The Radial Arm Maze (RAM), developed by Olton and Samuelson (1976) and quickly adapted in humans, is a high ecological spatial task, firstly used in a real environment and subsequently in the virtual one.
- human navigation
- virtual reality
- behavioral task
- spatial abilities
- large-scale task
1. Radial Arm Maze Task (RAM)
1.1. Free-Choice and Forced-Choice Version
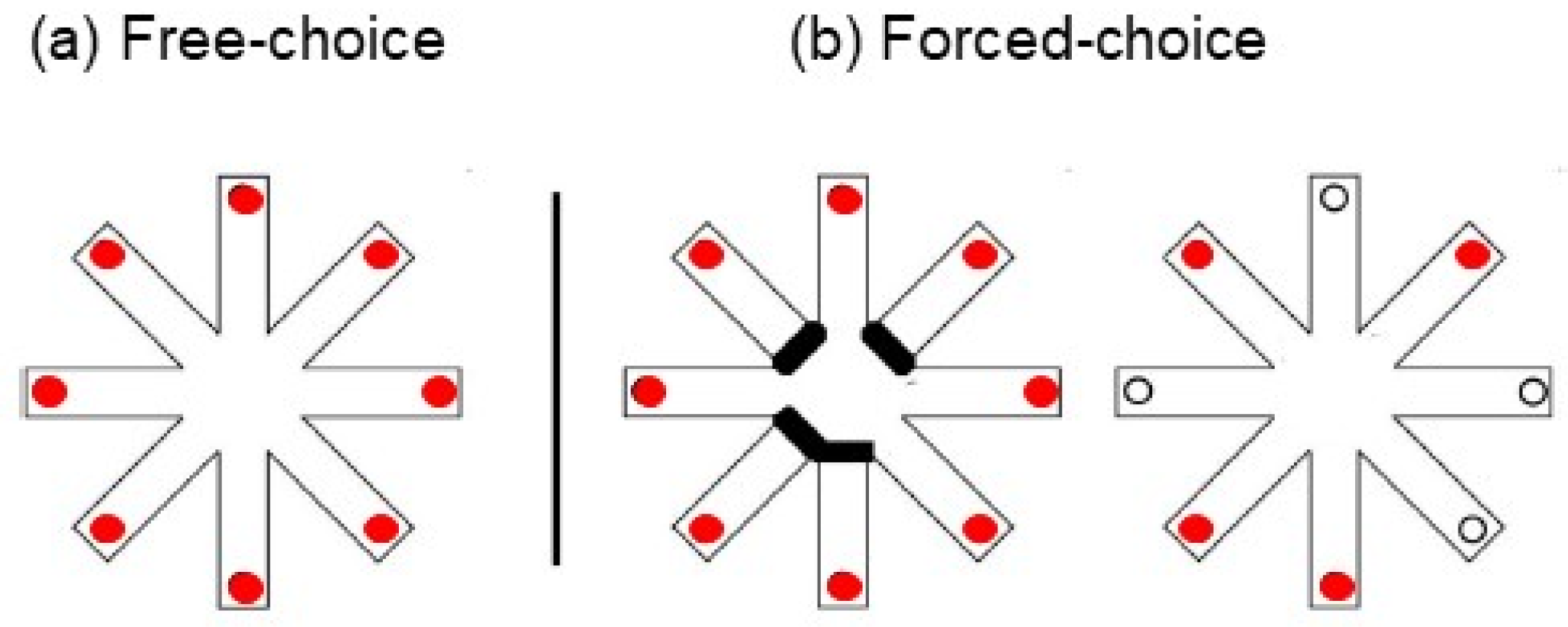
Free-Choice RAM Version | Forced-Choice RAM Version | (Referred to the Second Phase of the Task) | |||||
|---|---|---|---|---|---|---|---|
Total time to complete the entire task | Time to reach each reward | Total time to complete the second phase of the task | |||||
Latency to select the first arm | Latency to select the first arm | ||||||
Total entries (arms correct and incorrect visited) | Total entries (arms correct and incorrect visited) | ||||||
Distance travelled | Distance travelled | ||||||
Movement speed | Errors | ||||||
Frequency of successes/Percentage of correct visits/Search efficiency | Across-phase errors | ||||||
Errors/Error-free trials | Within-phase errors | ||||||
The longest sequence of correctly visited arms | The longest sequence of correctly visited arms | ||||||
Percentage of angles turned (45°, 90°, 135°, 180° or 360°)/Angle change/Strategy fixation | |||||||
Perseverations (consecutive entries into the same arm or the re-entries into a fixed sequence of arms) | |||||||
Declarative mastery |
1.2. Table RAM and Visuospatial Peripersonal Abilities
2. Applications of RAM Task in Real Environment
3. Potentiality and Applications of RAM Task in Virtual Environment
From the analysis of the studies carried out, it is clear that most of them use the forced choice paradigm, and this observation deserves careful consideration. Once again, the two paradigms allowed to evaluate different facets of spatial memory. Still, the forced choice method is more sensitive to the short memory components, and is also more challenging to perform. However, in virtual modality, it is easier to modify scenarios by reducing (or increasing) the complexity of the task. Perhaps this could be why forced choice in virtual modality is more frequent than free choice.
