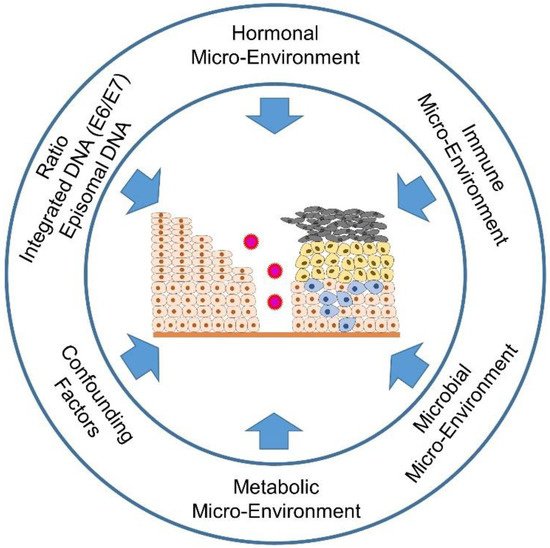
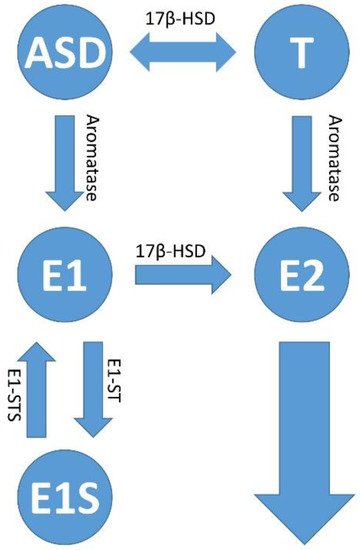
Figure 34. Estrogen synthesis pathways. In addition to estradiol (E2), estrone (E1) is also formed in the human body when androstenedione (ASD) is aromatized. This mainly happens in postmenopausal women. Estrone (E1) and/or estradiol (E2) are delivered to the tumor and its microenvironment via the bloodstream; or there is increased local production and retention of the hormones within the tumors. The latter occurs via increased activity of the cytochrome P450 enzyme, aromatase (CYP19A1), which converts androstenedione (ASD) to E1 and testosterone (T) to E2. The enzymatic activity of estrone (E1) sulfatase converts estrone sulfate (E1S) to estrone (E1); and 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1) activity is responsible for the conversion of E1 to E2, and androstenedione (ASD/AE) to testosterone (T), respectively. The sulfation of estradiol (E2) leads to the inactive form of estrogen, estrogen sulfate (E1S) via the activity of estrone sulfotransferase (E1-ST).
Estrone (E1) and estradiol (E2) are the starting molecules of estrogens, which are converted into metabolites in the liver and occur as hormones with different potency
[66][195]. Subsequently, the starting molecules and their metabolites are converted to glucuronides and sulfates by glucuronidation or sulfation in order to be excreted via the bile, the kidneys or the intestines
[67][196]. Just as estrogens are conjugated, they can also be de-conjugated by bacteria in the stomach and intestines. This occurs via β-glucuronidases (GUSB) or β-glucosidases from the class of glycosidases
[68][197]. The bacteria in the intestine recycle the estradiol, and this can possibly fuel neoplasia, the development of which depends on estrogen signaling. The intestinal bacteria de-conjugate the bound estrogens and release them via the secretion of the GUSB so that they can carry out their biological activity in the body. When there is an oversupply of free estrogen via estrogen signaling, this can lead to hyper-estrogenic pathologies such as endometriosis and cancer. On the other hand, reduced GUSB activity caused by dysbiosis can lead to hypo-estrogenic pathologies such as obesity, metabolic syndrome and cardiovascular and neurological diseases
[23][69][23,198]. This association between the β-glucuronidases (GUSB) produced by the gut microbiome, as part of the estrobolome that reactivates estrogens, has been confirmed
[70][199]. Unconjugated estrogens are transferred back into the bloodstream via the intestines by means of GUSB enzymes and can develop their hormonal effect elsewhere.
