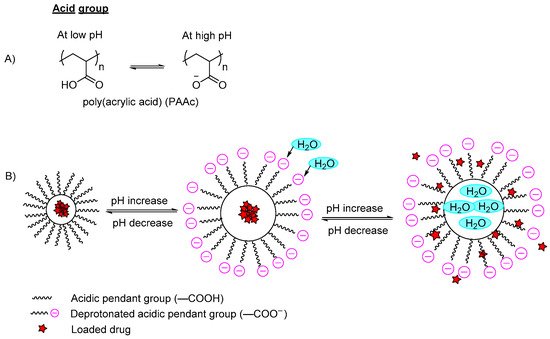
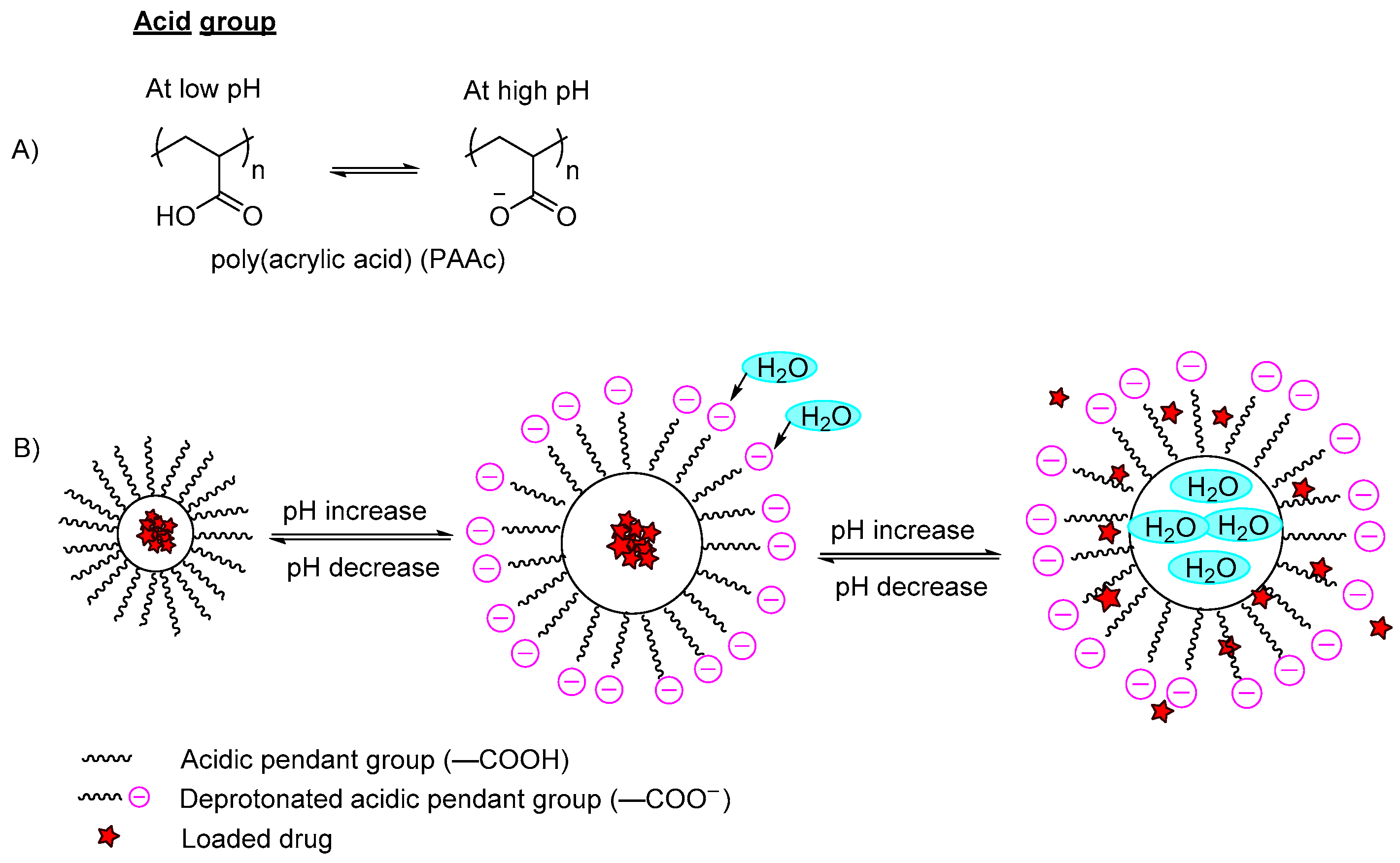
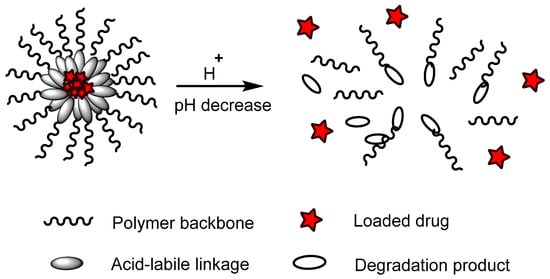
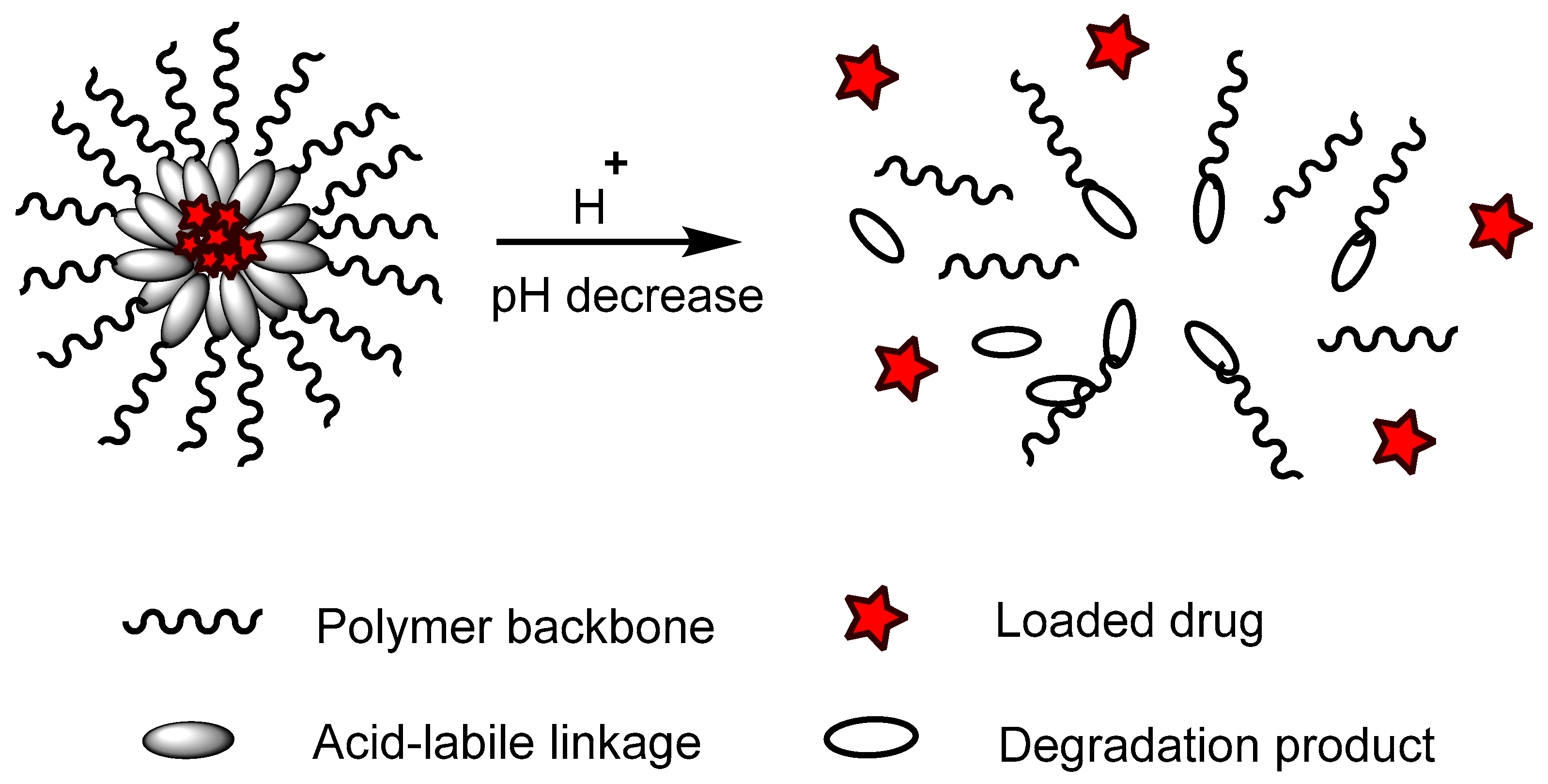
pH-responsive polymers are polymers that respond to changes in environmental pH. They can be classified into: (A) polymers with ionizable moieties; and (B) polymers that contain acid-labile linkages. pH-responsive polyurethanes demonstrated good biological response and sustainability in biomedical applications and drug delivery. They have been used as controlled drug delivery systems for oral administration, intravaginal administration, and targeted drug delivery systems for chemotherapy treatment.
