Third-generation solar cells, including dye-sensitized solar cells, bulk-heterojunction solar cells, and perovskite solar cells, are being intensively researched to obtain high efficiencies in converting solar energy into electricity. However, it is also important to note their stability over time and the devices’ thermal or operating temperature range. Today’s widely used polymeric materials are also used at various stages of the preparation of the complete device—it is worth mentioning that in dye-sensitized solar cells, suitable polymers can be used as flexible substrates counter-electrodes, gel electrolytes, and even dyes. In the case of bulk-heterojunction solar cells, they are used primarily as donor materials; however, there are reports in the literature of their use as acceptors. In perovskite devices, they are used as additives to improve the morphology of the perovskite, mainly as hole transport materials and also as additives to electron transport layers. Polymers, thanks to their numerous advantages, such as the possibility of practically any modification of their chemical structure and thus their physical and chemical properties, are increasingly used in devices that convert solar radiation into electrical energy.
- photovoltaics
- dye-sensitized solar cells
- bulk-heterojunction solar cells
- perovskite solar cells
- polymers
- thin layers
1. Introduction
2. Polymers in Dye-Sensitized Solar Cells (DSSCs)
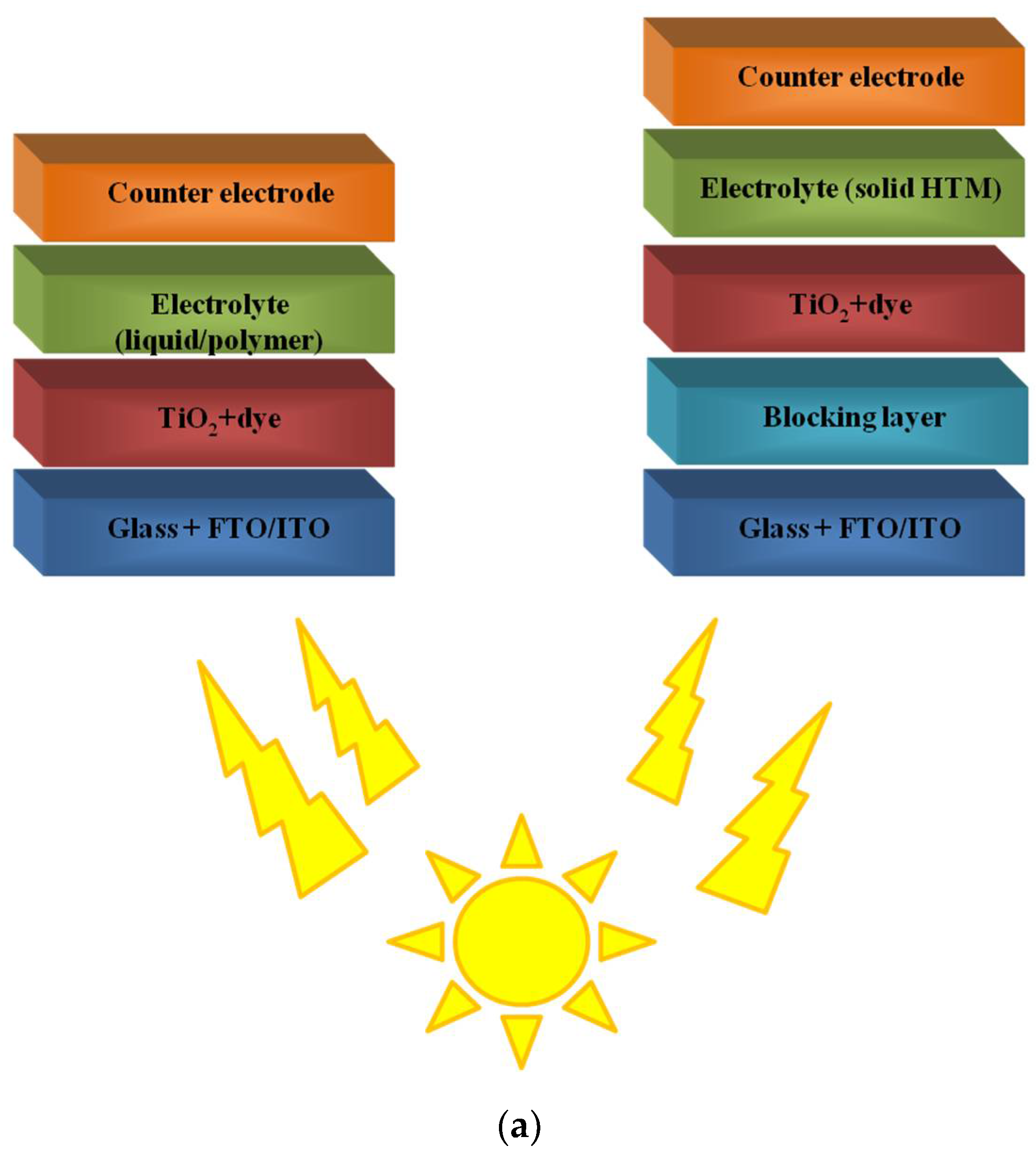
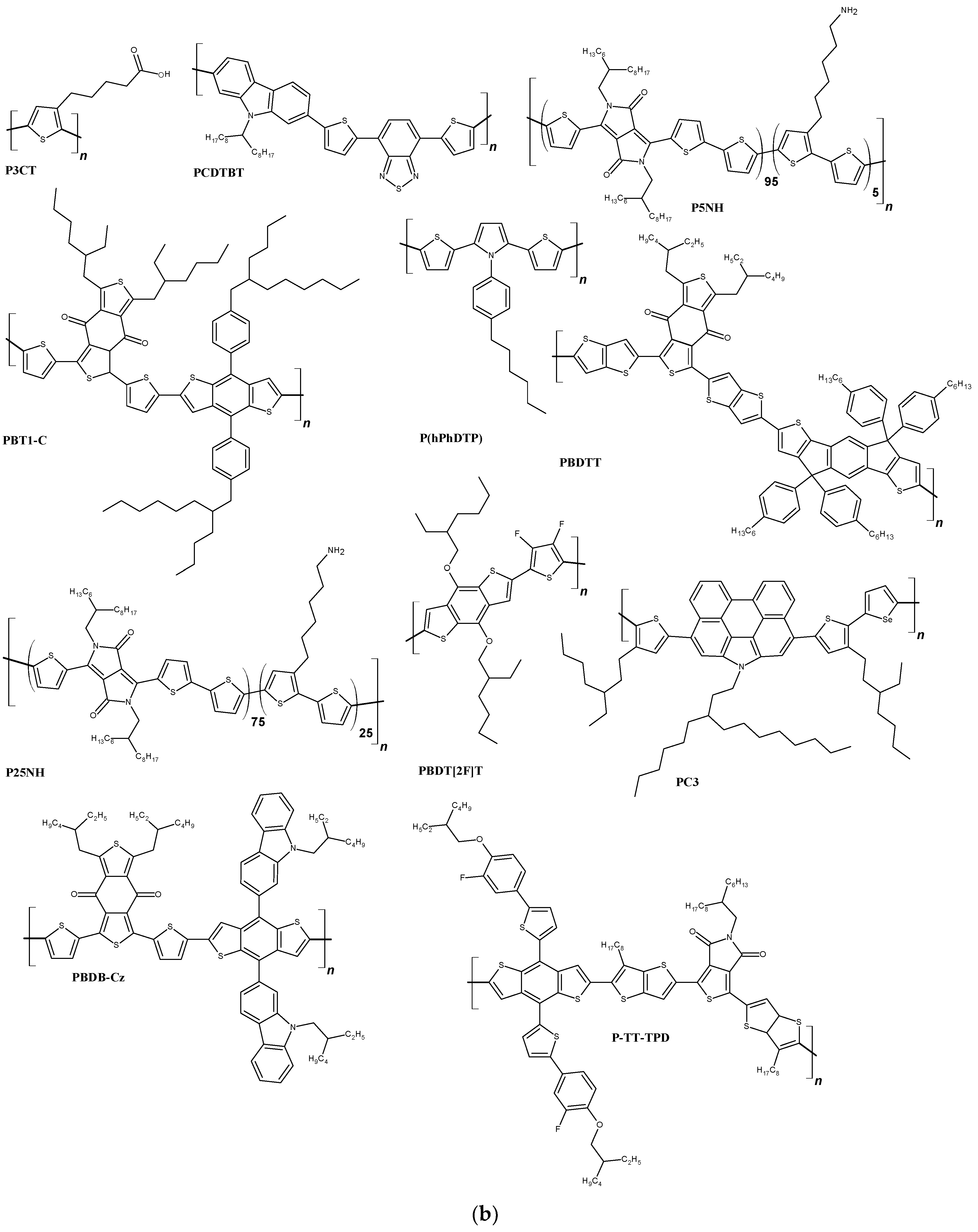
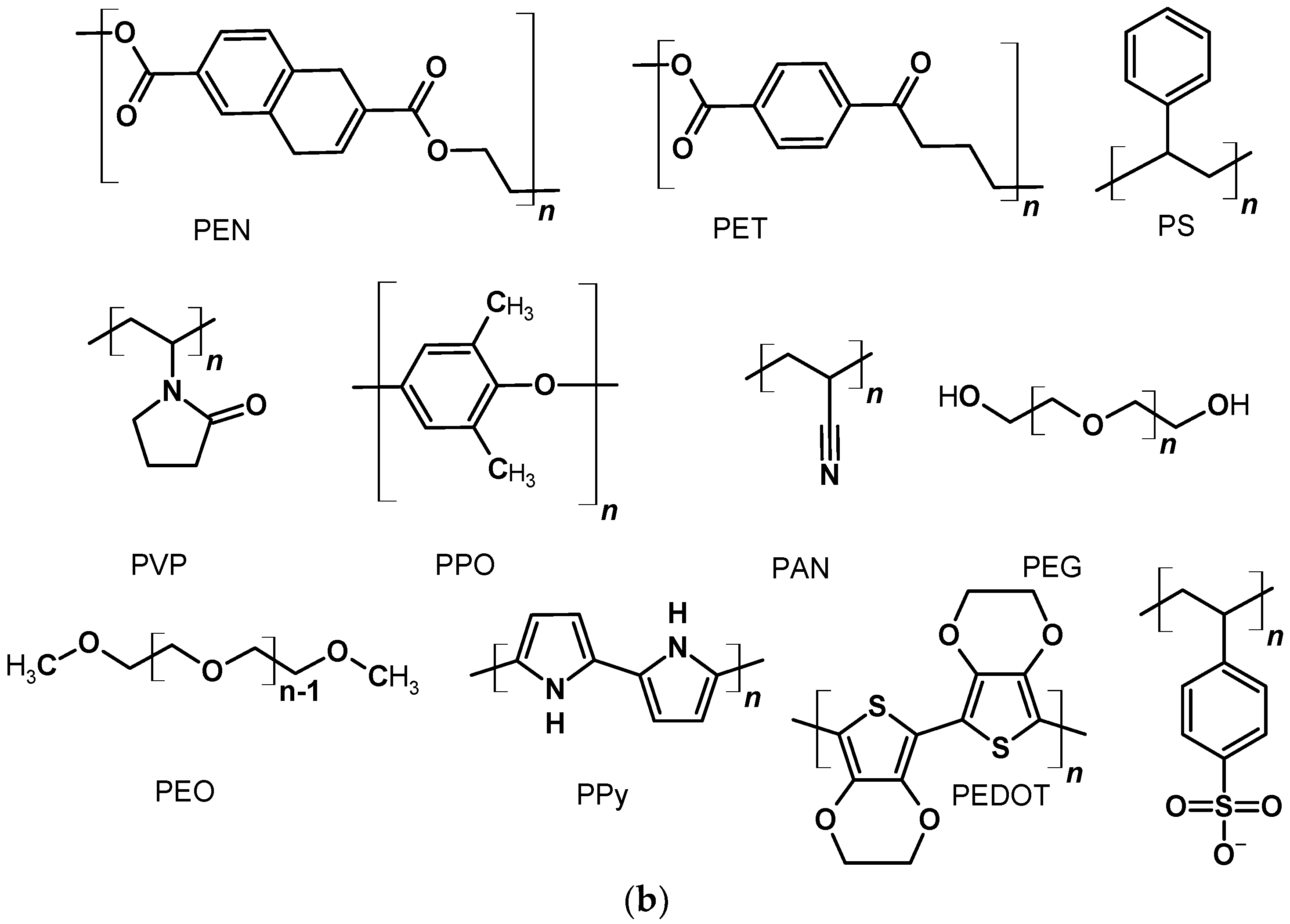 Figure 1. The structures of DSSCs with liquid and solid electrolyte (a) and polymer structures often used in this type of solar cell (b).