Funding
This research was supported by funding from the Department of Humanities, University of Naples Federico II (Fondi ricerca dipartimentale 30% and 70% 2020/2021) to L.M.
References
- L. Mandolesi; L. Petrosini; D. Menghini; F. Addona; S. Vicari; Children's radial arm maze performance as a function of age and sex. International Journal of Developmental Neuroscience 2009, 27, 789-797, 10.1016/j.ijdevneu.2009.08.010.
- Nigel P. Foreman; Margaret Arber; Joe Savage; Spatial memory in preschool infants. Developmental Psychobiology 1984, 17, 129-137, 10.1002/dev.420170204.
- W. H. Overman; Bobbi Jean Pate; Kim Moore; Andrea Peuster; Ontogeny of place learning in children as measured in the Radial Arm Maze, Morris Search Task, and Open Field Task.. Behavioral Neuroscience 1996, 110, 1205-1228, 10.1037//0735-7044.110.6.1205.
- Jan Aadland; William W. Beatty; Ruth H. Maki; Spatial memory of children and adults assessed in the radial maze. Developmental Psychobiology 1985, 18, 163-172, 10.1002/dev.420180208.
- Y. Leitner; D. Heldman; S. Harel; C.G. Pick; Deficits in spatial orientation of children with intrauterine growth retardation. Brain Research Bulletin 2005, 67, 13-18, 10.1016/j.brainresbull.2005.04.017.
- Léa Bertholet; Manuel Torres Escobar; Marion Depré; Camille F. Chavan; Fabienne Giuliani; Pascale Gisquet-Verrier; Delphine Preissmann; Françoise Schenk; Spatial radial maze procedures and setups to dissociate local and distal relational spatial frameworks in humans. Journal of Neuroscience Methods 2015, 253, 126-141, 10.1016/j.jneumeth.2015.06.012.
- R.Corey O'Connor; Robert B. Glassman; Human performance with a seventeen-arm radial maze analog. Brain Research Bulletin 1993, 30, 189-191, 10.1016/0361-9230(93)90058-j.
- Robert B. Glassman; Kimberly J. Garvey; Kimberly M. Elkins; Kimberly L. Kasal; Nicole L. Couillard; Spatial working memory score of humans in a large radial maze, similar to published score of rats, implies capacity close to the magical number 7 ± 2. Brain Research Bulletin 1994, 34, 151-159, 10.1016/0361-9230(94)90012-4.
- Robert B. Glassman; Kimberly M. Leniek; Tamara M. Haegerich; Human working memory capacity is 7 ± 2 in a radial maze with distracting interruption: possible implication for neural mechanisms of declarative and implicit long-term memory. Brain Research Bulletin 1998, 47, 249-256, 10.1016/s0361-9230(98)00083-5.
- Lauren J. Levy; Robert S. Astur; Karyn Frick; Men and Women Differ in Object Memory but Not Performance of a Virtual Radial Maze.. Behavioral Neuroscience 2005, 119, 853-862, 10.1037/0735-7044.119.4.853.
- Robert S Astur; Laughlin B Taylor; Adam N Mamelak; Linda Philpott; Robert J Sutherland; Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behavioural Brain Research 2001, 132, 77-84, 10.1016/s0166-4328(01)00399-0.
- Laura Mandolesi; M. G. Leggio; A. Graziano; P. Neri; L. Petrosini; Cerebellar contribution to spatial event processing: involvement in procedural and working memory components. European Journal of Neuroscience 2001, 14, 2011-2022, 10.1046/j.0953-816x.2001.01819.x.