- pH-responsive
- polyurethane
- biomedical
- drug delivery
1. Introduction
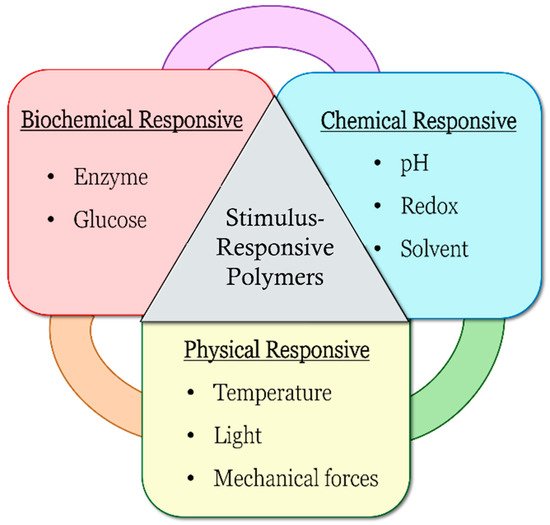
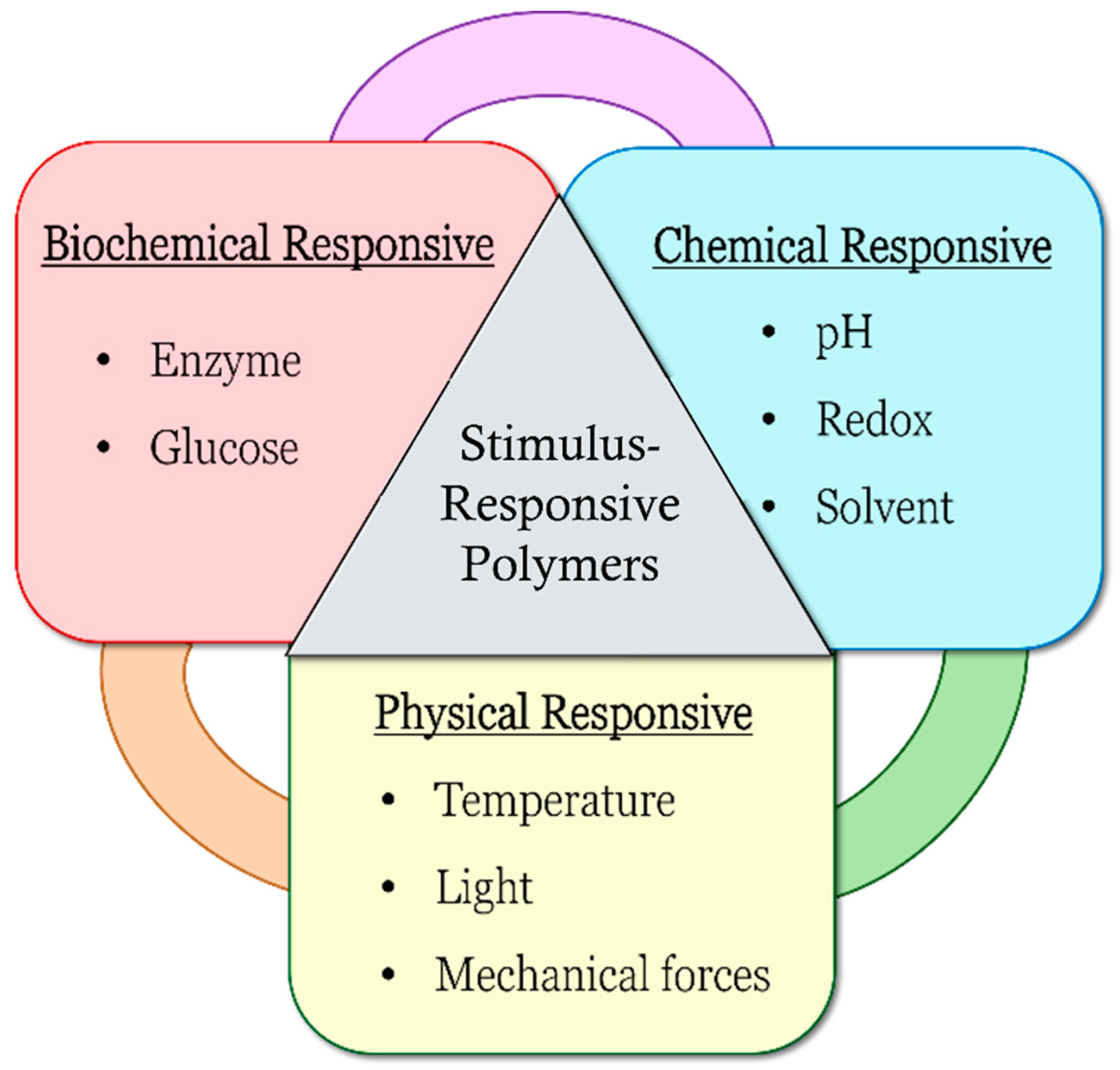
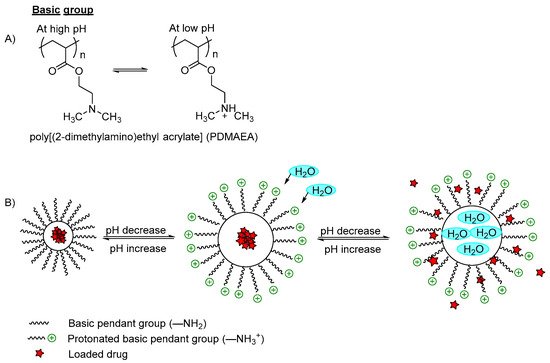
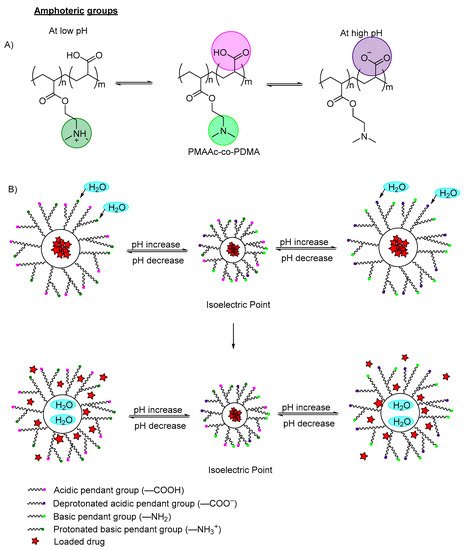
pH-Responsive Polymers
1.1. pH-Responsive Polymers

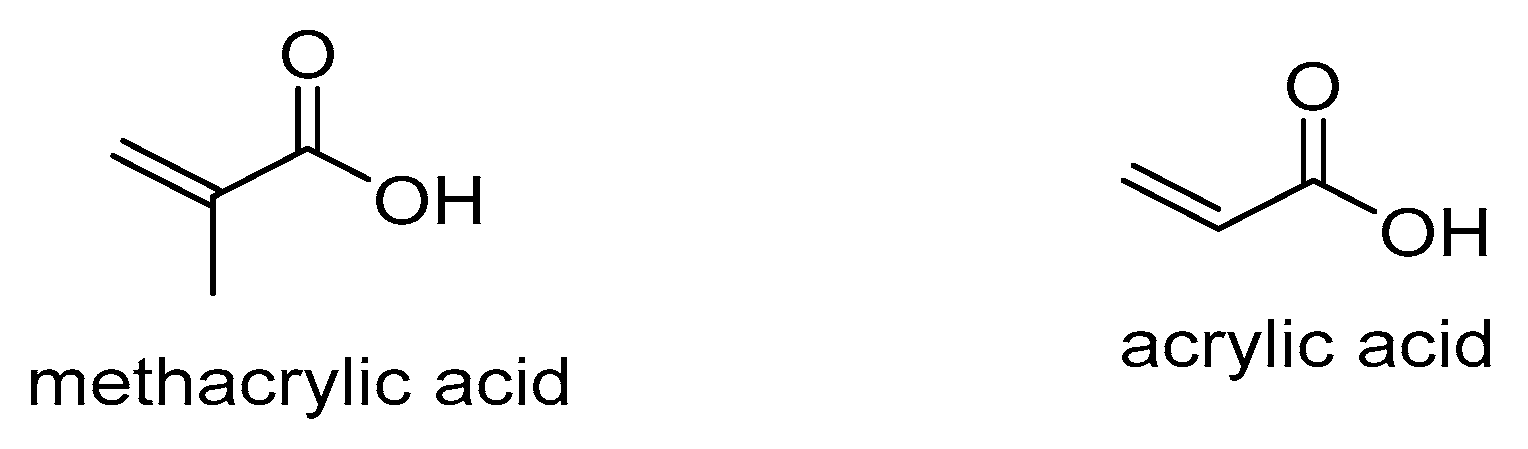
|
Mechanism |
Pendant Groups |
Ionizable Group/Linkage |
Responsive pH |
Polymer Type |
Type of Response |
Biocompatibility Evaluation Application |
Ref |
|---|---|---|---|---|---|---|---|
|
Type of pH-Responsive PU |
PU System |
Reactants Used in Synthesis of PU |
Ionizable Group/Linkage | Biodegradability Evaluation |
Applications |
Ref |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Additional Stimulus Response | pH Responsiveness |
* Applications |
Reference |
Reference Materials |
Improvement to the Reference Materials |
||||||||||||||||||
|
(A) Ionizable Moieties |
|||||||||||||||||||||||
|
Single |
Basic pendant groups, such as amine and its derivatives |
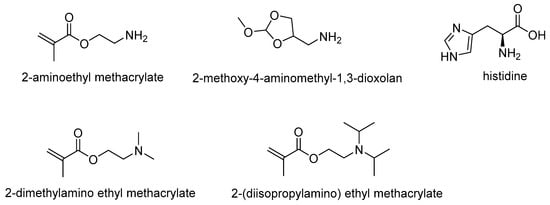
Thermo 2-aminoethyl methacrylate |
|||||||||||||||||||||
|
Single |
PU micelles |
A + C | 6.5 and 6.8 |
|
Poly(ether urethane) (PEU) Polyplex Nanoparticles (NPs) |
Hydrazone linkage
|
Improve cellular uptake and transfection efficiency |
Ex vivo in rodent model for hydrogel injectability and gelation |
Gene Delivery |
** N/A |
N/A |
pH ranging from 4.0–6.0, cleavage of hydrazone bond and degraded |
A [7] |
||||||||||
Controlled and triggered release drug | [ | , | |||||||||||||||||||||
] | N/A | N/A |
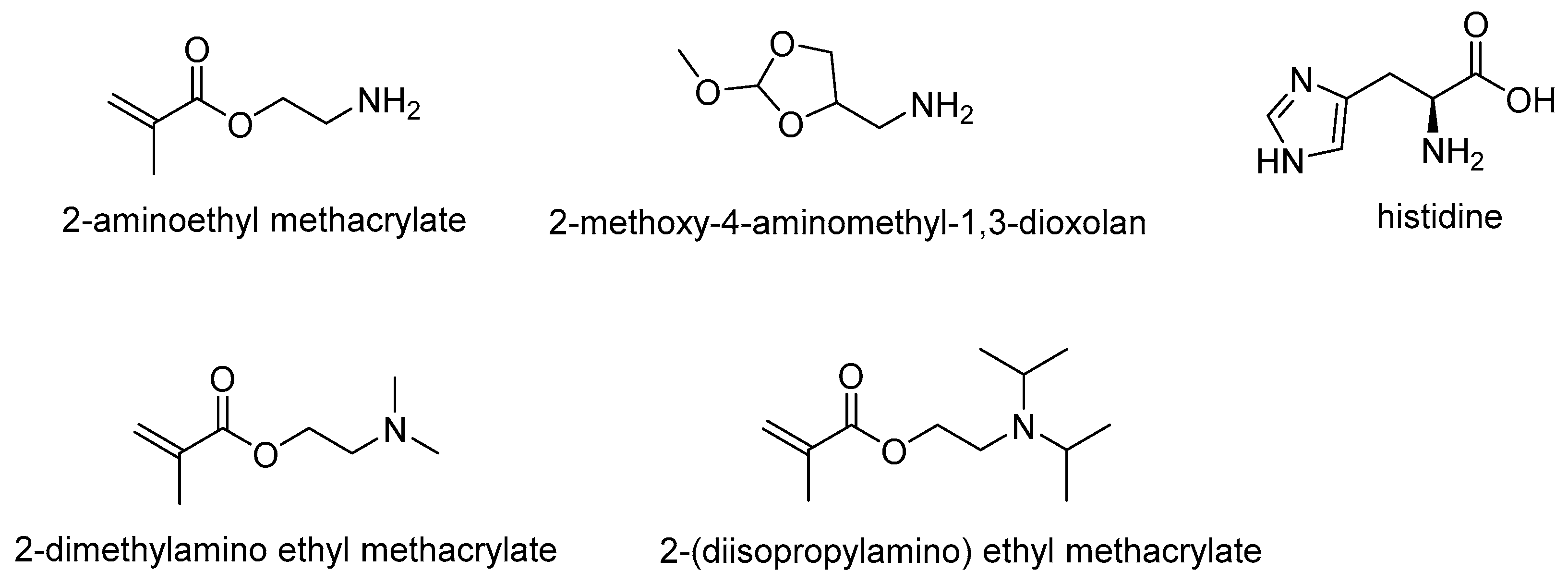
2-methoxy-4-aminomethyl-1,3-dioxolan |
Redox 5.5 |
A + C |
PU Nanoparticles |
| ||||||||||||||||
|
PU micelles |
|
|
Poly(vinyl alcohol) PVA |
Rapid drug release |
Drug carrier for tumor therapy |
|
Hydrazone linkage
| ||||||||||||||||
Degrade and reach 50% weight loss (polymers with increase of disulfide bonds) in 10 mM glutathione (GSH) after 14 days | Chemotherapy drug delivery | ||||||||||||||||||||||
N/A | At pH 4.4, particle size increase due to swelling, drug release to 98% |
A |
Same PU without hydrazone bond |
N/A |
Histidine |
Redox 5.0 |
PU/DEA copolymer A + C |
PU micelles with mPEG block and PLA block with disulfide bonds |
|
Poly(ethylene glycol)-polycaprolactone PEG-PCL |
|
mPEG-PUSS-mPEG Increase drug release |
2-(diethylamino) ethyl methacrylate
|
Chemotherapy |
N/A |
| |||||||
Decompose within 24 h in the presence of 10 mM dithiothreitol (DTT) | Anticancer drug delivery |
[ |
At pH 4.0, dynamic swelling and drug release 4] |
||||||||||||||||||||
A | [ |
Same PU without DEA monomers |
N/A |
2-dimethylamino ethyl methacrylate |
Redox 4.0 and 7.0 |
A + C Poly(lactic acid) PLA |
PU with disulfide bonds, pendant carboxyl groups, and primary amine group PU-SS-COOH-NH2 micellesSwells and speeds drug release |
|
Targeted drug delivery vehicles |
