dyn. 2005, 233, 1560–1570.
- Cabezas-Sáinz, P.; Pensado-López, A.; Sáinz, B.; Sánchez, L. Modeling Cancer Using Zebrafish Xenografts: Drawbacks for Mimicking the Human Microenvironment. Cells 2020, 9, 1978.
- He, S.; Lamers, G.E.; Beenakker, J.W.M.; Cui, C.; Ghotra, V.P.; Danen, E.H.; Meijer, A.H.; Spaink, H.P.; Snaar-Jagalska, B.E. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol. 2012, 227, 431–445.
- Roh-Johnson, M.; Shah, A.N.; Stonick, J.A.; Poudel, K.R.; Kargl, J.; Yang, G.H.; di Martino, J.; Hernandez, R.E.; Gast, C.E.; Zarour, L.R.; et al. Macrophage-Dependent Cytoplasmic Transfer during Melanoma Invasion In Vivo. Dev. Cell 2017, 43, 549–562.
- Hill, D.; Chen, L.; Snaar-Jagalska, E.; Chaudhry, B. Embryonic zebrafish xenograft assay of human cancer metastasis. F1000Research 2018, 7, 1682.
- Britto, D.D.; Wyroba, B.; Chen, W.; Lockwood, R.A.; Tran, K.B.; Shepherd, P.R.; Hall, C.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. Macrophages enhance Vegfa-driven angiogenesis in an embryonic zebrafish tumour xenograft model. DMM Dis. Model. Mech. 2018, 11, dmm035998.
- Allen, T.A.; Asad, D.; Amu, E.; Taylor Hensley, M.; Cores, J.; Vandergriff, A.; Tang, J.; Dinh, P.U.; Shen, D.; Qiao, L.; et al. Circulating tumor cells exit circulation while maintaining multicellularity, augmenting metastatic potential. J. Cell Sci. 2019, 132, jcs231563.
- Lal, S.; La Du, J.; Tanguay, R.L.; Greenwood, J.A. Calpain 2 is required for the invasion of glioblastoma cells in the zebrafish brain microenvironment. J. Neurosci. Res. 2012, 90, 769–781.
- Gamble, J.T.; Reed-Harris, Y.; Barton, C.L.; La Du, J.; Tanguay, R.; Greenwood, J.A. Quantification of glioblastoma progression in zebrafish xenografts: Adhesion to laminin alpha 5 promotes glioblastoma microtumor formation and inhibits cell invasion. Biochem. Biophys. Res. Commun. 2018, 506, 833–839.
- Zeng, A.; Ye, T.; Cao, D.; Huang, X.; Yang, Y.; Chen, X.; Xie, Y.; Yao, S.; Zhao, C. Identify a Blood-Brain Barrier Penetrating Drug-TNB using Zebrafish Orthotopic Glioblastoma Xenograft Model. Sci. Rep. 2017, 7, 14372.
- Asnaghi, L.; White, D.T.; Yoon, L.; Price, A.; Lee, G.Y.; Sahoo, A.; Mumm, J.S.; Eberhart, C.G. Downregulation of Nodal inhibits metastatic progression in retinoblastoma. Acta Neuropathol. Commun. 2019, 7, 137.
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.D.; Lerch, M.M.; Bagowski, C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2009, 9, 128.
- Usai, A.; Di Franco, G.; Colucci, P.; Pollina, L.E.; Vasile, E.; Funel, N.; Palmeri, M.; Dente, L.; Falcone, A.; Morelli, L.; et al. A model of a zebrafish avatar for co-clinical trials. Cancers 2020, 12, 677.
- Al-Samadi, A.; Tuomainen, K.; Kivimäki, A.; Salem, A.; Al-Kubati, S.; Hyytiäinen, A.; Parikka, M.; Mesimäki, K.; Wilkman, T.; Mäkitie, A.; et al. PCR-based zebrafish model for personalised medicine in head and neck cancer. J. Transl. Med. 2019, 17, 235.
- Peverelli, E.; Giardino, E.; Treppiedi, D.; Meregalli, M.; Belicchi, M.; Vaira, V.; Corbetta, S.; Verdelli, C.; Verrua, E.; Serban, A.L.; et al. Dopamine receptor type 2 (DRD2) and somatostatin receptor type 2 (SSTR2) agonists are effective in inhibiting proliferation of progenitor/stem-like cells isolated from nonfunctioning pituitary tumors. Int. J. Cancer 2017, 140, 1870–1880.