In addition to the estrogen-metabolizing gut microbiome, there is also a microbiome in the gut, as mentioned earlier, that metabolizes tryptophan instead of estrogens. This microbial community, which also includes
Klebsiella spp., does not compete with the host cells of the intestine for tryptophan and therefore promotes the production of melatonin. The gastric and intestinal cells and the endogenous metabolism in the liver produce melatonin as a secondary metabolite via pre-beta-lipoproteins (VLDL) and LDL
[71][200]. A well-known effect of melatonin is that it regulates the circadian rhythm. In addition, it shows oncostatic effects such as the inhibition of proliferation and stimulation of apoptosis, immunomodulation and anti-inflammatory effects; furthermore, it shows supportive effects for the anti-oxidation systems. In addition, it regulates blood vessel formation
[72][201]. The background to this is that ER signaling is blocked by melatonin, which is analogous to the effects of a selective estrogen receptor modulator (SERM)
[73][74][202,203]. However, melatonin also shows the properties of a selective estrogen enzyme modulator (SEEM)
[75][204]. Thus, melatonin inhibits the enzymatic activity of estrone (E1) sulfatase, which converts estrone sulfate (E1S) into estrone (E1), but also the activity of 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1), which is responsible for the conversion of E1 to E2. This subsequently reduces the circulating estradiol levels in the plasma
[76][205]. Melatonin also promotes the sulfation of estradiol to the inactive form of estrogen, estrogen sulfate (E1S), via the activity of estrone sulfotransferase (E1-ST), and thus shows an anti-estrogenic effect. The effects of estradiol (E2) are diverse; they promote, in addition to growth,
for e
xample.g., the cervical epithelium, the immunosuppression in the microenvironment of the intraepithelial cervical lesions via their genomic effect on the estrogen receptor alpha (ERα). This also affects the cells responsible for immunosuppression such as the regulatory T cells (Tregs), the myeloid-derived suppressor cells (MDSCs) and cancer-associated fibroblasts (CAFs)
[15] [15](Figure 3). These effects of E2 now bring us to the next subsection of our review, estrogen signaling.
2.2. Estrogen Signaling
Estradiol signals in a ligand-dependent and classical manner via the estrogen receptors α and β (ER α/β) and the G protein-coupled estrogen receptor 1 (GPER1), also known as G protein-coupled receptor 30 (GPR30). Independently, ligand signals in a non-classical way via other receptors such as the epidermal growth factor receptor (EGFR), the insulin growth factor receptor (IGFR) and the fibroblast growth factor receptor (FGFR)
[77][206]. In addition, a genomic pathway is distinguished from a non-genomic pathway. In the genomic pathway, the hormone-receptor complexes are translocated to the nucleus, where the receptor dimers bind to estrogen-responsive elements (EREs) on the target gene promoter, triggering gene activation and epigenetic changes. The estrogen receptor serves as a transcription factor that regulates gene expression and thus cell proliferation and cell survival. Tissues that respond to fluctuating estrogen levels may experience organic changes
[23]. As already mentioned in the last subchapter, one should not underestimate the indirect effects of estrogen signaling on the tumor microenvironment, such as the infiltrating immune cells, stroma cells and cancer-associated fibroblasts (CAFs)
[77][206].
2.3. Influence of Estrogen Signaling on the Microenvironment of Cervical Intraepithelial Lesion, Cervical Pre-Cancer and in Tumor
O
f all the work on cervical carcinogenesis, animal models have made their decisive contribution to elucidating the mechanisms of the course of infection with the high-risk HPV types and the resulting transformation of the cervical lesions. In a frequently cited
onework by Arbeit et al. from 1996
[78][207],
it
wahe authors investigated the processes of carcinogenesis in a transgenic mouse model (K14-HPV16-E6/E7), which was permanently exposed to estradiol
[78][207]. As already mentioned, chronic unresolved infection with one of the high-risk HPV strains is necessary for the start of the malignant transformation. However, further supporting co-factors, such as estradiol, are required for the full development of invasive carcinoma
[79][80][81][208,209,210]. Sirtuin-1 (SIRT-1) cooperates with estrogen signaling in breast cancer tumorigenesis and progression
[82][211]. SIRT-1 is also up-regulated in cervical carcinogenesis and leads via deacetylation to the destabilization of the tumor suppressor protein Werner syndrome protein (WRN). In HPV16 cervical cells, the destabilization of WRN resulted in increased basal cell proliferation, damage to DNA and epithelial thickening. This led to increased DNA replication via E1 and E2 and, as a result, to an increase in the proliferation of keratinocytes and the HPV life cycle in the lesions of the cervical neoplastic tissue
[83][212]. It is also assumed that the likely use of birth control drugs and multiple maternities increases the risk of developing cervical squamous cell carcinoma (SCC)
[84][85][86][87][88][213,214,215,216,217]. The association between viral infection and the effects of cofactors such as hormonal contraceptives in cervical carcinogenesis was examined by Marks MA et al.