- Laura Mandolesi; M. G. Leggio; F. Spirito; L. Petrosini; Cerebellar contribution to spatial event processing: do spatial procedures contribute to formation of spatial declarative knowledge?. European Journal of Neuroscience 2003, 18, 2618-2626, 10.1046/j.1460-9568.2003.02990.x.
- L. Mandolesi; F. Addona; F. Foti; D. Menghini; L. Petrosini; S. Vicari; Spatial competences in Williams syndrome: a radial arm maze study. International Journal of Developmental Neuroscience 2009, 27, 205-213, 10.1016/j.ijdevneu.2009.01.004.
- Aryeh Routtenberg; John O'keefe; Lynn Nadel; The Hippocampus as a Cognitive Map. The American Journal of Psychology 1980, 93, 177, 10.2307/1422119.
- Leonard E. Jarrard; On the role of the hippocampus in learning and memory in the rat. Behavioral and Neural Biology 1993, 60, 9-26, 10.1016/0163-1047(93)90664-4.
- Liana Palermo; Francesca Foti; Fabio Ferlazzo; Cecilia Guariglia; Laura Petrosini; I find my way in a maze but not in my own territory! Navigational processing in developmental topographical disorientation.. Neuropsychology 2014, 28, 135-146, 10.1037/neu0000021.
- Emily K. Farran; Harry R. M. Purser; Yannick Courbois; Marine Ballé; Pascal Sockeel; Daniel Mellier; Mark Blades; Route knowledge and configural knowledge in typical and atypical development: a comparison of sparse and rich environments.. Journal of Neurodevelopmental Disorders 2015, 7, 37, 10.1186/s11689-015-9133-6.
- Michael McLaren-Gradinaru; Ford Burles; Inderpreet Dhillon; Adam Retsinas; Alberto Umiltà; Jaimy Hannah; Kira Dolhan; Giuseppe Iaria; A Novel Training Program to Improve Human Spatial Orientation: Preliminary Findings. Frontiers in Human Neuroscience 2020, 14, 5, 10.3389/fnhum.2020.00005.
- Francesca Foti; Pierpaolo Sorrentino; Deny Menghini; Simone Montuori; Matteo Pesoli; Patrizia Turriziani; Stefano Vicari; Laura Petrosini; Laura Mandolesi; Peripersonal Visuospatial Abilities in Williams Syndrome Analyzed by a Table Radial Arm Maze Task. Frontiers in Human Neuroscience 2020, 14, 254, 10.3389/fnhum.2020.00254.
- Andrea Serino; Peripersonal space (PPS) as a multisensory interface between the individual and the environment, defining the space of the self. Neuroscience & Biobehavioral Reviews 2019, 99, 138-159, 10.1016/j.neubiorev.2019.01.016.
- Enrique Moraleda; Cristina Broglio; Fernando Rodríguez; Development of different spatial frames of reference for orientation in small-scale environments. Psicothema 2013, 25, 468-475, 10.7334/PSICOTHEMA2013.29.
- Nigel Foreman; Raphael Gillett; Sandra Jones; Choice autonomy and memory for spatial locations in six-year-old children. British Journal of Psychology 1994, 85, 17-27, 10.1111/j.2044-8295.1994.tb02505.x.
- N Foreman; R Warry; P Murray; Development of reference and working spatial memory in preschool children.. The Journal of General Psychology 1990, 117, 267-276.
- Francesca Foti; Deny Menghini; Laura Petrosini; Giuliana Valerio; Antonino Crinò; Stefano Vicari; Teresa Grimaldi; Laura Mandolesi; Spatial Competences in Prader–Willi Syndrome: A Radial Arm Maze Study. Behavior Genetics 2011, 41, 445-456, 10.1007/s10519-011-9471-4.
- Francesca Foti; Domenico Martone; Stefania Orrù; Simone Montuori; Esther Imperlini; Pasqualina Buono; Laura Petrosini; Laura Mandolesi; Are young children able to learn exploratory strategies by observation?. Psychological Research 2017, 82, 1212-1223, 10.1007/s00426-017-0896-0.
- Bohbot, V.D.; Jech, R.; Ruèicka, E.; Nadel, L.; Kanilna, M.; Stepankova, K.; Bures, J.; Rat Spatial Memory Tasks Adapted for Humans: Characterization in Subjects with Intact Brain and Subjects with Medial Temporal Lobe Lesions. Physiol. Res. 2002, 51, S49–S56.