- Liverani, C.; La Manna, F.; Groenewoud, A.; Mercatali, L.; Van Der Pluijm, G.; Pieri, F.; Cavaliere, D.; De Vita, A.; Spadazzi, C.; Miserocchi, G.; et al. Innovative approaches to establish and characterize primary cultures: An ex vivo 3D system and the zebrafish model. Biol. Open 2017, 6, 133–140.
- Bentley, V.L.; Veinotte, C.J.; Corkery, D.P.; Pinder, J.B.; Leblanc, M.A.; Bedard, K.; Weng, A.P.; Berman, J.N.; Dellaire, G. Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia. Haematologica 2015, 100, 70–76.
- Peterson, R.T.; Link, B.A.; Dowling, J.E.; Schreiber, S.L. Small molecule developmental screens reveal the logic and timing of vertebrate development. Natl. Acad Sci. 2020, 97, 12965–12969.
- Gianoncelli, A.; Guarienti, M.; Fragni, M.; Bertuzzi, M.; Rossini, E.; Abate, A.; Basnet, R.M.; Zizioli, D.; Bono, F.; Terzolo, M.; et al. Adrenocortical Carcinoma Xenograft in Zebrafish Embryos as a Model To Study the In Vivo Cytotoxicity of Abiraterone Acetate. Endocrinology 2019, 160, 2620–2629.
- Cabezas-Sainz, P.; Guerra-Varela, J.; Carreira, M.J.; Mariscal, J.; Roel, M.; Rubiolo, J.A.; Sciara, A.A.; Abal, M.; Botana, L.M.; López, R.; et al. Improving zebrafish embryo xenotransplantation conditions by increasing incubation temperature and establishing a proliferation index with ZFtool. BMC Cancer 2018, 18, 3.
- Cornet, C.; Dyballa, S.; Terriente, J.; Di Giacomo, V. ZeOncoTest: Refining and Automating the Zebrafish Xenograft Model for Drug Discovery in Cancer. Pharmaceuticals 2019, 13, 1.
- ZeClinics. Powering Discovery with Zebrafish. Available online: https://www.zeclinics.com/ (accessed on 7 May 2020).
- Li, X.; Huang, L.; Wu, J.; He, M.; Zhu, S.; Zhan, P.; Lv, T.; Song, Y. Zebrafish Xenograft Model of Human Lung Cancer for Evaluating Osimertinib Resistance. BioMed Res. Int. 2019, 2019, 3129748.
- Precazzini, F.; Pancher, M.; Gatto, P.; Tushe, A.; Adami, V.; Anelli, V.; Mione, M.C. Automated in vivo screen in zebrafish identifies Clotrimazole as targeting a metabolic vulnerability in a melanoma model. Dev. Biol. 2020, 457, 215–225.
- Xu, Z.; Hu, C.; Chen, S.; Zhang, C.; Yu, J.; Wang, X.; Lv, H.; Cheng, X. Apatinib enhances chemosensitivity of gastric cancer to paclitaxel and 5-fluorouracil. Cancer Manag. Res. 2019, 11, 4905–4915.
- Costa, B.; Ferreira, S.; Póvoa, V.; Cardoso, M.J.; Vieira, S.; Stroom, J.; Fidalgo, P.; Rio-Tinto, R.; Figueiredo, N.; Parés, O.; et al. Developments in zebrafish avatars as radiotherapy sensitivity reporters—Towards personalized medicine. EBioMedicine 2020, 51, 102578.
- Ai, N.; Chong, C.M.; Chen, W.; Hu, Z.; Su, H.; Chen, G.; Wong, Q.W.L.; Ge, W. Ponatinib exerts anti-angiogenic effects in the zebrafish and human umbilical vein endothelial cells via blocking VEGFR signaling pathway. Oncotarget 2018, 9, 31958–31970.
- Reddy, V.G.; Reddy, T.S.; Jadala, C.; Reddy, M.S.; Sultana, F.; Akunuri, R.; Bhargava, S.K.; Wlodkowic, D.; Srihari, P.; Kamal, A. Pyrazolo-benzothiazole hybrids: Synthesis, anticancer properties and evaluation of antiangiogenic activity using in vitro VEGFR-2 kinase and in vivo transgenic zebrafish model. Eur. J. Med. Chem. 2019, 182, 111609.