[89][218]. Analyzing cervical secretions from elderly women with HPV infection it was found that markers associated with anti-inflammation and allergies (IL-5, IL-9, IL-13, IL-17, EOTAXIN, GM-CSF and MIP-1α) were increased compared to HPV-negative women. In addition, T cell cytokines were shifted from interleukin 2 (IL-2) towards Eotaxin. This suggests immunosuppressive T cell responses via type 2 T helper cells (TH2 lymphocytes)
[90][219]. Since estradiol, together with other factors, seems to suppress the immune response in cervical carcinogenesis, it is important to determine the estradiol concentrations in the blood plasma but also in the cervical secretions, namely the cervical mucus in postmenopausal HPV-infected women. During pregnancy, elevated levels of estradiol are found in blood plasma.
A study from Denmark found a higher rate of mortality with cervical cancer during pregnancy or shortly after pregnancy
[91][220].
2.3.1. Estrogen Distribution in the Tumor and the Surrounding Microenvironment
Estrone (E1) and/or estradiol (E2) are delivered to the tumor and its microenvironment via the bloodstream or there is an increased local production and retention of the hormones within the tumors. The latter occurs via the increased activity of the cytochrome P450 enzyme, aromatase (CYP19A1), which converts androstenedione and testosterone into E2
[92][221]. Estradiol levels have been shown to increase in the tumor microenvironment (TME) of cervical carcinoma while present at regular levels in blood plasma. In the transformed cervical keratinocytes, estradiol was distributed within the cytoplasm and in the infiltrating immune cells, and in the stroma in both the cytoplasm and the nucleus
[93][94][222,223]. Compared to estradiol levels, aromatase showed an analogous distribution, suggesting that the hormone is synthesized within the tumor microenvironment and tumor
[93][95][222,224]. As an alternative to the synthesis pathways via aromatase, estradiol can also be synthesized in the tissue via other pathways. Estrone sulfate (E1S) is converted into estrone by means of estrone sulfatase (E1-STS) and then further into estradiol (E2) by means of the enzyme 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1). The two enzymes E1-STS and 17β-HSD were detected in vitro in the HPV-positive cervical carcinoma cell line Hela, but not in vivo or in xenografts from patients
[65][96][194,225].
2.3.2. Different Estrogen Signaling Pathways Causing Both, Pro- and Anti-Tumorigenic Effects in HPV-Positive Lesions of the Cervical Epithelium
Depending on the respective concentration of estrogens in the tissue of the cervix, there is a proliferation of the cancer cells and the suppression of their apoptosis induction at physiological hormone levels
[97][98][99][100][226,227,228,229]. On the other hand, an opposite effect can also set in with a higher distribution of estradiol in the tissue, which disrupts protein translation on the ribosomes and leads to apoptotic cancer cell death
[101][102][230,231]. The latter pathway, at high hormone concentrations, via estradiol-induced phosphodiesterase 3A (PDE3A) activation and transactivation of Schlafen, a family member 12 (SLFN12) protein that is predicted to act upstream or within the negative regulation of cell proliferation, leads to the inhibition of mitochondrial activity and the blockade of the apoptotic proteins Bcl2 and Mcl1 for cancer cell apoptosis
[103][232].
In addition to the need for E2, the transgenic mouse models K14-HPV16/HPV18-E6/E7 were also dependent on the expression of the estrogen receptor α (ERα)
[78][104][207,233]. It was not surprising that selective estrogen receptor modulators (SERMs) and selective estrogen receptor disruptors (SERDs) inhibited the growth of precancerous lesions and carcinomas in animal models
[78][80][105][106][107][108][207,209,234,235,236,237]. It was surprising to see that in the transformed cervical keratinocytes from healthy tissue in the direction of malignant development, the expression of ERα was gradually lost, and in mature cancer, no ERα expression could be detected, while, in contrast, the expression of ERβ was preserved and both ERα and ERβ expression were determined in the surrounding stroma
[109][110][111][112][113][114][238,239,240,241,242,243]. If tumor cells cannot use estradiol via the genomic pathway, the estradiol originating from the tumor microenvironment can also signal in the tumor cell via non-genomic pathways
[77][206]. The pro-tumor and anti-tumor effects of estradiol on malignant lesions due to HPV infection are not contradictory
[13]. Since carcinogenesis always depends on the interactions between the developing malignancy and its tumor microenvironment
[27][115][27,121],
itwe must focus
theour attention primarily on the bidirectional crosstalk between the tumor and its tumor microenvironment. These primarily include the HPV-infected keratinocytes of the cervical lesion, but also the immune cells infiltrating the tumor stroma, which play a key role in deciding the fate of the lesion towards malignancy
[108][116][117][237,244,245].