N/A |
Drug release at pH 7.0 |
A |
[76 | ||||||||||
|
| ||||||||||||||||||||||
|
PU micelles |
|
** N/A |
Chemotherapy drug delivery |
||||||||||||||||||||
|
Diethanolamine |
|
|
[26 |
** N/A |
N/A ][24Drug delivery |
At pH 5.5, highest drug release ] |
||||||||||||||||
A | [ | ] | |||||||||||||||||||||
[ | ] | N/A | N/A |
2-dimethylamino ethyl methacrylate |
Light 5.0 |
B + C |
|||||||||||||||||
|
PU copolymer |
| PCL Serinol-based PU nanoparticles |
Swells and enhances drug release |
|
Drug delivery |
** N/A |
HEP | ||||||||||||||||
N/A |
At pH 4.5, swelled twofold and close to zero drug release; however, sodium diclofenac incorporated release at elevation to pH 7.0 | Nanoparticle count rate decrease ~>30% after 15 min of UV irradiation |
A Nanocarrier for controlled drug release |
[3, |
|||||||||||||||||||
] | [ | ] | [ | 72] |
PEG-HD-MDI-HD without HEP monomers |
Reversible and sharp switch between “on” and “off” drug release, serves as window membrane in reservoir-type intravaginal rings |
Chitosan |
Shape memory |
N/A | ||||||||||||||
|
PU copolymer NPs | 5.5 |
|
Shape memory polyurethane (SMPU) |
Poly(lactic-co-glycolic acid) |
| PLGA |
|
Efficient release |
|
Tacrolimus delivery |
Commercial SMPU, MM3520 | ||||||||||||
N/A | ** N/A |
PU containing higher HEP ratio, swelled and highest drug release at pH 5.0 ** N/A |
Endovascular embolization |
||||||||||||||||||||
A | [ | ][73] |
N/A |
N/A |
Chitosan |
Dual | 5.5 |
Shape memory + water |
N/A |
Thermoplastic PU/hydroxyethyl cotton cellulose nanofibers (TPU/CNF-C/CNTs) |
Commercial TPU, BT-70ARYU |
** N/A |
** N/A |
||||||||||
|
PU copolymer hydrogel |
|
Poly(N-isopropylacrylamide)-co-itaconic acid NIPAM |
Fast release |
Local breast cancer therapy |
|||||||||||||||||||
] | [ | ] | Sensors, actuators |
2-(diisopropylamino) ethyl methacrylate | |||||||||||||||||||
DMPA | N/A | N/A |
(DPA) |
6.5 |
Hydroxyethyl methacrylate-co-DPA copolymers |
Increased drug release |
Thermo + light Ocular drug delivery |
||||||||||||||||
|
PU hydrogels- |
| [ | ][28] |
||||||||||||||||||||
A + C |
|
PUA Nanoparticles |
|
|
Weight loss of approximately >~10% in 28 days |
|
3D cell-laden bioprinting |
[ |
Poly(azomethine-urethane) (PAMU) |
N/A |
Highest swelling degree at pH 3.0; increase of PAMU, swelling degree increase, release of drug increase |
A |
N/A |
N/A |
Carboxylic acid pendant groups |
||||||||
|
PU nanomicelles |
Methacrylic acid | ||||||||||||||||||||||
|
Thermo + shape memory |
| (MAA) |
|
A + C |
| 7.4 |
|
PCL-based PU (PCLAU/Fe3O4) Amine-modified bimodal mesopores silica |
|
2-[N,N-bis (2-hydroxy-ethyl)] aminoethanesulfonic acid sodium salt (BES-Na)
|
Swells and speeds drug release |
N/A |
|
Drug release rate: pH 5.0 > pH 6.8 > pH 7.4 Drug delivery carrier |
Weight loss of 67% after 13 weeks |
A |
|||||||
Vascular stents | [ | 12] |
N/A |
N/A |
Acrylic acid |
||||||||||||||||||