- Mercurio, A.; Sharples, L.; Corbo, F.; Franchini, C.; Vacca, A.; Catalano, A.; Carocci, A.; Kamm, R.D.; Pavesi, A.; Adriani, G. Phthalimide Derivative Shows Anti-angiogenic Activity in a 3D Microfluidic Model and No Teratogenicity in Zebrafish Embryos. Front. Pharmacol. 2019, 10, 349.
- Beedie, S.; Rore, M.H.; Barnett, S.; Chau, H.C.; Luo, W.; Greig, N.H.; Figg, W.D.; Vargesson, N. In vivo screening and discovery of novel candidate thalidomide analogs in the zebrafish embryo and chicken embryo model systems. Oncotarget 2016, 7, 33237–33245.
- Yang, B.; Wang, N.; Wang, S.; Li, X.; Zheng, Y.; Li, M.; Song, J.; Zhang, F.; Mei, W.; Lin, Y.; et al. Network-pharmacology-based identification of caveolin-1 as a key target of Oldenlandia diffusa to suppress breast cancer metastasis. Biomed. Pharmacother. 2019, 112, 108607.
- Song, Z.; Zhang, Y.; Zhang, H.; Rajendran, R.S.; Wang, R.; Hsiao, C.-D.; Li, J.; Xia, Q.; Liu, K. Isoliquiritigenin triggers developmental toxicity and oxidative stress–mediated apoptosis in zebrafish embryos/larvae via Nrf2-HO1/JNK-ERK/mitochondrion pathway. Chemosphere 2020, 246, 125727.
- Wang, G.; Xiao, Q.; Wu, Y.; Wei, Y.; Jing, Y.; Cao, X.; Gong, Z. Design and synthesis of novel celastrol derivative and its antitumor activity in hepatoma cells and antiangiogenic activity in zebrafish. J. Cell. Physiol. 2019, 234, 16431–16446.
- Yang, Y.; Hao, E.; Pan, X.; Tan, D.; Du, Z.; Xie, J.; Hou, X.; Deng, J.; Wei, K. Gomisin M2 from Baizuan suppresses breast cancer stem cell proliferation in a zebrafish xenograft model. Aging (Albany NY) 2019, 11, 8347–8361.
- Liu, J.S.; Huo, C.Y.; Cao, H.H.; Fan, C.L.; Hu, J.Y.; Deng, L.J.; Lu, Z.-B.; Yang, H.Y.; Yu, L.Z.; Mo, Z.X.; et al. Aloperine induces apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma cells through the PI3K/Akt signaling pathway. Phytomedicine 2019, 61, 152843.
- Wu, Q.; Zheng, K.; Huang, X.; Li, L.; Mei, W. Tanshinone-IIA-Based Analogues of Imidazole Alkaloid Act as Potent Inhibitors to Block Breast Cancer Invasion and Metastasis in Vivo. J. Med. Chem. 2018, 61, 10488–10501.
- Zhang, Y.; Hou, Q.; Li, X.; Zhu, J.; Wang, W.; Li, B.; Zhao, L.; Xia, H. Enrichment of novel quinazoline derivatives with high antitumor activity in mitochondria tracked by its self-fluorescence. Eur. J. Med. Chem. 2019, 178, 417–432.
- Lin, H.-S.; Huang, Y.-L.; Wang, Y.-R.S.; Hsiao, E.; Hsu, T.-A.; Shiao, H.-Y.; Jiaang, W.-T.; Sampurna, B.P.; Lin, K.-H.; Wu, M.-S.; et al. Identification of Novel Anti-Liver Cancer Small Molecules with Better Therapeutic Index Than Sorafenib via Zebrafish Drug Screening Platform. Cancers 2019, 11, 739.
- Costa, M.; Rosa, F.; Ribeiro, T.; Hernandez-Bautista, R.; Bonaldo, M.; Silva, N.G.; Eiríksson, F.; Thorsteinsdóttir, M.; Ussar, S.; Urbatzka, R. Identification of cyanobacterial strains with potential for the treatment of obesity-related co-morbidities by bioactivity, toxicity evaluation and metabolite profiling. Mar. Drugs 2019, 17, 280.
- Carrillo, P.; Martínez-Poveda, B.; Cheng-Sánchez, I.; Guerra, J.; Tobia, C.; López-Romero, J.M.; Sarabia, F.; Medina, M.Á.; Quesada, A.R. Exploring the Antiangiogenic Potential of Solomonamide A Bioactive Precursors: In Vitro and in Vivo Evidences of the Inhibitory Activity of Solo F-OH During Angiogenesis. Mar. Drugs 2019, 17, 228.