It We will
now discuss the hormonal effects on the tumor stroma and its effects on the infiltration of immune-modulating cells of the immune response
(Figure 3).
2.3.3. The Importance of the ERα for the Tumor Stroma and the Tumor Microenvironment in Relation to the Development of Precancerous Lesions up to the Invasive Form of Cervical Carcinoma
Many benign cells of the cervical stroma express the estrogen receptor α. On the other hand, adequate estradiol levels are not produced in the blood plasma of women who are currently in their menstrual phase. This suggests that the hormone levels appropriate for ERα expression are produced by the hormone-sensitive tissues themselves
[109][112][113][238,241,242]. The estrogen receptor α was expressed in 30% to more than 50% of the stromal cells surrounding the tumor from precancerous lesions and invasive cervical carcinoma, but not in the tumor itself. Immunohistochemistry showed inequality in the distribution of the receptor in the tumor stroma. It was present in all tumors, regardless of cancer grade, in the intracellular spaces between tumor cells in cancer-associated fibroblasts (CAFs), MDSCs and other subtypes of lymphocytes. The tumor cells themselves, on the other hand, did not show any ERα expression
[93][94][113][118][119][120][222,223,242,246,247,248]. This relationship between the estrogen signaling of cervical squamous tumor cells and the surrounding stromal cells could only be explained by a paracrine delivery of estrogen signaling-derived signals from the stromal cells. In fact, a special procedure applied within the related transgenic mouse models helped to remove the ERα only from the stromal cells in order to demonstrate these paracrine mechanisms of estrogen signaling
[110][121][239,249]. In addition to the results from the animal models, it was shown, in the human model of cervical carcinoma, that cancer-associated fibroblasts (CAFs), which were cultivated ex vivo, were estrogen receptor α positive. Thus, ERα genomic signaling in the CAFs induced the secretion of soluble molecules, which, in a paracrine manner, directly benefited malignant cell proliferation and migration, vascular angiogenesis and the epithelial–mesenchymal transition (EMT) of the metastatic tumor, and indirectly supported inflammatory processes within the cervical lesions that promoted cervical carcinogenesis
[94][223]. Treating CAFs with a selective estrogen receptor modulator (SERM), in this case, methylpiperidinopyrazole (MPP), or a selective estrogen receptor disruptor (SERD), in this case, fulvestrant (Faslodex, ICI-182780), suppressed cell cycle- and metabolism-related genes in their expression. This attenuated tumorigenesis and angiogenesis as well as the functionality of estrogen signaling
[94][223]. However, since the stromal genes, which are up-regulated via ERα signaling, are extremely important for cervical carcinogenesis
[94][108][110][122][223,237,239,250], this implicates, conversely, that these genes should be therapeutically targeted
[123][251].
2.3.4. Estrogen Signaling in the Infiltrating Cells of the Immune Response in Cervical Carcinogenesis
Since the cervical epithelium slowly loses its ERα expression in the course of carcinogenesis via the various stages of cervical intraepithelial neoplasia (CIN1–3) to invasive CxCa, estradiol (E2) likely initially acts via the classical genomic pathway, with decreasing ERα-Expression levels within the transformed cells possibly via the non-classical, non-genomic pathways. Furthermore, E2 promotes an anti-inflammatory and regulatory immune microenvironment, which contributes decisively to the success of cervical carcinogenesis
[89][218] (Figure 3).
Erythrocytes, granulocytes, monocytes and thrombocytes are formed during the myeloid hematopoiesis that takes place in the bone marrow. Estrogens induce this process and also enhance the mobility of MDSCs and their inherent immunosuppressive character
[124][252]. In pregnant women, it has been shown that the increased estradiol levels in the blood plasma caused by pregnancy were sufficient to stimulate myeloid hematopoiesis and increase the mobility of MDSCs from the site of origin, the bone marrow, towards the spleen and local tumors. The ERα signaling of granulocytic myeloid suppressor cells (GrMDSCs) contributed to potentiating the level of immunosuppression within the tumor. This resulted in the progression of cervical carcinoma tumor cells that were ERα-negative on their own
[118][246]. Ex vivo and in an orthotopic animal model of cervical carcinoma, the estrogen receptor antagonist fulvestrant was shown to be able to abrogate the immunosuppressive function of the tumor-infiltrating GrMDSCs
[118][246].