|
Thermo + enzyme |
7.4 |
B + C Poly(ethylene glycol) PEG |
Poly(ester urethane) Nanoparticles Increased drug release |
Targeted drug delivery |
|||||||||||||||||||
| |||||||||||||||||||||||
|
PU-sodium alginate (SA) blend |
|
Sodium Alginate |
|
N/A |
Swelled at pH 7.4, sustained and prolonged release of incorporated protein or insulin |
A |
N/A |
BHET derived from PET waste, biocompatible |
Mechanism |
Multi |
Acid-Labile Groups |
Responsive pH |
Polymer Type |
Type of Response |
Thermo + shape + water |
A + C |
PU/nanoporous cellulose gel (PU/NCG) |
| |||||
|
Cellulose crosslinked PU |
|
|
N/A |
|
Application |
** N/A | |||||||||||||||||
Ref | |||||||||||||||||||||||
** N/A |
All 3 PUs swelled and highest release of incorporated drugs at pH 7.4 |
A |
[80 | Biomaterials, sensors |
][78] |
N/A |
Control release rate by changing chain extender; Drug release rate LAPU > DAPU > GAPU |
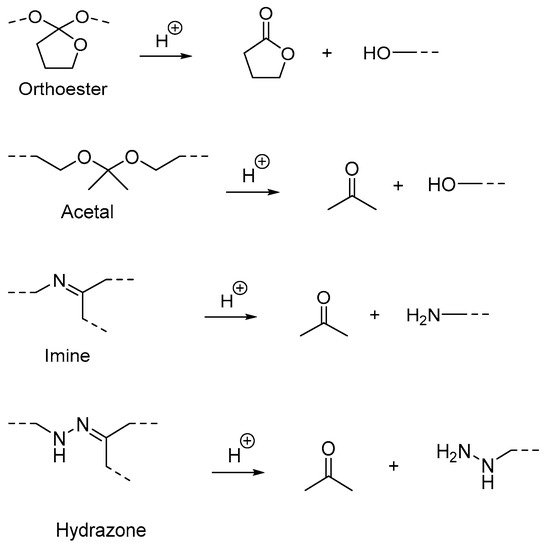
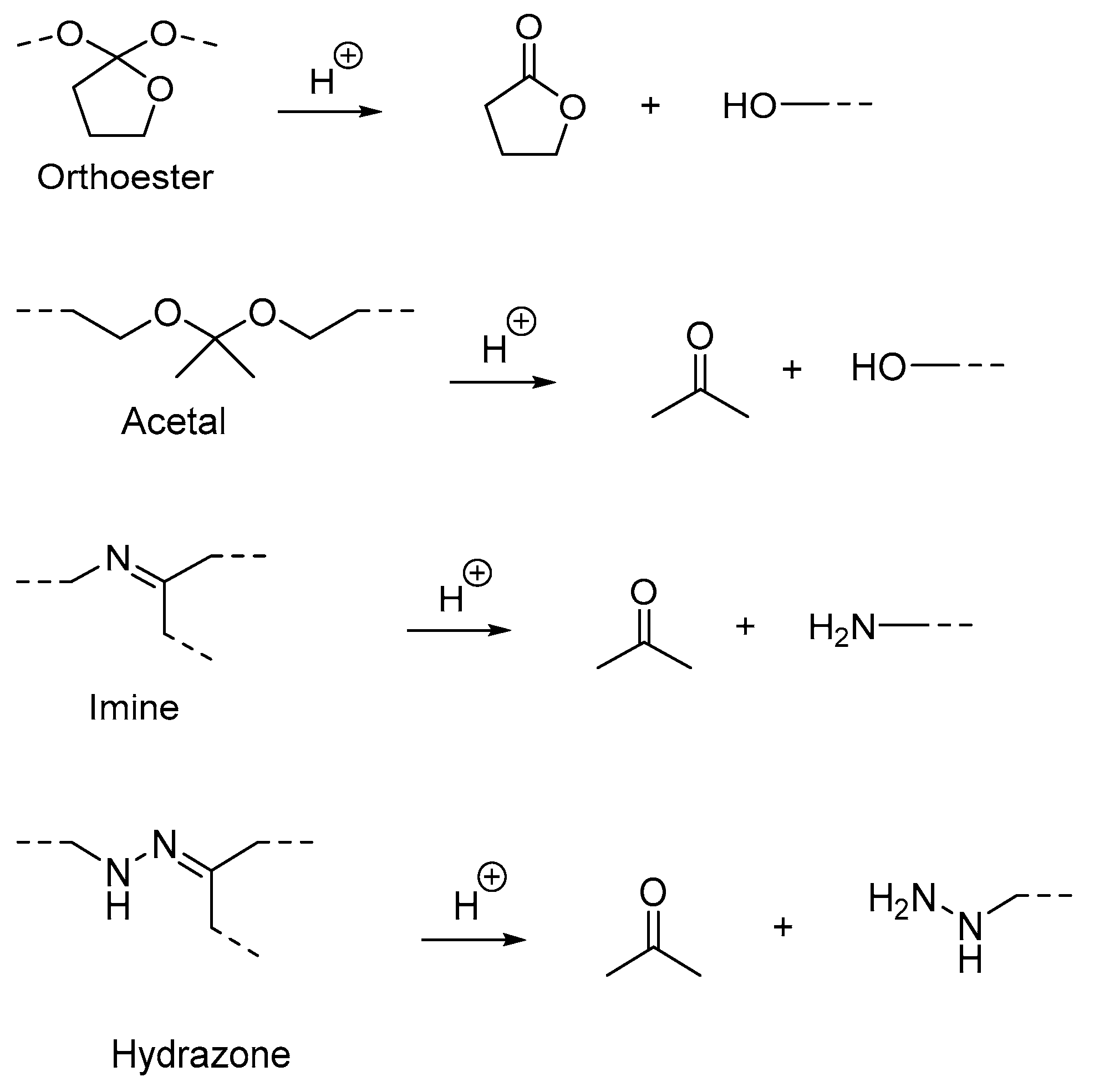
(B) Acid-labile linkages |
β-Thiopropionate | ||||||||||||||
|
PEG-HTPB (g-COOH)-PEG triblock copolymer |
|
5.0 |
Mercaptoacetic acid | PEG |
N/A Increased drug release |
Targeted cancer cell treatment |
At pH 7.4, micelles swelled rapidly and released drug |
||||||||||||||||
A | [ | ][79] |
N/A |
N/A |
β-Thiopropionate |
||||||||||||||||||
|
PU/cellulose acetate phthalate (CAP) fibers |
5.0 |
Commercial PU |
Poly(beta-thioether ester)-PEG |
Rapid drug release |
Nanocarrier for drug delivery |
CAP |
N/A |
Rapid release of Rhodamine B at pH 7.4 within 1 min |
|||||||||||||||
A | [ | ][80] |
- Pure CAP - Pure PU fibers |
Improved tensile strength compared to previously reported CAP fibers |
Hydrazone linkage |
||||||||||||||||||
|
PU films | 5.5 |
|
Lipid Polymer Hybrid NPs |
|
Fast drug release |
|
Biomedical and chemotherapy |
N/A |
PU-Arginine shows highest drug release at pH 4.4; All PU shows average drug release of 64% at pH 10.4 |
||||||||||||||
A | [ | ][81] |
N/A |
N/A |
Hydrazone linkage |
||||||||||||||||||
|
PEG-PU copolymers | 5.4 |
|
N-isopropylacrylamide-co-glycidyl methacrylate |
| (NIPAM-co-GMA) |
|
Increased drug release |
N/A Chemotherapy drug delivery |
Highest pH buffering capacity 7.02, specific responsiveness not mentioned |
||||||||||||||
B | [ | ][82] |
N/A |
N/A |
Hydrazone linkage |
||||||||||||||||||
|
Dual |
5.6 |
PU Poly(β-benzyl malate) |
|
Rapid drug release |
|
Antitumor drug carrier |
| ||||||||||||||||
|
Thermo-responsive |
PU-MDEA: Swells at pH 4.0–5.5 PU-DMPA: Swells at pH 8.5–10.0 |
N/A |
N/A |
N/A |
Oxazoline |
6.0 |
Poly(lactic acid)-poly(β-amino ester) |
Increased drug release |
Colon cancer adjuvant therapy |
|||||||||||||
|
PU Micelles |
|
|
Thermo-responsive |
HDI-MDEA and HDI-BDEA, rapid drug release at pH 4.0 | |||||||||||||||||||
A | [ | ][84] |
N/A |
N/A |
Boron-ester linkage |
6.8 |
|||||||||||||||||
|
PU/DPA |
|
Polymer dots |