- Long, W.; Wang, M.; Luo, X.; Huang, G.; Chen, J. Murrangatin suppresses angiogenesis induced by tumor cell–derived media and inhibits AKT activation in zebrafish and endothelial cells. Drug Des. Devel. Ther. 2018, 12, 3107–3115.
- Jiang, J.; Wu, S.; Lv, L.; Liu, X.; Chen, L.; Zhao, X.; Wang, Q. Mitochondrial dysfunction, apoptosis and transcriptomic alterations induced by four strobilurins in zebrafish (Danio rerio) early life stages. Environ. Pollut. 2019, 253, 722–730.
- Bousquet, M.S.; Ma, J.J.; Ratnayake, R.; Havre, P.A.; Yao, J.; Dang, N.H.; Paul, V.J.; Carney, T.J.; Dang, L.H.; Luesch, H. Multidimensional Screening Platform for Simultaneously Targeting Oncogenic KRAS and Hypoxia-Inducible Factors Pathways in Colorectal Cancer. ACS Chem. Biol. 2016, 11, 1322–1331.
- Yang, Q.; Salim, L.; Yan, C.; Gong, Z. Rapid Analysis of Effects of Environmental Toxicants on Tumorigenesis and Inflammation Using a Transgenic Zebrafish Model for Liver Cancer. Mar. Biotechnol. 2019, 21, 396–405.
- Xiao, Y.F.; Jie, M.M.; Li, B.S.; Hu, C.J.; Xie, R.; Tang, B.; Yang, S.M. Peptide-Based Treatment: A Promising Cancer Therapy. J. Immunol. Res. 2015, 2015, 761820.
- Wu, D.; Gao, Y.; Qi, Y.; Chen, L.; Ma, Y.; Li, Y. Peptide-based cancer therapy: Opportunity and challenge. Cancer Lett. 2014, 351, 13–22.
- Varas, M.A.; Muñoz-Montecinos, C.; Kallens, V.; Simon, V.; Allende, M.L.; Marcoleta, A.E.; Lagos, R. Exploiting Zebrafish Xenografts for Testing the in vivo Antitumorigenic Activity of Microcin E492 Against Human Colorectal Cancer Cells. Front. Microbiol. 2020, 11, 405.
- Hsieh, T.H.; Hsu, C.Y.; Tsai, C.F.; Chiu, C.C.; Liang, S.S.; Wang, T.N.; Kuo, P.L.; Long, C.Y.; Tsai, E.M. A novel cell-penetrating peptide suppresses breast tumorigenesis by inhibiting β-catenin/LEF-1 signaling. Sci. Rep. 2016, 6, 19156.
- Pearce, M.C.; Gamble, J.T.; Kopparapu, P.R.; O’Donnell, E.F.; Mueller, M.J.; Jang, H.S.; Greenwood, J.A.; Satterthwait, A.C.; Tanguay, R.L.; Zhang, X.K.; et al. Induction of apoptosis and suppression of tumor growth by Nur77-derived Bcl-2 converting peptide in chemoresistant lung cancer cells. Oncotarget 2018, 9, 26072–26085.
- Cordeiro, M.; Carvalho, L.; Silva, J.; Saúde, L.; Fernandes, A.R.; Baptista, P.V. Gold Nanobeacons for Tracking Gene Silencing in Zebrafish. Nanomaterials 2017, 7, 10.
- Chiavacci, E.; Rizzo, M.; Pitto, L.; Patella, F.; Evangelista, M.; Mariani, L.; Rainaldi, G. The zebrafish/tumor xenograft angiogenesis assay as a tool for screening anti-angiogenic miRNAs. Cytotechnology 2014, 67, 969–975.
- Kiener, M.; Chen, L.; Krebs, M.; Grosjean, J.; Klima, I.; Kalogirou, C.; Riedmiller, H.; Kneitz, B.; Thalmann, G.N.; Snaar-Jagalska, E.; et al. MiR-221-5p regulates proliferation and migration in human prostate cancer cells and reduces tumor growth in vivo. BMC Cancer 2019, 19, 627.
- Bayer, V. An Overview of Monoclonal Antibodies. Semin. Oncol. Nurs. 2019, 35, 150927.
- Fior, R.; Póvoa, V.; Mendes, R.V.; Carvalho, T.; Gomes, A.; Figueiredo, N.; Ferreira, F.R. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl. Acad. Sci. USA 2017, 114, E8234–E8243.