Figure 1. The structures of DSSCs with liquid and solid electrolyte (a) and polymer structures often used in this type of solar cell (b).3. Polymers in Bulk-Heterojunction Solar Cells (BHJ)
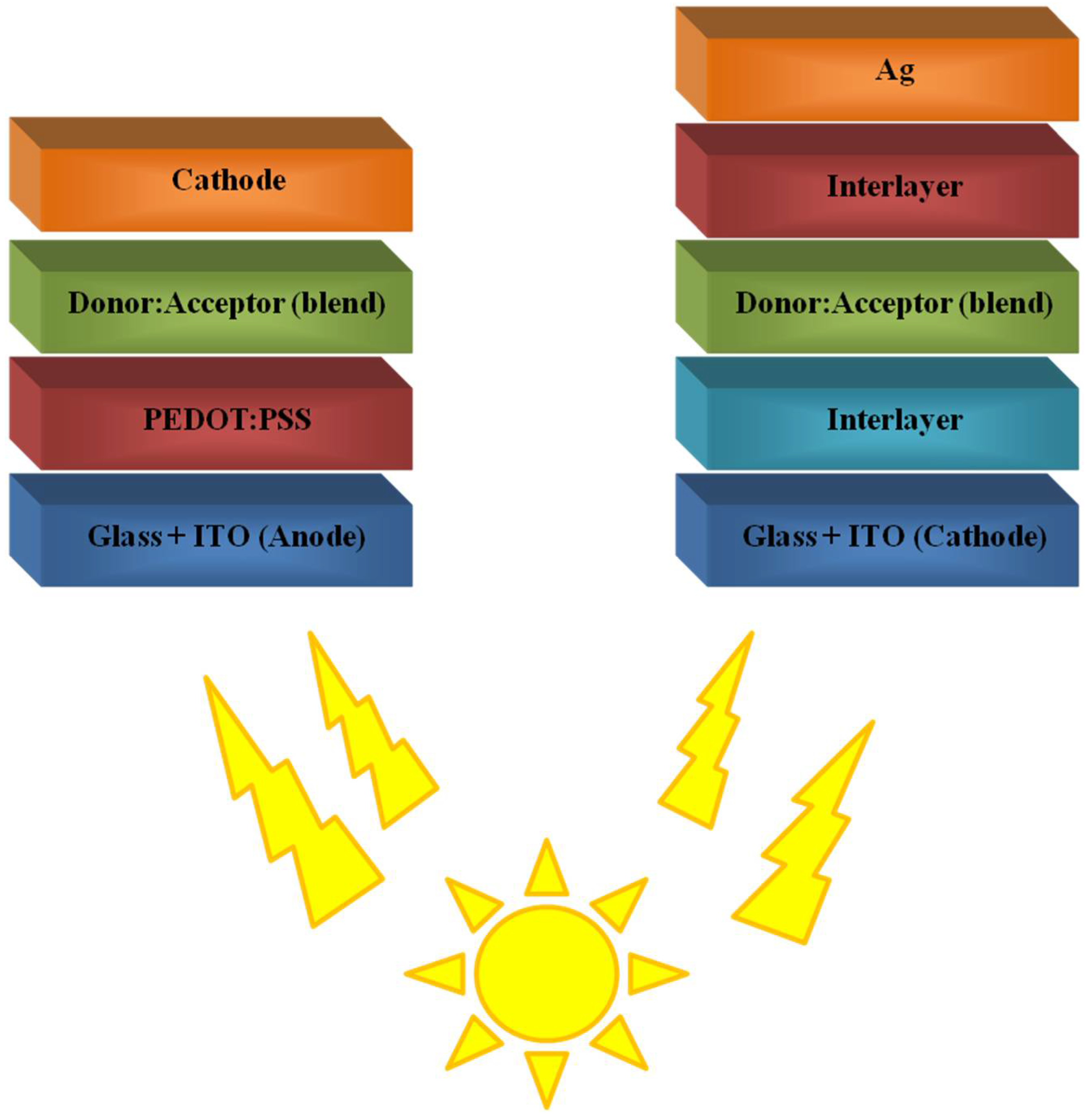
4. Polymers in Perovskite Solar Cells (PSCs)
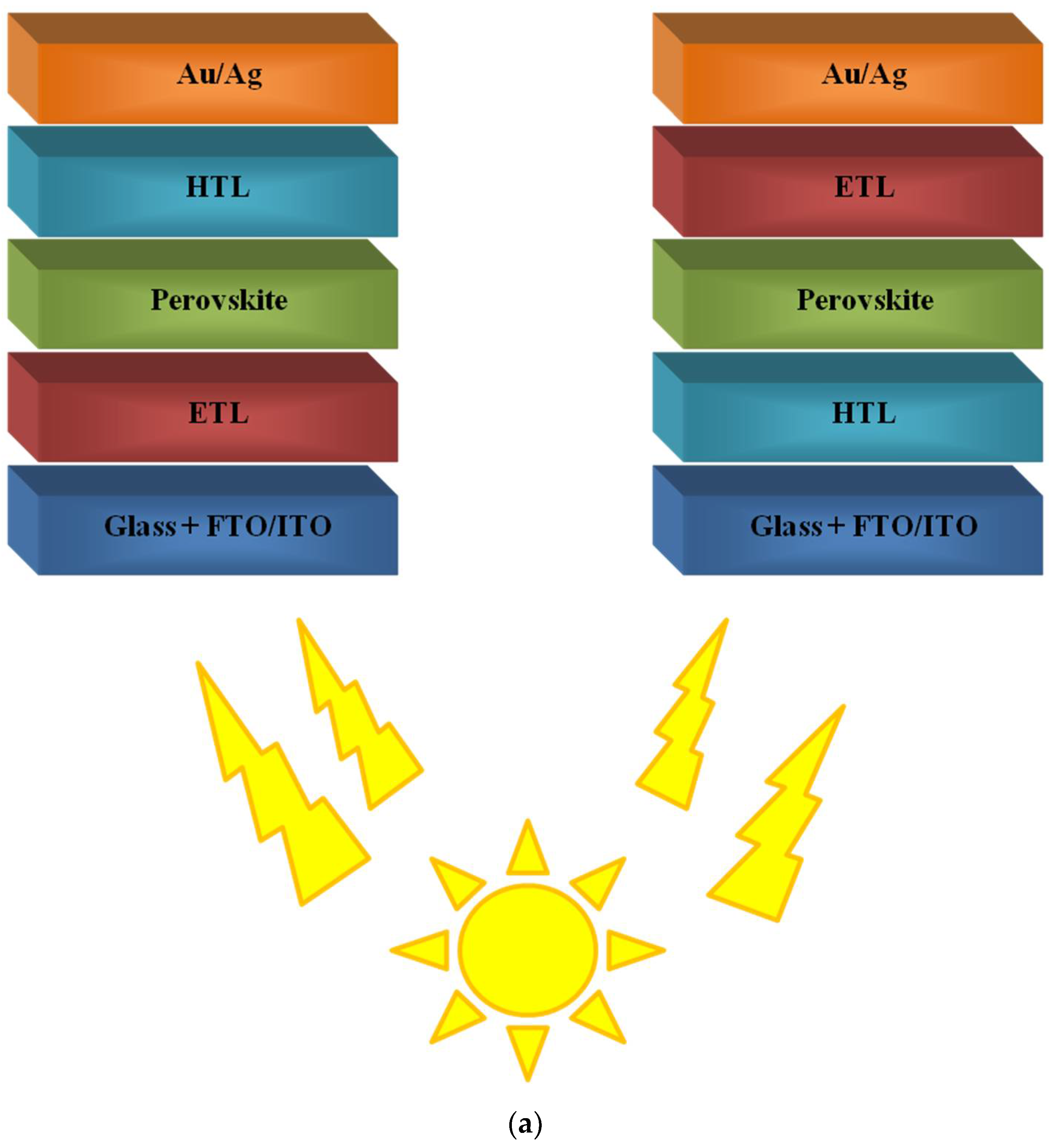
5. Summary and Conclusions
References
- Grätzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798.
- Khatibi, A.; Razi Astaraei, F.; Ahmadi, M.H. Generation and combination of the solar cells: A current model review. Energy Sci. Eng. 2019, 7, 305–322.
- Palm, J.; Probst, V.; Karg, F.H. Second generation CIS solar modules. Sol. Energy 2004, 77, 757–765.
- El Chaar, L.; Lamont, L.A.; El Zein, N. Review of photovoltaic technologies. Renew. Sustain. Energy Rev. 2011, 15, 2165–2175.
- Parida, B.; Iniyan, S.; Goic, R. A review of solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2011, 15, 1625–1636.
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, W.C. Photovoltaic materials: Present efficiencies and future challenges. Science 2016, 352, aad4424.