Better fluorescent intensity |
Bioimaging probe |
|||||||||||||||||||
|
Borate-ester linkage |
5.5 |
PNIPAAm Poly(N-isopropylacrylamide) |
Rapid drug release |
Cancer therapy |
|||||||||||||||||||
|
Others |
Metal ligand (Fe3+) |
5.0 |
PEG-PLGA |
Rapid drug release |
MRI-guided therapy |
||||||||||||||||||
2. Stimulus-Responsive PU
2.1. Polyurethane
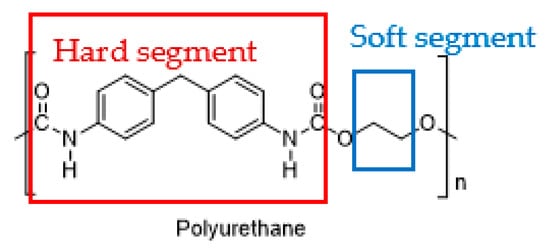
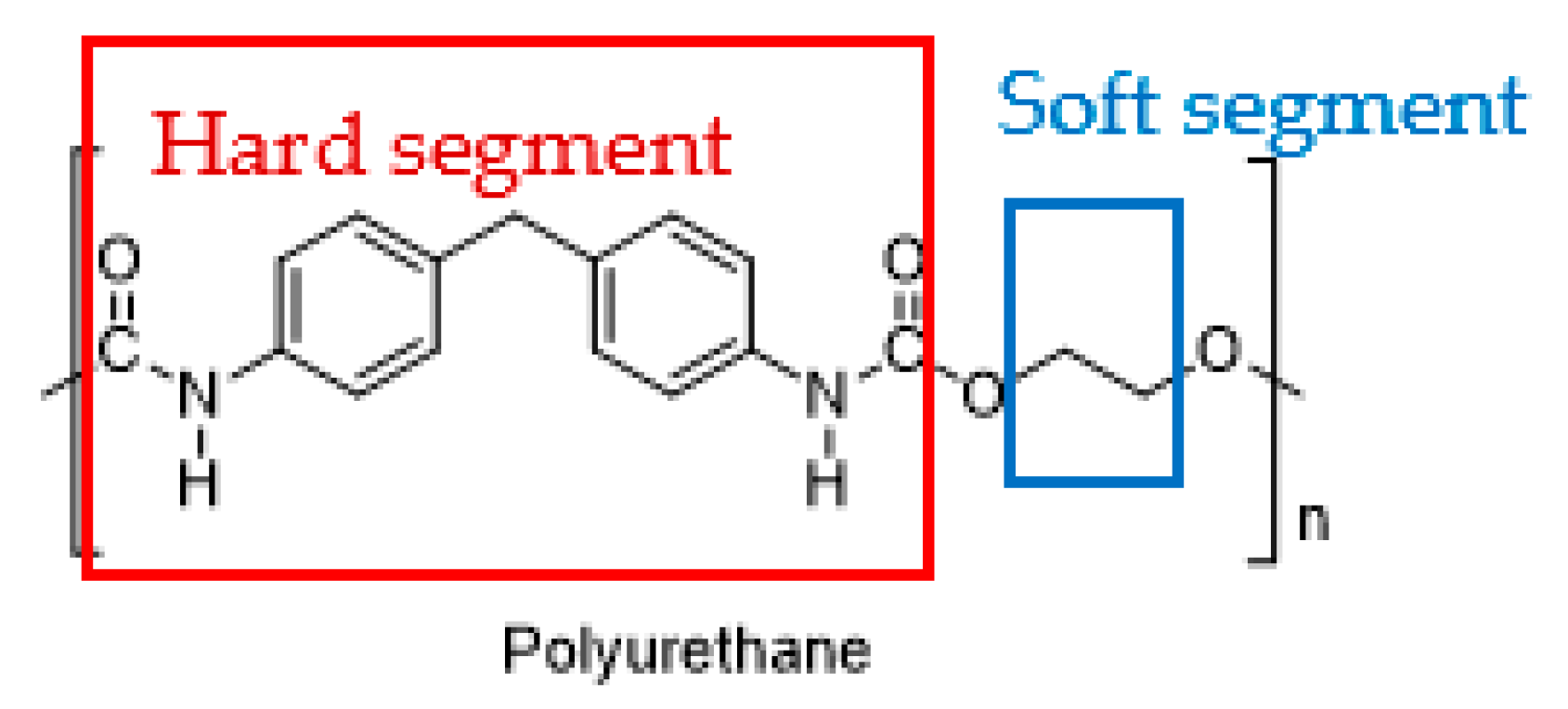
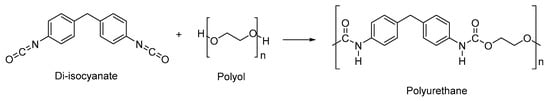
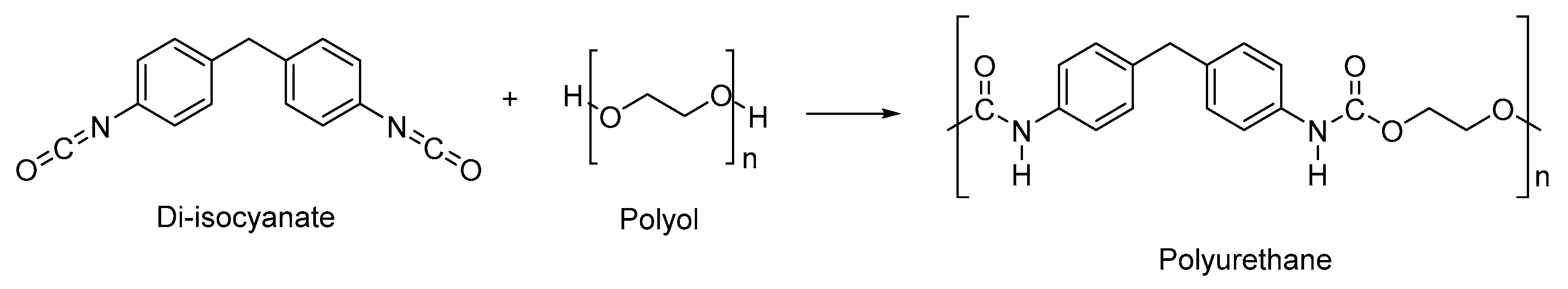
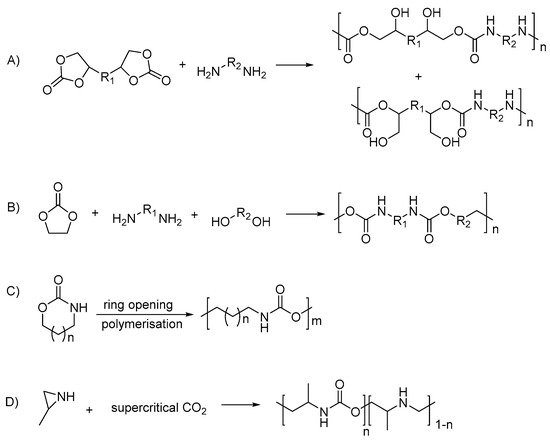
2.2. Types of Stimuli/Stimulus-Responsive Polyurethanes Used in Biomedical and Drug Delivery Applications
|
Type of Stimuli/Stimulus-Responsive PU |
Type of Stimuli/Stimulus |
* PU Type |
PU Name |
Reactants for Synthesis of PU |
|---|
* PU Type: (A) isocyanate-based PU, (B) non-isocyanate-based PU, (C) PU synthesized with petrochemical-based polyols and (D) synthesized from bio-based polyols. ** N/A: information not available.
3. pH-Responsive Polyurethane
| |||||||||
| |||||||||
| |||||||||
DPA | |||||||||
Thermo-responsive | Increase of DPA, results in highest swelling degree at pH 4.0 |
A |
Same PU without addition of DPA/PPGDA/PEGMA mixture |
N/A |
|||||
|
PEG-PCL based PU blend with cellulose nanocrystals (CNC) |
|
|
Shape memory |
CNC-COOH; At pH 4.0, folded strip of CNC-C6H4NO2 recovers to straight |
C |
N/A |
N/A |
||
|
PU |
|
Pyridine |
Shape memory |
Swells at pH 1.3, drug release and shape recovers |
A, C |
[10] |
N/A |
N/A |
|
|
Azo-cationic waterborne polyurethane (CWPU) |
|
|
Photo-responsive |
Shows different color in different pH medium |
B |
N/A |
N/A |
||
|
PU micelles with disulfide linkage |
|
MDEA |
Reduction-responsive |
Rapid drug release at pH 5.5 |
A |
N/A |
N/A |
||
|
PU with disulfide bonds |
|
Poly(2-ethyl-2-oxazoline)(PEOz) |
Reduction-responsive |
Drug release rate higher at pH 5.0 |
A |
- End-group-carboxylated PEOz-PLA - PEOz-hydrazone-DOX |
Cumulative drug release increase with presence of 1, 4-dithio-D, L-threitol (DTT) |
||
|
MPEG/PU triblock copolymers with disulfide linkage |
|
Bis-1,4-(hydroxy-ethyl) piperazine (HEP) |
Reduction-responsive |
pH 5.5 and 6.8, swells and faster drug release |
A |
N/A |
N/A |
||
|
Multi |
PU |
|
DMPA |
Thermo-responsive and shape memory |
For PEG-30%-MDI-DMPA, fixes deformed shape at pH 2.0, recovers shape at pH 9.0 |
N/A |
N/A |
N/A |
* Applications: (A) Biomedical and drug delivery, (B) optical imaging, and (C) biomaterials.
References
- García-Fernández, L.; Mora-Boza, A.; Reyes-Ortega, F. pH-Responsive Polymers: Properties, Synthesis, and Applications. In Smart Polymers and Their Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–86.
- Polo Fonseca, L.; Trinca, R.B.; Felisberti, M.I. Amphiphilic polyurethane hydrogels as smart carriers for acidic hydrophobic drugs. Int. J. Pharm. 2018, 546, 106–114.
