Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Ileana Rubio.
Thyroid cancer (TC) is one of the most frequent endocrine cancers. TC derived from follicular cells includes differentiated thyroid cancer (DTC) with papillary (PTC) or follicular (FTC) histology (80% of cases) and undifferentiated (anaplastic-ATC) and poorly differentiated TC (1–2% of cases). Metformin is the most used drug for type 2 diabetes (T2DM). Its antitumor activity has been described by clinical studies showing reduced risk of cancer development in T2DM patients, as well as management of T2DM compared with those receiving other glucose-lowering drugs. Metformin has a plethora of molecular actions in cancer cells.
- thyroid cancer
- cell lines
- metformin
1. Introduction
Since the first reported use of metformin for diabetes treatment in 1957, millions of people have been taking this synthetic biguanide for type 2 diabetes management worldwide [1]. Attention has been brought to this safe and inexpensive drug for its antitumor activity from a clinical report in 2005 that associated the use of metformin with the decreased risk of cancer in patients with type 2 diabetes [2]. Since then, observational clinical studies, metanalyses and some clinical trials have associated metformin with improved cancer outcomes, reduced cancer mortality and reduced incidence across different types of cancer [3][4]. Likewise, extensive in vivo and in vitro laboratory studies have shown experimental evidence of the antineoplastic activity and underlying mechanisms of metformin in cancer, although they are not completely understood.
The action of metformin is multifactorial. In cancer, its action can be credited to the inhibition of insulin mitogenic activity and activation of adenosine monophosphate-activated protein kinase (AMPK), an energy-sensing complex that regulates cellular and whole-body energy balance [5]. AMPK is activated through the tumor suppressor liver kinase B1, resulting in inhibition of the mammalian target of rapamycin pathway (mTOR), activated in many human cancers [6]. Still, metformin antitumor activity is also mediated through AMPK-independent pathways [5]. In thiRes review, we earchers aimed to summarize the available data on in vitro studies of the effects of metformin on human thyroid, prostate and head and neck cancers. Figure 1 summarizes the molecular pathways modulated by metformin and how it influences the cancer hallmarks [7][8][9].
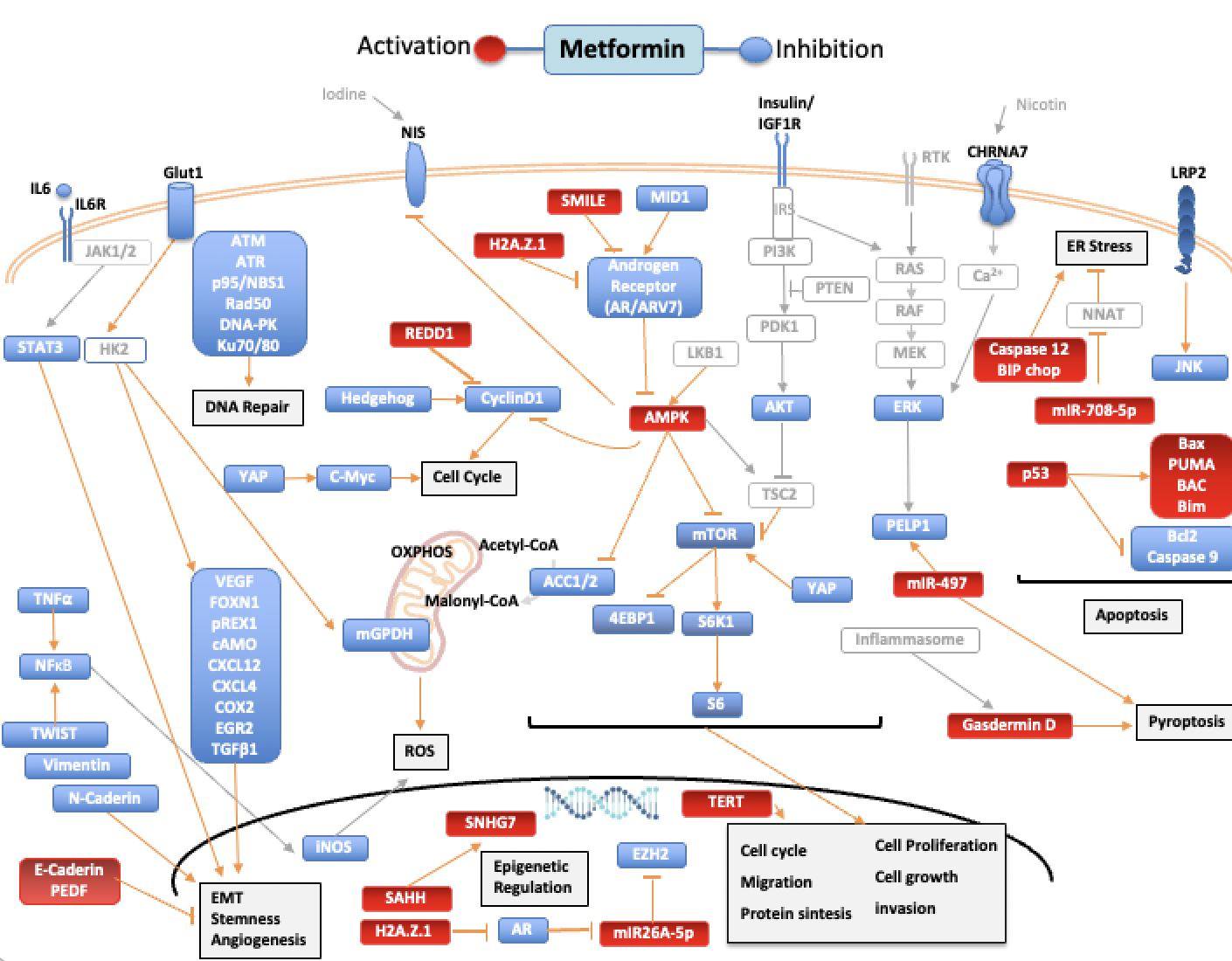