- NREL. Best Research-Cell Efficiencies: Rev. 04-06-2020. Best Res. Effic. Chart|Photovolt. Res.|NREL. 2020. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 15 February 2022).
- Roncali, J.; Leriche, P.; Blanchard, P. Molecular materials for organic photovoltaics: Small is beautiful. Adv. Mater. 2014, 26, 3821–3838.
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162.
- Gnida, P.; Libera, M.; Pająk, A.; Schab-Balcerzak, E. Examination of the Effect of Selected Factors on the Photovoltaic Response of Dye-Sensitized Solar Cells. Energy Fuels 2020, 34, 14344–14355.
- Song, L.; Du, P.; Xiong, J.; Ko, F.; Cui, C. Efficiency enhancement of dye-sensitized solar cells by optimization of electrospun ZnO nanowire/nanoparticle hybrid photoanode and combined modification. Electrochim. Acta 2015, 163, 330–337.
- Nath, B.; Pradhan, B.; Panda, S.K. Optical tunability of lead free double perovskite Cs2AgInCl6: Via composition variation. New J. Chem. 2020, 44, 18656–18661.
- Ji, J.M.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-sensitizing Novel Thienoindole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 2000124.
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897.
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275.
- Han, Y.W.; Jeon, S.J.; Lee, H.S.; Park, H.; Kim, K.S.; Lee, H.W.; Moon, D.K. Evaporation-Free Nonfullerene Flexible Organic Solar Cell Modules Manufactured by An All-Solution Process. Adv. Energy Mater. 2019, 9, 1902065.
- Kim, J.Y.; Park, S.; Lee, S.; Ahn, H.; Joe, S.Y.; Kim, B.J.; Son, H.J. Low-Temperature Processable High-Performance D–A-Type Random Copolymers for Nonfullerene Polymer Solar Cells and Application to Flexible Devices. Adv. Energy Mater. 2018, 8, 1801601.
- Gong, S.C.; Jang, S.K.; Ryu, S.O.; Jeon, H.; Park, H.H.; Chang, H.J. Post annealing effect of flexible polymer solar cells to improve their electrical properties. Curr. Appl. Phys. 2010, 10, e192–e196.
- Jung, H.S.; Han, G.S.; Park, N.G.; Ko, M.J. Flexible Perovskite Solar Cells. Joule 2019, 3, 1850–1880.
- Feng, J.; Zhu, X.; Yang, Z.; Zhang, X.; Niu, J.; Wang, Z.; Zuo, S.; Priya, S.; Liu, S.F.; Yang, D. Record Efficiency Stable Flexible Perovskite Solar Cell Using Effective Additive Assistant Strategy. Adv. Mater. 2018, 30, e1801418.
- Yang, D.; Yang, R.; Priya, S.; Liu, S.F. Recent Advances in Flexible Perovskite Solar Cells: Fabrication and Applications. Angew. Chemie—Int. Ed. 2019, 58, 4466–4483.
- Noorasid, N.S.; Arith, F.; Mustafa, A.N.; Azam, M.A.; Mahalingam, S.; Chelvanathan, P.; Amin, N. Current advancement of flexible dye sensitized solar cell: A review. Optik 2022, 254, 168089.
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. The integration of flexible dye-sensitized solar cells and storage devices towards wearable self-charging power systems: A review. Renew. Sustain. Energy Rev. 2022, 159, 112252.
- Fan, X. Flexible dye-sensitized solar cells assisted with lead-free perovskite halide. J. Mater. Res. 2022, 37, 866–875.
- Aydin, E.; De Bastiani, M.; De Wolf, S. Defect and Contact Passivation for Perovskite Solar Cells. Adv. Mater. 2019, 31, e1900428.
- Zhao, X.; Wang, M. Organic hole-transporting materials for efficient perovskite solar cells. Mater. Today Energy 2018, 7, 208–220.
- Bakr, Z.H.; Wali, Q.; Fakharuddin, A.; Schmidt-Mende, L.; Brown, T.M.; Jose, R. Advances in hole transport materials engineering for stable and efficient perovskite solar cells. Nano Energy 2017, 34, 271–305.
- Yin, X.; Song, Z.; Li, Z.; Tang, W. Toward ideal hole transport materials: A review on recent progress in dopant-free hole transport materials for fabricating efficient and stable perovskite solar cells. Energy Environ. Sci. 2020, 13, 4057–4086.