- Laura Serra; Fondazione Santa Lucia Neuroimaging Laboratory; Sara Raimondi; Carlotta di Domenico; Silvia Maffei; Anna Lardone; Marianna Liparoti; Pierpaolo Sorrentino; Carlo Caltagirone; Laura Petrosini; et al.Laura MandolesiFondazione Santa Lucia Laboratory Of Experimental And Behavioural Neurophysiology The beneficial effects of physical exercise on visuospatial working memory in preadolescent children. AIMS Neuroscience 2021, 8, 496-509, 10.3934/neuroscience.2021026.
- Qazi Rahman; Johanna Koerting; Sexual orientation-related differences in allocentric spatial memory tasks. Hippocampus 2007, 18, 55-63, 10.1002/hipo.20375.
- Gaën Plancher; A. Tirard; V. Gyselinck; S. Nicolas; P. Piolino; Using virtual reality to characterize episodic memory profiles in amnestic mild cognitive impairment and Alzheimer's disease: Influence of active and passive encoding. Neuropsychologia 2012, 50, 592-602, 10.1016/j.neuropsychologia.2011.12.013.
- Eleanor A. Maguire; Rory Nannery; Hugo Spiers; Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain 2006, 129, 2894-2907, 10.1093/brain/awl286.
- Giuseppe Iaria; Michael Petrides; Alain Dagher; Bruce Pike; Véronique D. Bohbot; Cognitive Strategies Dependent on the Hippocampus and Caudate Nucleus in Human Navigation: Variability and Change with Practice. The Journal of Neuroscience 2003, 23, 5945-5952, 10.1523/jneurosci.23-13-05945.2003.
- Véronique D. Bohbot; Giuseppe Iaria; Michael Petrides; Hippocampal Function and Spatial Memory: Evidence From Functional Neuroimaging in Healthy Participants and Performance of Patients With Medial Temporal Lobe Resections.. Neuropsychology 2004, 18, 418-425, 10.1037/0894-4105.18.3.418.
- Harrison Banner; Venkataramana Bhat; Nicole Etchamendy; Ridha Joober; Véronique D. Bohbot; The brain-derived neurotrophic factor Val66Met polymorphism is associated with reduced functional magnetic resonance imaging activity in the hippocampus and increased use of caudate nucleus-dependent strategies in a human virtual navigation task. European Journal of Neuroscience 2011, 33, 968-977, 10.1111/j.1460-9568.2010.07550.x.
- Rachel Marsh; Xuejun Hao; Dongrong Xu; Zhishun Wang; Yunsuo Duan; Jun Liu; Alayar Kangarlu; Diana Martinez; Felix Garcia; Gregory Z. Tau; et al.Shan YuMark G. PackardBradley S. Peterson A virtual reality-based FMRI study of reward-based spatial learning. Neuropsychologia 2010, 48, 2912-2921, 10.1016/j.neuropsychologia.2010.05.033.
- Naomi J. Goodrich-Hunsaker; Ramona O. Hopkins; Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage.. Behavioral Neuroscience 2010, 124, 405-413, 10.1037/a0019193.
- Véronique D. Bohbot; Jason Lerch; Brook Thorndycraft; Giuseppe Iaria; Alex P. Zijdenbos; Gray Matter Differences Correlate with Spontaneous Strategies in a Human Virtual Navigation Task. The Journal of Neuroscience 2007, 27, 10078-10083, 10.1523/jneurosci.1763-07.2007.
- Robert S Astur; Jennifer Tropp; Simona Sava; R.Todd Constable; Etan J Markus; Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behavioural Brain Research 2004, 151, 103-115, 10.1016/j.bbr.2003.08.024.
- Veronique D. Bohbot; Sam McKenzie; Kyoko Konishi; Celine Fouquet; Vanessa Kurdi; Russell Schachar; Michel Boivin; Philippe Robaey; Virtual navigation strategies from childhood to senescence: evidence for changes across the life span. Frontiers in Aging Neuroscience 2012, 4, 28, 10.3389/fnagi.2012.00028.
- Julia Anglen Bauer; Birgit Claus Henn; Christine Austin; Silvia Zoni; Chiara Fedrighi; Giuseppa Cagna; Donatella Placidi; Roberta F. White; Qiong Yang; Brent A. Coull; et al.Donald SmithRoberto LucchiniRobert WrightManish Arora Manganese in teeth and neurobehavior: Sex-specific windows of susceptibility. Environment International 2017, 108, 299-308, 10.1016/j.envint.2017.08.013.