- Kim, S.; Chen, Y.; Ho, E.A.; Liu, S. Reversibly pH-responsive polyurethane membranes for on-demand intravaginal drug delivery. Acta Biomater. 2017, 47, 100–112.
- Yu, C.; Tan, X.; Xu, Z.; Zhu, G.; Teng, W.; Zhao, Q.; Liang, Z.; Wu, Z.; Xiong, D. Smart drug carrier based on polyurethane material for enhanced and controlled DOX release triggered by redox stimulus. React. Funct. Polym. 2020, 148, 104507.
- Zhang, C.; Wen, X.; Vyavahare, N.R.; Boland, T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 2008, 29, 3781–3791.
- Yang, P.; Zhu, F.; Zhang, Z.; Cheng, Y.; Wang, Z.; Li, Y. Stimuli-responsive polydopamine-based smart materials. Chem. Soc. Rev. 2021, 50, 8319–8343.
- Ooi, Y.J.; Wen, Y.; Zhu, J.; Song, X.; Li, J. Surface Charge Switchable Polymer/DNA Nanoparticles Responsive to Tumor Extracellular pH for Tumor-Triggered Enhanced Gene Delivery. Biomacromolecules 2020, 21, 1136–1148.
- Chen, K.; Gou, W.; Wang, X.; Zeng, C.; Ge, F.; Dong, Z.; Wang, C. UV-Cured Fluoride-Free Polyurethane Functionalized Textile with pH-Induced Switchable Superhydrophobicity and Underwater Superoleophobicity for Controllable Oil/Water Separation. ACS Sustain. Chem. Eng. 2018, 6, 16616–16628.
- Yang, L.; Wang, C.; Li, L.; Zhu, F.; Ren, X.; Huang, Q.; Cheng, Y.; Li, Y. Bioinspired Integration of Naturally Occurring Molecules towards Universal and Smart Antibacterial Coatings. Adv. Funct. Mater. 2022, 32, 2108749.
- Chen, H.; Li, Y.; Liu, Y.; Gong, T.; Wang, L.; Zhou, S. Highly pH-sensitive polyurethane exhibiting shape memory and drug release. Polym. Chem. 2014, 5, 5168–5174.
- Pardini, F.; Faccia, P.; Amalvy, J. Evaluation of pH-sensitive polyurethane/2-diethylaminoethyl methacrylate hybrids potentially useful for drug delivery developments. J. Drug Deliv. Sci. Technol. 2015, 30, 199–208.
- Song, Y.; Chai, Y.; Xu, K.; Zhang, P. Functional polyurethane nanomicelle with pH-responsive drug delivery property. E-Polymers 2018, 18, 409–417.
- Sgreccia, E.; Pasquini, L.; Ercolani, G.; Knauth, P.; Di Vona, M.L. Stimuli-responsive amphoteric ion exchange polymers bearing carboxylic and amine groups grafted to a cross-linkable silica network. Eur. Polym. J. 2019, 112, 255–262.
- Luo, Y.; Peng, H.; Wu, J.; Sun, J.; Wang, Y. Novel amphoteric pH-sensitive hydrogels derived from ethylenediaminetetraacetic dianhydride, butanediamine and amino-terminated poly(ethylene glycol): Design, synthesis and swelling behavior. Eur. Polym. J. 2011, 47, 40–47.
- Abou Taleb, M.F. Radiation synthesis of multifunctional polymeric hydrogels for oral delivery of insulin. Int. J. Biol. Macromol. 2013, 62, 341–347.
- Binauld, S.; Stenzel, M.H. Acid-degradable polymers for drug delivery: A decade of innovation. Chem. Commun. 2013, 49, 2082–2102.
- Pang, J.; Xing, H.; Sun, Y.; Feng, S.; Wang, S. Non-small cell lung cancer combination therapy: Hyaluronic acid modified, epidermal growth factor receptor targeted, pH sensitive lipid-polymer hybrid nanoparticles for the delivery of erlotinib plus bevacizumab. Biomed. Pharmacother. 2020, 125, 109861.
- Cui, T.; Sihao, Z.; Sun, H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment. Oncol. Rep. 2017, 37, 1253–1260.
- Kumar, A.; Montemagno, C.; Choi, H.J. Smart Microparticles with a pH-responsive Macropore for Targeted Oral Drug Delivery. Sci. Rep. 2017, 7, 3059.
- Li, Y.; Gao, J.; Zhang, C.; Cao, Z.; Cheng, D.; Liu, J.; Shuai, X. Stimuli-Responsive Polymeric Nanocarriers for Efficient Gene Delivery. Top. Curr. Chem. 2017, 375, 167–215.
- Yang, W.; Bai, T.; Carr, L.R.; Keefe, A.J.; Xu, J.; Xue, H.; Irvin, C.A.; Chen, S.; Wang, J.; Jiang, S. The effect of lightly crosslinked poly(carboxybetaine) hydrogel coating on the performance of sensors in whole blood. Biomaterials 2012, 33, 7945–7951.
- Li, D.; Qin, J.; Sun, M.; Yan, G.; Tang, R. pH-sensitive, dynamic graft polymer micelles via simple synthesis for enhanced chemotherapeutic efficacy. J. Biomater. Appl. 2020, 34, 1059–1070.
- Wang, P.; Liu, W.; Liu, S.; Yang, R.; Pu, Y.; Zhang, W.; Wang, X.; Liu, X.; Ren, Y.; Chi, B. pH-responsive nanomicelles of poly(ethylene glycol)-poly(ε-caprolactone)-poly(L-histidine) for targeted drug delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 277–292.
- Ifra; Saha, S. Fabrication of topologically anisotropic microparticles and their surface modification with pH responsive polymer brush. Mater. Sci. Eng. C 2019, 104, 109894.
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-Assembly of Partially Alkylated Dextran- graft-poly Copolymer Facilitating Hydrophobic/Hydrophilic Drug Delivery and Improving Conetwork Hydrogel Properties. Biomacromolecules 2018, 19, 1142–1153.
- Mansouri, A.; Abnous, K.; Alibolandi, M.; Taghdisi, S.M.; Ramezani, M. Targeted delivery of tacrolimus to T cells by pH-responsive aptamer-chitosan-poly(lactic-co-glycolic acid) nanocomplex. J. Cell. Physiol. 2019, 234, 18262–18271.
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964.
- Faccia, P.A.; Pardini, F.M.; Amalvy, J.I. Uptake and release of Dexamethasone using pH-responsive poly(2-hydroxyethyl methacrylate-co-2-(diisopropylamino)ethyl methacrylate) hydrogels for potential use in ocular drug delivery. J. Drug Deliv. Sci. Technol. 2019, 51, 45–54.
- Ma, J.Y.; Han, J.; Sun, J.H.; Fan, L.; Bai, S.Y.; Jiao, Y.W. pH-sensitive controlled release in vitro and pharmacokinetics of ibuprofen from hybrid nanocomposite using amine-modified bimodal mesopores silica as core and poly(methylacrylic acid) as shell. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 1023–1033.
- Yue, Z.; Che, Y.J.; Jin, Z.; Wang, S.; Ma, Q.; Zhang, Q.; Tan, Y.; Meng, F. A facile method to fabricate thermo- and pH-sensitive hydrogels with good mechanical performance based on poly(ethylene glycol) methyl ether methacrylate and acrylic acid as a potential drug carriers. J. Biomater. Sci. Polym. Ed. 2019, 30, 1375–1398.
- Zhang, H.; Zhou, T.; Yu, Q.; Yang, Z.; Sun, Y.; Cai, Z.; Cang, H. pH-Sensitive betulinic acid polymer prodrug nanoparticles for efficient and targeted cancer cells treatment. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 659–668.
- Wu, W.X.; Yang, X.L.; Liu, B.Y.; Deng, Q.F.; Xun, M.M.; Wang, N.; Yu, X.Q. Lipase-catalyzed synthesis of oxidation-responsive poly(ethylene glycol)-b-poly(β-thioether ester) amphiphilic block copolymers. RSC Adv. 2016, 6, 11870–11879.
- Wang, J. Combination treatment of cervical cancer using folate-decorated, pH-sensitive, carboplatin and paclitaxel co-loaded lipid-polymer hybrid nanoparticles. Drug Des. Devel. Ther. 2020, 14, 823.
- Pourjavadi, A.; Kohestanian, M.; Streb, C. pH and thermal dual-responsive poly(NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery. Mater. Sci. Eng. C 2020, 108, 110418.
- Qiao, Y.; Wang, C.; Liu, B.; Peng, Y.; Meng, H.; Yang, T.; Zhou, Q.; Guo, S.; Wu, H. Erratum: Enhanced endocytic and pH-sensitive poly(malic acid) micelles for antitumor drug delivery. J. Biomed. Nanotechnol. 2019, 15, 28–41.
- Hao, D.L.; Xie, R.; De, G.J.; Yi, H.; Zang, C.; Yang, M.Y.; Liu, L.; Ma, H.; Cai, W.Y.; Zhao, Q.H.; et al. PH-responsive artesunate polymer prodrugs with enhanced ablation effect on rodent xenograft colon cancer. Int. J. Nanomed. 2020, 15, 1771.
- Kim, S.G.; Ryplida, B.; Phuong, P.T.M.; Won, H.J.; Lee, G.; Bhang, S.H.; Park, S.Y. Reduction-triggered paclitaxel release nano-hybrid system based on core-crosslinked polymer dots with a pH-responsive shell-cleavable colorimetric biosensor. Int. J. Mol. Sci. 2019, 20, 5368.