In addition to the MDSCs, the regulatory T cells (Tregs or suppressor T cells) also suppress the immune response by infiltrating the tumor. Estradiol causes the expansion of the Tregs and induces the FOXP3 gene promoter, which is mainly responsible for the regulation of Tregs in mice
[125][126][253,254]. However, it has been demonstrated that estradiol occurs in the tumor, its stroma and the infiltrating immune cells in human CxCa
[93][95][222,224]. In comparison to other cells of the immune system, the intracellular estradiol levels were highest in the circulating, intratumoral Tregs and the Tregs of the draining lymph nodes
[93][222]. In addition to the induction of the FOXP3 gene promoter, the associated FOXP3 expression and the maintenance of the control function of the Tregs, the immunosuppressive TGF-β and IL-10 cytokines were also released via the cell contact of the immunosuppressive Tregs. This was all controlled via ERα signaling
[93][222]. It was not surprising that eight possible estrogen-responsive elements (EREs) were found in the FOXP3 locus of Foxp3+ regulatory T cells (FOXP3+ Tregs). The complex of the estrogen receptor α and estradiol was able to bind the translated FOXP3 protein product in the Tregs in humans
[93][222]. Next to this classic genomic pathway of estrogen signaling, it could also be shown that Tregs could also signal via non-genomic (fast) signaling via the G protein-coupled estrogen receptor 1 (GPER1, GPR30) or the membrane-bound ERα. This led, via protein kinase B (Akt/PKB) phosphorylation, to the activation of programmed cell death protein 1 (PD1) signaling and/or increased expression of perforin. Perforin, a cytolytic protein, is found in the granules of cytotoxic T cells and NK cells, but also in Tregs. After degranulation, the cell membrane of the target cell is perforated. A pore is formed (hence the name perforin). Granzyme B enters the target cell and subsequently causes apoptotic cell death
[127][255]. Thus, Tregs encountering cytotoxic T cells or NK cells in the tumor microenvironment could use the same mechanism to induce their apoptotic death by granzyme B and perforin, thus suppressing the immune response. Granzyme B and perforin are thus important for both the clearance of the malignant transformed tissue and its progression in vivo, and help to determine whether the pro- or anti-tumorigenic forces of the immune microenvironment fight for dominance within the cervical intraepithelial lesions and the tumor and its microenvironment prevail
[127][128][129][130][131][132][255,256,257,258,259,260].
In contrast, selective estrogen receptor disruptors (SERDs) such as ICI 182,780 (fulvestrant, 7α,17β-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol) or RU 58,668 (11β,17β)-11-[4-[[5-[(4,4,5,5,5-pentafluoropentyl)sulfonyl]pentyl]-oxy]phenylestra-1,3,5,(10)-triene-3,17-diol) were used as antagonists of estrogen signaling. This resulted in the abrogation of the effects of estradiol on tumor-infiltrating Treg cells (CD4+CD25highCD127low) and the concomitant destruction of ERα and suppression of FOXP3 expression to terminate the suppressive effects of Tregs on both CD8+ and CD4+(CD4+CD25int) effector T cell subsets
[93][133][222,261].
Another important type of immune cell is the M2 tumor-associated macrophages (M2-TAMs). These likewise immunosuppressive, tumor-promoting cells of the innate immune system are stimulated, at least in breast, ovarian and lung carcinomas, via the effect of estradiol to migrate into the tumors and secrete VEGF. The latter leads to positive feedback that prompts M2-TAMs to infiltrate the tumors in even larger numbers
[77][206]. The proteinase inhibitor 9 (PI9) expression is increased via estrogen signaling in the immune cells, which suppresses the secretion of granzyme B (GrB) both endo- and exogenously
[134][135][262,263]. The expression levels of the granzyme gene family are also down-regulated by the interaction of the HR HPV16 E7 oncoprotein with estrogen signaling
[136][264]. Granzyme B is also secreted by keratinocytes. This suggests that estrogen signaling inhibits granzyme B expression via a similar effect in HPV-infected keratinocytes of the cervical epithelium in collaboration with HPV oncoproteins
[137][265]. As
it w
ase mentioned earlier, perforin and granzyme B promote apoptosis
[127][255]. Granzyme B also promotes the degradation of collagen, an extremely important component of the extracellular matrix (ECM). This mechanism paves the way for the infiltration of the tumor microenvironment by cytotoxic T lymphocytes
[138][139][266,267]. However, since granzyme B expression is now down-regulated via the collaboration of E2 with the HR HPV16 E7 oncoprotein, it saves the developing transformed keratinocyte from apoptotic cell death and protects it by preventing the invasion of effector T cells into the premalignant lesion prior to recognition and elimination by the immune system
[135][140][263,268]. Chemokines such as CC chemokine ligand 2 (CCL2) and CCL5 promote tumor progression. Their expression is also induced by the action of estradiol, at least in breast cancer
[141][269]. Both CD8+ cytotoxic effector T lymphocytes (CTLs), the NK cell mobilizing CD4+ T lymphocytes (T helper cells) and the regulatory T cells (Tregs) show estrogen receptor α (ERα) expression, but Tregs, compared to the other lymphocyte subgroups, show a higher ERα expression
[93][222].