- Marilyn Cyr; Zhishun Wang; Gregory Z. Tau; Guihu Zhao; Eve Friedl; Mihaela Stefan; Kate Terranova; Rachel Marsh; Reward-Based Spatial Learning in Teens With Bulimia Nervosa. Journal of the American Academy of Child & Adolescent Psychiatry 2016, 55, 962-971.e3, 10.1016/j.jaac.2016.07.778.
- E.M. Migo; O. O’Daly; M. Mitterschiffthaler; E. Antonova; G.R. Dawson; C.T. Dourish; K.J. Craig; A. Simmons; G.K. Wilcock; E. McCulloch; et al.S.H.D. JacksonM.D. KopelmanS.C.R. WilliamsR.G. Morris Investigating virtual reality navigation in amnestic mild cognitive impairment using fMRI. Aging, Neuropsychology, and Cognition 2015, 23, 196-217, 10.1080/13825585.2015.1073218.
- Philippe Robaey; Sam McKenzie; Russel Schachar; Michel Boivin; Veronique D. Bohbot; Stop and look! Evidence for a bias towards virtual navigation response strategies in children with ADHD symptoms. Behavioural Brain Research 2016, 298, 48-54, 10.1016/j.bbr.2015.08.019.
- Rachel Marsh; Gregory Z. Tau; Zhishun Wang; Yuankai Huo; Ge Liu; Xuejun Hao; Mark G. Packard; Bradley S. Peterson; H. Blair Simpson; Reward-Based Spatial Learning in Unmedicated Adults With Obsessive-Compulsive Disorder. American Journal of Psychiatry 2015, 172, 383-392, 10.1176/appi.ajp.2014.13121700.
- Jun-Young Lee; Sooyeon Kho; Hye Bin Yoo; Soowon Park; Jung-Seok Choi; Jun Soo Kwon; Kyung Ryeol Cha; Hee-Yeon Jung; Spatial memory impairments in amnestic mild cognitive impairment in a virtual radial arm maze. Neuropsychiatric Disease and Treatment 2014, 10, 653-660, 10.2147/ndt.s58185.
- Eva Pirogovsky; Heather M. Holden; Cecily Jenkins; Guerry M. Peavy; David P. Salmon; Uglas R. Galasko; Paul E. Gilbert; Temporal sequence learning in healthy aging and amnestic mild cognitive impairment.. Experimental Aging Research 2013, 39, 371-81, 10.1080/0361073X.2013.808122.
- Leanne K. Wilkins; Todd A. Girard; Kyoko Konishi; Matthew King; Katherine A. Herdman; Jelena King; Bruce Christensen; Veronique D. Bohbot; Selective deficit in spatial memory strategies contrast to intact response strategies in patients with schizophrenia spectrum disorders tested in a virtual navigation task. Hippocampus 2013, 23, 1015-1024, 10.1002/hipo.22189.
- Elena A. Spieker; Robert S. Astur; Jeffrey T. West; Jacqueline A. Griego; Laura M. Rowland; Spatial memory deficits in a virtual reality eight-arm radial maze in schizophrenia. Schizophrenia Research 2012, 135, 84-89, 10.1016/j.schres.2011.11.014.
- Eva Pirogovsky; Jody Goldstein; Guerry Peavy; Mark W. Jacobson; Jody Corey-Bloom; Paul E. Gilbert; Temporal order memory deficits prior to clinical diagnosis in Huntington’s disease. Journal of the International Neuropsychological Society 2009, 15, 662-670, 10.1017/s1355617709990427.
- Federica Somma; Paolo Bartolomeo; Federica Vallone; Antonietta Argiuolo; Antonio Cerrato; Orazio Miglino; Laura Mandolesi; Maria Clelia Zurlo; Onofrio Gigliotta; Further to the left. Stress-induced increase of spatial pseudoneglect during the COVID-19 lockdown. null 2020, 12, 573846, 10.31234/osf.io/xb954.
- Hamed Taheri Gorji; Michela Leocadi; Francesco Grassi; Gaspare Galati; The art gallery maze: a novel tool to assess human navigational abilities. Cognitive Processing 2021, 22, 501-514, 10.1007/s10339-021-01022-9.