- Wu, J.Q.; Fan, R.Y.; Zhang, S.R.; Li, C.Y.; Shen, L.Z.; Wei, P.; He, Z.H.; He, M.F. A systematical comparison of anti-angiogenesis and anti-cancer efficacy of ramucirumab, apatinib, regorafenib and cabozantinib in zebrafish model. Life Sci. 2020, 247, 117402.
- Jin, Y.; Wei, L.; Jiang, Q.; Song, X.; Teng, C.; Fan, C.; Lv, Y.; Liu, Y.; Shen, W.; Li, L.; et al. Comparison of efficacy and toxicity of bevacizumab, endostar and apatinib in transgenic and human lung cancer xenograftzebrafish model. Sci. Rep. 2018, 8, 15837.
- Zhang, J.; Gao, B.; Zhang, W.; Qian, Z.; Xiang, Y. Monitoring antiangiogenesis of bevacizumab in zebrafish. Drug Des. Devel. Ther. 2018, 12, 2423–2430.
- Gomes-Silva, D.; Ramos, C.A. Cancer Immunotherapy Using CAR-T Cells: From the Research Bench to the Assembly Line. Biotechnol. J. 2018, 13, 1700097.
- Wang, Z.; Wu, Z.; Liu, Y.; Han, W. New development in CAR-T cell therapy. J. Hematol. Oncol. 2017, 10, 53.
- Pascoal, S.; Salzer, B.; Scheuringer, E.; Wenninger-Weinzierl, A.; Sturtzel, C.; Holter, W.; Taschner-Mandl, S.; Lehner, M.; Distel, M. A preclinical embryonic zebrafish xenograft model to investigate CAR T cells in vivo. Cancers 2020, 12, 567.
- Ramachandran, R.; Krishnaraj, C.; Sivakumar, A.S.; Prasannakumar, P.; Abhay Kumar, V.K.; Shim, K.S.; Song, C.-G.; Yun, S.-I. Anticancer activity of biologically synthesized silver and gold nanoparticles on mouse myoblast cancer cells and their toxicity against embryonic zebrafish. Mater. Sci. Eng. C 2017, 73, 674–683.
- Calienni, M.N.; Cagel, M.; Montanari, J.; Moretton, M.A.; Prieto, M.J.; Chiappetta, D.A.; Alonso, S.d.V. Zebrafish (Danio rerio) model as an early stage screening tool to study the biodistribution and toxicity profile of doxorubicin-loaded mixed micelles. Toxicol. Appl. Pharmacol. 2018, 357, 106–114.
- Wu, Y.; Ge, P.; Xu, W.; Li, M.; Kang, Q.; Zhang, X.; Xie, J. Cancer-targeted and intracellular delivery of Bcl-2-converting peptide with functional macroporous silica nanoparticles for biosafe treatment. Mater. Sci. Eng. C 2020, 108, 110386.
- Haque, E.; Ward, A.C. Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials 2018, 8, 561.
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65.
- Jia, H.R.; Zhu, Y.X.; Duan, Q.Y.; Chen, Z.; Wu, F.G. Nanomaterials meet zebrafish: Toxicity evaluation and drug delivery applications. J. Control. Release 2019, 311–312, 301–318.
- Chang, H.; Yhee, J.Y.; Jang, G.H.; You, D.G.; Ryu, J.H.; Choi, Y.; Na, J.H.; Park, J.H.; Lee, K.H.; Choi, K.; et al. Predicting the in vivo accumulation of nanoparticles in tumor based on in vitro macrophage uptake and circulation in zebrafish. J. Control. Release 2016, 244, 205–213.
- Gundersen, E.T.; Førde, J.L.; Tislevoll, B.S.; Leitch, C.; Barratt, G.; Gjertsen, B.T.; Herfindal, L. Repurposing chlorpromazine for anti-leukaemic therapy by nanoparticle encapsulation. Int. J. Pharm. 2022, 612, 121296.
- Gong, C.; Deng, S.; Wu, Q.; Xiang, M.; Wei, X.; Li, L.; Gao, X.; Wang, B.; Sun, L.; Chen, Y.; et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials 2013, 34, 1413–1432.
- Askes, S.H.C.; Bossert, N.; Bussmann, J.; Talens, V.S.; Meijer, M.S.; Kieltyka, R.E.; Kros, A.; Bonnet, S.; Heinrich, D. Dynamics of dual-fluorescent polymersomes with durable integrity in living cancer cells and zebrafish embryos. Biomaterials 2018, 168, 54–63.