2.4. The Effect of ER Antagonists in Modulating the Immune Microenvironment of the Premalignant Cervical Lesion and CxCa
The use of selective estrogen receptor disruptors (SERDs, ER antagonists, ICIs) has shown to counteract the effects of estradiol on estrogen signaling and subsequently on the immune response cells infiltrating the tumor and its stroma, including cancer-associated fibroblasts (CAFs), MDSCs and regulatory (Th2) T lymphocytes (Tregs) in cervical carcinoma
[80][93][94][118][209,222,223,246].
As already mentioned, estradiol (E2) acts both classically and ligand-dependently via the classical ERs and membrane-bound receptors such as GPER1 (GPR30), and non-classically and ligand-independently via other receptors such as EGFR, IGFR and FGFR. This is also dependent and independent of FOXP3 expression and dependent and independent of the programmed cell death 1 ligand 1 (PD-L1)/programmed cell death protein 1 (PD1) pathway
[125][128][129][130][132][253,256,257,258,260]. When using ICIs such as fulvestrant, under the action of exogenously supplied E2, after some time there was a lifting of the suppression of ER signaling and a renewed secretion of cytokines, probably via signals transmitted via extranuclear activity pathways (RAS/MAPK and PI3K/AKT). This happened non-canonically, and further,
for e
xample.g., via transcription factors such as the activating protein-1 (AP-1) and the specific protein-1 (Sp1)
[54][93][142][143][144][183,222,270,271,272]. Given the variety of pathways in which effects can be exerted via estrogen signaling, it might make more sense to tackle the problem of estrogen signaling inhibition “at the root”, which is hormone synthesis. In addition to therapy with ER antagonists, the use of a group of anti-estrogens such as aromatase inhibitors (AIs) could offer a more effective approach in the therapy of CxCa.
FIn a study o
rn patients with breast cancer, it was found that continued therapy with aromatase inhibitors subsequently also reduced the incidence of malignant changes in the cervix. A 10-year incidence of high-grade cervical dysplasia (CIN2–3) was found in the group that has been screened regularly (HR = 0.49; 95% CI, 0.27 to 0.90;
p = 0.0212), especially in women over 50 years of age (HR = 0.34; 95% CI, 0.14 to 0.80;
p = 0.014). The protective effect of tamoxifen monotherapy against low-grade cervical dysplasia (CIN1) was found only in young women
[145][273]. In another
study, the main load of CxCa (43%) was found in postmenopausal women who also showed a high E2 concentration in the tumor
[93][222]. This reinforces the importance of inhibition of local synthesis of E2 by AIs in the affected tissue
[145][273]. Letrozole and anastrozole, two drugs from the group of AIs, led to a reduction in Th2 cytokines and an increase in Th1 cytokines as well as a simultaneous reduction in forkhead box protein P3 (FOXP3+) regulatory T cells (Tregs) in animal experiments as well as in humans
[146][147][274,275]. Thus, the inhibition of E2 synthesis via the action of the AIs led to the reactivation of the immune function of (Th1) cytotoxic T lymphocytes (CTLs)
[128][148][256,276] and the natural killer (NK) cells
[140][268] within the tumor microenvironment. AIs abrogate the function of aromatase only between the conversion of androstenedione to estrone (E1) and the conversion of testosterone to estradiol (E2). It is important to remember that, especially in postmenopausal women, E2 is also synthesized via the conversion of the predominant form of the hormone in these women from estrone sulfate (E1S) to E1 by estrone sulphatase and further by type 1 17β-hydroxysteroid dehydrogenase (17β-HSD, HSD17B)
[15][65][149][150][15,194,277,278].