- Zhou, P.; Wu, S.; Hegazy, M.; Li, H.; Xu, X.; Lu, H.; Huang, X. Engineered borate ester conjugated protein-polymer nanoconjugates for pH-responsive drug delivery. Mater. Sci. Eng. C 2019, 104, 109914.
- Zhang, C.; Li, J.; Yang, C.; Gong, S.; Jiang, H.; Sun, M.; Qian, C. A pH-sensitive coordination polymer network-based nanoplatform for magnetic resonance imaging-guided cancer chemo-photothermal synergistic therapy. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102071.
- Shoaib, M.; Bahadur, A.; ur Rahman, M.S.; Iqbal, S.; Arshad, M.I.; Tahir, M.A.; Mahmooda, T. Sustained drug delivery of doxorubicin as a function of pH, releasing media, and NCO contents in polyurethane urea elastomers. J. Drug Deliv. Sci. Technol. 2017, 39, 277–282.
- Aksoy, E.A.; Taskor, G.; Gultekinoglu, M.; Kara, F.; Ulubayram, K. Synthesis of biodegradable polyurethanes chain-extended with (2S)-bis(2-hydroxypropyl) 2-aminopentane dioate. J. Appl. Polym. Sci. 2018, 135, 45764.
- Yeoh, F.H.; Lee, C.S.; Kang, Y.B.; Wong, S.F.; Cheng, S.F.; Ng, W.S. Production of biodegradable palm oil-based polyurethane as potential biomaterial for biomedical applications. Polymers 2020, 12, 1842.
- Arévalo-Alquichire, S.; Valero, M. Castor Oil Polyurethanes as Biomaterials. In Elastomers; Çankaya, N., Ed.; IntechOpen: London, UK, 2017; pp. 137–157.
- Poussard, L.; Mariage, J.; Grignard, B.; Detrembleur, C.; Jéroîme, C.; Calberg, C.; Heinrichs, B.; De Winter, J.; Gerbaux, P.; Raquez, J.M.; et al. Non-Isocyanate Polyurethanes from Carbonated Soybean Oil Using Monomeric or Oligomeric Diamines to Achieve Thermosets or Thermoplastics. Macromolecules 2016, 49, 2162–2171.
- Uscátegui, Y.L.; Díaz, L.E.; Gómez-Tejedor, J.A.; Vallés-Lluch, A.; Vilariño-Feltrer, G.; Serrano, M.A.; Valero, M.F. Candidate polyurethanes based on castor oil (ricinus communis), with polycaprolactone diol and chitosan additions, for use in biomedical applications. Molecules 2019, 24, 237.
- Doley, S.; Dolui, S.K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168.
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased polyurethanes prepared from different vegetable oils. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233.
- Barksby, N.; Dormish, J.F.; Haider, K.W. Encyclopedia of Polymeric Nanomaterials; Springer: Berlin, Germany, 2020; pp. 1–14.
- Szycher, M. Structure–Property Relations in Polyurethanes. In Szycher’s Handbook of Polyurethanes; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439839584.
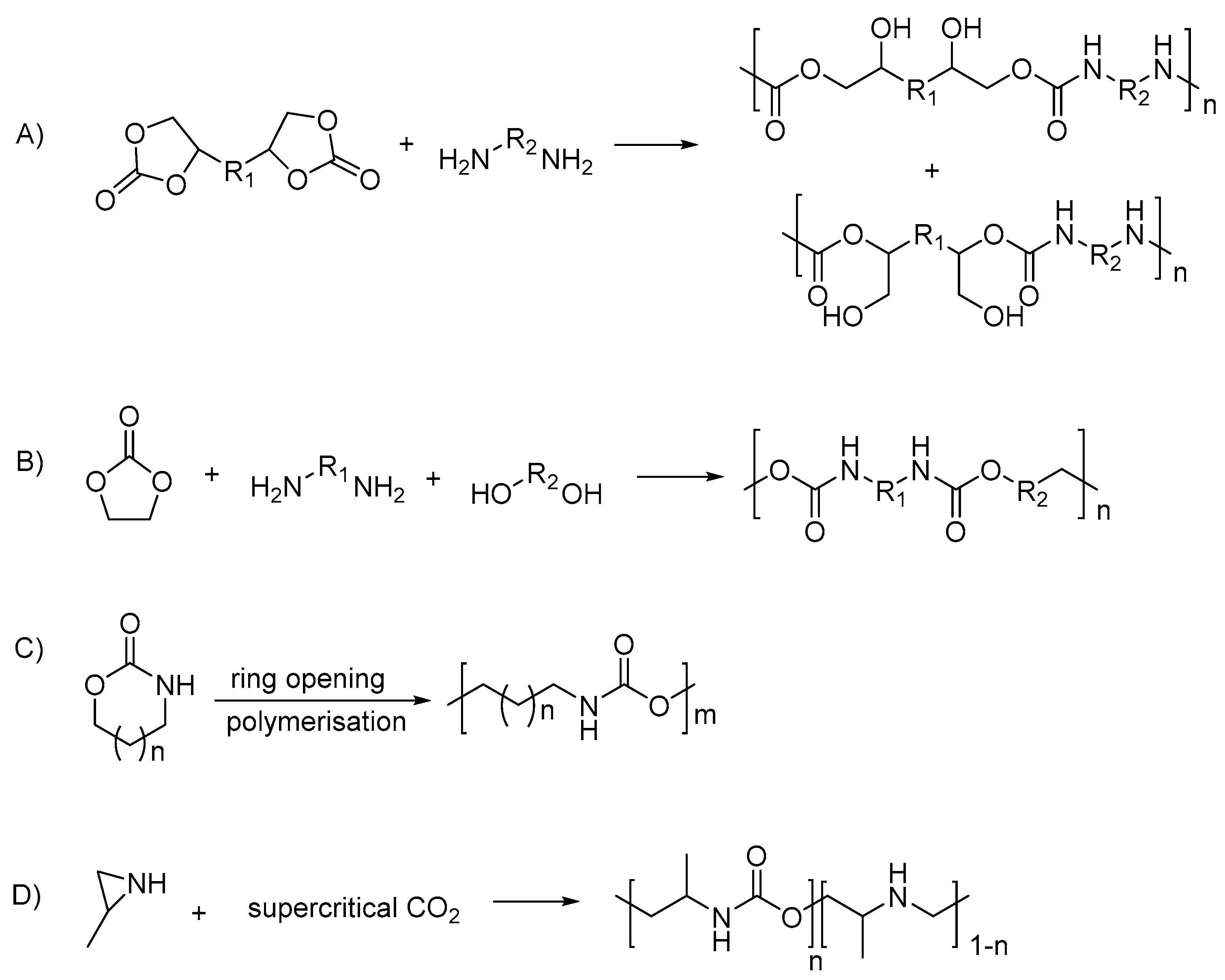
- Keul, H.; Mommer, S.; Möller, M. Poly(amide urethane)s with functional/reactive side groups based on a bis-cyclic bio-based monomer/coupling agent. Eur. Polym. J. 2013, 49, 853–864.
- Rokicki, G.; Piotrowska, A. A new route to polyurethanes from ethylene carbonate, diamines and diols. Polymer 2002, 43, 2927–2935.
- Neffgen, S.; Keul, H.; Höcker, H. Cationic Ring-Opening Polymerization of Trimethylene Urethane: A Mechanistic Study. Macromolecules 1997, 30, 1289–1297.
- Ihata, O.; Kayaki, Y.; Ikariya, T. Aliphatic Poly(urethane−amine)s Synthesized by Copolymerization of Aziridines and Supercritical Carbon Dioxide. Macromolecules 2005, 38, 6429–6434.
- Iyer, R.; Nguyen, T.; Padanilam, D.; Xu, C.; Saha, D.; Nguyen, K.T.; Hong, Y. Glutathione-responsive biodegradable polyurethane nanoparticles for lung cancer treatment. J. Control. Release 2020, 321, 363–371.
- Tamariz, E.; Rios-Ramrez, A. Biodegradation of Medical Purpose Polymeric Materials and Their Impact on Biocompatibility. In Biodegradation—Life of Science; Chamy, R., Ed.; IntechOpen: London, UK, 2013; pp. 3–30.
- Ghosh, T.; Karak, N. Silicone-Containing Biodegradable Smart Elastomeric Thermoplastic Hyperbranched Polyurethane. ACS Omega 2018, 3, 6849–6859.
- Zhao, L.; Liu, C.; Qiao, Z.; Yao, Y.; Luo, J. Reduction responsive and surface charge switchable polyurethane micelles with acid cleavable crosslinks for intracellular drug delivery. RSC Adv. 2018, 8, 17888–17897.
- Aluri, R.; Saxena, S.; Joshi, D.C.; Jayakannan, M. Multistimuli-Responsive Amphiphilic Poly(ester-urethane) Nanoassemblies Based on l-Tyrosine for Intracellular Drug Delivery to Cancer Cells. Biomacromolecules 2018, 19, 2166–2181.
- Zhou, L.; Yu, L.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q. Synthesis and characterization of pH-sensitive biodegradable polyurethane for potential drug delivery applications. Macromolecules 2011, 44, 857–864.