- Elza Rechtman; Paul Curtin; Demetrios M. Papazaharias; Stefano Renzetti; Giuseppa Cagna; Marco Peli; Yuri Levin-Schwartz; Donatella Placidi; Donald R. Smith; Roberto G. Lucchini; et al.Robert O. WrightMegan K. Horton Sex-specific associations between co-exposure to multiple metals and visuospatial learning in early adolescence. Translational Psychiatry 2020, 10, 1-10, 10.1038/s41398-020-01041-8.
- Devin J. Sodums; Véronique D. Bohbot; Negative correlation between grey matter in the hippocampus and caudate nucleus in healthy aging. Hippocampus 2020, 30, 892-908, 10.1002/hipo.23210.
- Louisa Dahmani; Blandine Courcot; Jamie Near; Raihaan Patel; Robert S. C. Amaral; M. Mallar Chakravarty; Véronique D. Bohbot; Fimbria-Fornix Volume Is Associated With Spatial Memory and Olfactory Identification in Humans. Frontiers in Systems Neuroscience 2020, 13, 87, 10.3389/fnsys.2019.00087.
- Jarid Goodman; Mason McClay; Joseph E. Dunsmoor; Threat-induced modulation of hippocampal and striatal memory systems during navigation of a virtual environment. Neurobiology of Learning and Memory 2020, 168, 107160-107160, 10.1016/j.nlm.2020.107160.
- Yingying Yang; Edward C. Merrill; Qi Wang; Children’s response, landmark, and metric strategies in spatial navigation. Journal of Experimental Child Psychology 2019, 181, 75-101, 10.1016/j.jecp.2019.01.005.
- Jeremy B Caplan; Eric Lg Legge; Bevin Cheng; Christopher R Madan; Effectiveness of the method of loci is only minimally related to factors that should influence imagined navigation.. Quarterly Journal of Experimental Psychology 2019, 72, 2541-2553, 10.1177/1747021819858041.
- Étienne Aumont; Martin Arguin; Véronique Bohbot; Greg L. West; Increased flanker task and forward digit span performance in caudate-nucleus-dependent response strategies. Brain and Cognition 2019, 135, 103576, 10.1016/j.bandc.2019.05.014.
- Étienne Aumont; Caroll-Ann Blanchette; Veronique D. Bohbot; Greg L. West; Caudate nucleus-dependent navigation strategies are associated with increased risk-taking and set-shifting behavior. Learning & Memory 2019, 26, 101-108, 10.1101/lm.048306.118.
- Somayeh Raiesdana; Modeling the interaction of navigational systems in a reward-based virtual navigation task. Journal of Integrative Neuroscience 2018, 17, 45-67, 10.3233/JIN-170036.
- Louisa Dahmani; Raihaan M. Patel; Yiling Yang; M. Mallar Chakravarty; Lesley K. Fellows; Véronique D. Bohbot; An intrinsic association between olfactory identification and spatial memory in humans. Nature Communications 2018, 9, 1-12, 10.1038/s41467-018-06569-4.
- Étienne Aumont; Veronique D. Bohbot; Gregory L. West; Spatial learners display enhanced oculomotor performance. Journal of Cognitive Psychology 2018, 30, 872-879, 10.1080/20445911.2018.1526178.
- Kyoko Konishi; Ridha Joober; Judes Poirier; Kathleen MacDonald; Mallar Chakravarty; Raihaan Patel; John Breitner; Véronique D. Bohbot; Healthy versus Entorhinal Cortical Atrophy Identification in Asymptomatic APOE4 Carriers at Risk for Alzheimer’s Disease. Journal of Alzheimer's Disease 2018, 61, 1493-1507, 10.3233/JAD-170540.
- Hyunjeong Kim; Jin Young Park; Kwanguk (Kenny) Kim; Spatial Learning and Memory Using a Radial Arm Maze with a Head-Mounted Display. Psychiatry Investigation 2018, 15, 935-944, 10.30773/pi.2018.06.28.3.
- Tavor Ben-Zeev; Inbal Weiss; Saar Ashri; Yuval Heled; Itay Ketko; Ran Yanovich; Eitan Okun; Mild Physical Activity Does Not Improve Spatial Learning in a Virtual Environment. Frontiers in Behavioral Neuroscience 2020, 14, 584052, 10.3389/fnbeh.2020.584052.