- Wang, Q.; Liu, P.; Sun, Y.; Gong, T.; Zhu, M.; Sun, X.; Zhang, Z.; Duan, Y. Preparation and properties of biocompatible PS-PEG/calcium phosphate nanospheres. Nanotoxicology 2015, 9, 190–200.
- Evensen, L.; Johansen, P.L.; Koster, G.; Zhu, K.; Herfindal, L.; Speth, M.; Fenaroli, F.; Hildahl, J.; Bagherifam, S.; Tulotta, C.; et al. Zebrafish as a model system for characterization of nanoparticles against cancer. Nanoscale 2016, 8, 862–877.
- Crecente-Campo, J.; Guerra-Varela, J.; Peleteiro, M.; Gutiérrez-Lovera, C.; Fernández-Mariño, I.; Diéguez-Docampo, A.; González-Fernández, Á.; Sánchez, L.; Alonso, M.J. The size and composition of polymeric nanocapsules dictate their interaction with macrophages and biodistribution in zebrafish. J. Control. Release 2019, 308, 98–108.
- Jia, H.R.; Zhu, Y.X.; Xu, K.F.; Pan, G.Y.; Liu, X.; Qiao, Y.; Wu, F.G. Efficient cell surface labelling of live zebrafish embryos: Wash-free fluorescence imaging for cellular dynamics tracking and nanotoxicity evaluation. Chem. Sci. 2019, 10, 4062–4068.
- Mimeault, M.; Batra, S.K. Emergence of zebrafish models in oncology for validating novel anticancer drug targets and nanomaterials. Drug Discov. Today 2013, 18, 128–140.
- Liu, H.-n.; Guo, N.-n.; Guo, W.-w.; Huang-Fu, M.-y.; Vakili, M.R.; Chen, J.-j.; Xu, W.-h.; Wei, Q.-c.; Han, M.; Lavasanifar, A.; et al. Delivery of mitochondriotropic doxorubicin derivatives using self-assembling hyaluronic acid nanocarriers in doxorubicin-resistant breast cancer. Acta Pharmacol. Sin. 2018, 39, 1681–1692.
- Gao, X.; Wang, S.; Wang, B.L.; Deng, S.; Liu, X.; Zhang, X.N.; Luo, L.L.; Fan, R.R.; Xiang, M.L.; You, C.; et al. Improving the anti-ovarian cancer activity of docetaxel with biodegradable self-assembly micelles through various evaluations. Biomaterials 2015, 53, 646–658.
- Xie, Z.; Guo, W.; Guo, N.; Huangfu, M.; Liu, H.; Lin, M.; Xu, W.H.; Chen, J.; Wang, T.T.; Wei, Q.; et al. Targeting tumor hypoxia with stimulus-responsive nanocarriers in overcoming drug resistance and monitoring anticancer efficacy. Acta Biomater. 2018, 71, 351–362.
- Saraiva, S.M.; Gutiérrez-Lovera, C.; Martínez-Val, J.; Lores, S.; Bouzo, B.L.; Díez-Villares, S.; Alijas, S.; Pensado-López, A.; Vázquez-Ríos, A.J.; Sánchez, L.; et al. Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model. Sci. Rep. 2021, 11, 9873.
- Moret, F.; Conte, C.; Esposito, D.; Dal Poggetto, G.; Avitabile, C.; Ungaro, F.; Tiso, N.; Romanelli, A.; Laurienzo, P.; Reddi, E.; et al. Biodegradable nanoparticles combining cancer cell targeting and anti-angiogenic activity for synergistic chemotherapy in epithelial cancer. Drug Deliv. Transl. Res. 2022, 1–13.
- Deng, S.; Wu, Q.; Zhao, Y.; Zheng, X.; Wu, N.; Pang, J.; Li, X.; Bi, C.; Liu, X.; Yang, L.; et al. Biodegradable polymeric micelle-encapsulated doxorubicin suppresses tumor metastasis by killing circulating tumor cells. Nanoscale 2015, 7, 5270–5280.
- Zhou, Q.; Li, Y.; Zhu, Y.; Yu, C.; Jia, H.; Bao, B.; Hu, H.; Xiao, C.; Zhang, J.; Zeng, X.; et al. Co-delivery nanoparticle to overcome metastasis promoted by insufficient chemotherapy. J. Control. Release 2018, 275, 67–77.
More