- Bera, A.; Singh Chandel, A.K.; Uday Kumar, C.; Jewrajka, S.K. Degradable/cytocompatible and pH responsive amphiphilic conetwork gels based on agarose-graft copolymers and polycaprolactone. J. Mater. Chem. B 2015, 3, 8548–8557.
- Zhou, L.; Liang, D.; He, X.; Li, J.; Tan, H.; Li, J.; Fu, Q.; Gu, Q. The degradation and biocompatibility of pH-sensitive biodegradable polyurethanes for intracellular multifunctional antitumor drug delivery. Biomaterials 2012, 33, 2734–2745.
- Boffito, M.; Torchio, A.; Tonda-Turo, C.; Laurano, R.; Gisbert-Garzarán, M.; Berkmann, J.C.; Cassino, C.; Manzano, M.; Duda, G.N.; Vallet-Regí, M.; et al. Hybrid Injectable Sol-Gel Systems Based on Thermo-Sensitive Polyurethane Hydrogels Carrying pH-Sensitive Mesoporous Silica Nanoparticles for the Controlled and Triggered Release of Therapeutic Agents. Front. Bioeng. Biotechnol. 2020, 8, 384.
- Sun, J.; Rust, T.; Kuckling, D. Light-Responsive Serinol-Based Polyurethane Nanocarrier for Controlled Drug Release. Macromol. Rapid Commun. 2019, 40, 1900348.
- Kashyap, D.; Kishore Kumar, P.; Kanagaraj, S. 4D printed porous radiopaque shape memory polyurethane for endovascular embolization. Addit. Manuf. 2018, 24, 687–695.
- Wu, G.; Gu, Y.; Hou, X.; Li, R.; Ke, H.; Xiao, X. Hybrid nanocomposites of cellulose/carbon-nanotubes/polyurethane with rapidly water sensitive shape memory effect and strain sensing performance. Polymers 2019, 11, 1586.
- Hsiao, S.H.; Hsu, S.H. Synthesis and Characterization of Dual Stimuli-Sensitive Biodegradable Polyurethane Soft Hydrogels for 3D Cell-Laden Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 29273–29287.
- Gu, S.Y.; Chang, K.; Jin, S.P. A dual-induced self-expandable stent based on biodegradable shape memory polyurethane nanocomposites (PCLAU/Fe3O4) triggered around body temperature. J. Appl. Polym. Sci. 2018, 135, 45686.
- Li, K.; Wei, P.; Huang, J.; Xu, D.; Zhong, Y.; Hu, L.; Zhang, L.; Cai, J. Mechanically Strong Shape-Memory and Solvent-Resistant Double-Network Polyurethane/Nanoporous Cellulose Gel Nanocomposites. ACS Sustain. Chem. Eng. 2019, 7, 15974–15982.
- Song, N.; Zhou, L.; Li, J.; Pan, Z.; He, X.; Tan, H.; Wan, X.; Li, J.; Ran, R.; Fu, Q. Inspired by nonenveloped viruses escaping from endo-lysosomes: A pH-sensitive polyurethane micelle for effective intracellular trafficking. Nanoscale 2016, 8, 7711–7722.
- Pardini, F.M.; Amalvy, J.I. Synthesis and swelling behavior of pH-responsive polyurethane/poly hybrid materials. J. Appl. Polym. Sci. 2014, 131, 39799.
- Yao, Y.; Xu, D.; Liu, C.; Guan, Y.; Zhang, J.; Su, Y.; Zhao, L.; Meng, F.; Luo, J. Biodegradable pH-sensitive polyurethane micelles with different polyethylene glycol (PEG) locations for anti-cancer drug carrier applications. RSC Adv. 2016, 6, 97684–97693.
- Kim, S.; Traore, Y.L.; Ho, E.A.; Shafiq, M.; Kim, S.H.; Liu, S. Design and development of pH-responsive polyurethane membranes for intravaginal release of nanomedicines. Acta Biomater. 2018, 82, 12–23.
- He, W.; Zheng, X.; Zhao, Q.; Duan, L.; Lv, Q.; Gao, G.H.; Yu, S. pH-Triggered Charge-Reversal Polyurethane Micelles for Controlled Release of Doxorubicin. Macromol. Biosci. 2016, 16, 925–935.
- Kim, S.; Traore, Y.L.; Chen, Y.; Ho, E.A.; Liu, S. Switchable on-demand release of a nanocarrier from a segmented reservoir type intravaginal ring filled with a pH-responsive supramolecular polyurethane hydrogel. ACS Appl. Bio Mater. 2018, 1, 652–662.
- Duru Kamacı, U.; Kamacı, M. Preparation of polyvinyl alcohol, chitosan and polyurethane-based pH-sensitive and biodegradable hydrogels for controlled drug release applications. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 1167–1177.
- Bhattacharyya, A.; Mukhopadhyay, P.; Kundu, P.P. Synthesis of a novel pH-sensitive polyurethane-alginate blend with poly(ethylene terephthalate) waste for the oral delivery of protein. J. Appl. Polym. Sci. 2014, 131, 40650.
- Bhattacharyya, A.; Mukhopadhyay, P.; Pramanik, N.; Kundu, P.P. Effect of Polyethylene Glycol on Bis(2-hydroxyethyl) terephthalate-Based Polyurethane/Alginate pH-Sensitive Blend for Oral Protein Delivery. Adv. Polym. Technol. 2016, 35, 21525.
- Solanki, A.; Thakore, S. Cellulose crosslinked pH-responsive polyurethanes for drug delivery: α-hydroxy acids as drug release modifiers. Int. J. Biol. Macromol. 2015, 80, 683–691.
- Nabid, M.R.; Omrani, I. Facile preparation of pH-responsive polyurethane nanocarrier for oral delivery. Mater. Sci. Eng. C 2016, 69, 532–537.
- Hua, D.; Liu, Z.; Wang, F.; Gao, B.; Chen, F.; Zhang, Q.; Xiong, R.; Han, J.; Samal, S.K.; De Smedt, S.C.; et al. pH responsive polyurethane (core) and cellulose acetate phthalate (shell) electrospun fibers for intravaginal drug delivery. Carbohydr. Polym. 2016, 151, 1240–1244.
- Shoaib, M.; Bahadur, A.; Saeed, A.; ur Rahman, M.S.; Naseer, M.M. Biocompatible, pH-responsive, and biodegradable polyurethanes as smart anti-cancer drug delivery carriers. React. Funct. Polym. 2018, 127, 153–160.
- Yang, H.Y.; Zhang, X.M.; Duan, L.J.; Zhang, M.Y.; Gao, G.H.; Zhang, H.X. Environmental pH-responsive fluorescent PEG-polyurethane for potential optical imaging. J. Appl. Polym. Sci. 2013, 129, 846–852.
- Zhou, H.; Xun, R.; Liu, Q.; Fan, H.; Liu, Y. Preparation of thermal and pH dually sensitive polyurethane membranes and their properties. J. Macromol. Sci. Part B Phys. 2014, 53, 398–411.
- Wang, A.; Gao, H.; Sun, Y.; Sun, Y.L.; Yang, Y.W.; Wu, G.; Wang, Y.; Fan, Y.; Ma, J. Temperature- and pH-responsive nanoparticles of biocompatible polyurethanes for doxorubicin delivery. Int. J. Pharm. 2013, 441, 30–39.
- Pardini, F.M.; Faccia, P.A.; Pardini, O.R.; Amalvy, J.I. Thermal and pH dual responsive polyurethane/2-(diisopropylamino)ethyl methacrylate hybrids: Synthesis, characterization, and swelling behavior. Int. J. Polym. Anal. Charact. 2018, 23, 207–225.
- Li, Y.; Chen, H.; Liu, D.; Wang, W.; Liu, Y.; Zhou, S. PH-Responsive Shape Memory Poly(ethylene glycol)-Poly(ε-caprolactone)-based Polyurethane/Cellulose Nanocrystals Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 12988–12999.
- Li, J.; Zhang, X.; Gooch, J.; Sun, W.; Wang, H.; Wang, K. Photo- and pH-sensitive azo-containing cationic waterborne polyurethane. Polym. Bull. 2015, 72, 881–895.
- Guan, Y.; Su, Y.; Zhao, L.; Meng, F.; Wang, Q.; Yao, Y.; Luo, J. Biodegradable polyurethane micelles with pH and reduction responsive properties for intracellular drug delivery. Mater. Sci. Eng. C 2017, 75, 1221–1230.
- Bu, L.; Zhang, H.; Xu, K.; Du, B.; Zhu, C.; Li, Y. pH and reduction dual-responsive micelles based on novel polyurethanes with detachable poly(2-ethyl-2-oxazoline) shell for controlled release of doxorubicin. Drug Deliv. 2019, 26, 300–308.
- Yu, S.; He, C.; Ding, J.; Cheng, Y.; Song, W.; Zhuang, X.; Chen, X. PH and reduction dual responsive polyurethane triblock copolymers for efficient intracellular drug delivery. Soft Matter 2013, 9, 2637–2645.
- Yu, S.; He, C.; Lv, Q.; Sun, H.; Chen, X. PH and reduction dual responsive cross-linked polyurethane micelles as an intracellular drug delivery system. RSC Adv. 2014, 4, 63070–63078.
- Song, Q.; Chen, H.; Zhou, S.; Zhao, K.; Wang, B.; Hu, P. Thermo- and pH-sensitive shape memory polyurethane containing carboxyl groups. Polym. Chem. 2016, 7, 1739–1746.