Figure 1. Pathways regulated by metformin in thyroid, prostate, and head and neck cancers. Blue targets show inhibited or downregulated, and red targets show activated or upregulated by metformin. Signaling molecules and arrows in gray have no direct relationship described with metformin. Acetyl-CoA carboxylase (ACC), AKT Serine/Threonine Kinase (AKT), AMPK (adenosine monophosphate-activated protein kinase), androgen receptor (AR), Androgen receptor variant 7(ARV7), Ataxia Telangiectasia Mutated (ATM), Ataxia Telangiectasia And Rad3-Related Protein (ATR), Na+/I- symporter (NIS), BCL2 Associated X, Apoptosis Regulator (BAX), Bcl-2 Interacting Mediator Of Cell Death (BIM), B-Cell CLL/Lymphoma 2 (BCL2), Binding-Immunoglobulin Protein (BIP), Cholinergic Receptor Nicotinic Alpha 7 Subunit (CHRNA7), C/EBP Homologous Protein (CHOP), cyclic AMP (cAMP), C-X-C motif chemokine receptor 4 (CXCR4), C-X-C Motif Chemokine Ligand 12 (CXCL12), Cyclooxygenase 2 (COX2), DNA-dependent protein kinase (DNA-PK), Endoplasmic reticulum (ER), Early Growth Response 2 (EGR2), Epithelial–mesenchymal transition (EMT), Eukaryotic Translation Initiation Factor 4E Binding Protein 1 (4EBP1), Extracellular Signal-Regulated Kinase 2 (ERK), Forkhead Box M1 (FOXM1), Glucose transporter 1 (Glut1), H2A.Z variant histone 1 (H2AZ1), Hexokinase-2 (HK2), Interleukin-6 (IL6), Interleukin-6 receptor (IL6R), Insulin Like Growth Factor 1 Receptor (IGF1R), Insulin Receptor Substrate (IRS), Janus Kinase (JAK), JUN N-Terminal Kinase (JNK), LDL Receptor Related Protein 2 (LRP2), X-Ray Repair Cross Complementing 6 (Ku70), X-Ray Repair Cross Complementing 5 (Ku80), Mammalian target of rapamycin (mTOR), MAPK/ERK Kinase 1 (MEK), Midline-1 (MID1), Mitochondrial GAPDH (mGPDH), MYC Proto-Oncogene, BHLH Transcription Factor (C-Myc), Neuronatin (NNAT), Nijmegen breakage syndrome 1 protein (NBS1), RAD50 Double Strand Break Repair (Rad50), Nuclear Factor Kappa B (NFκB), ZFP42 Zinc Finger (REX1), oxidative phosphorylation (OXPHOS), Pigment epithelium-derived factor (PEDF), Phosphoinositide 3-kinase (PI3K), Phosphatase And Tensin Homolog (PTEN), Proline, Glutamate And Leucine Rich Protein 1 (PELP1), Pyruvate Dehydrogenase Kinase 1 (PDK1), P53 Up-Regulated Modulator Of Apoptosis (PUMA),Raf-1 Proto-Oncogene, Serine/Threonine Kinase (RAF), Ras Proto-Oncogene GTPase (RAS), Regulated in development and DNA damage response 1 (REDD1), Ribosomal Protein S6 Kinase B1 (S6K1), Ribosomal protein S6 (S6), Small heterodimer partner–interacting leucine zipper (SMILE), Signal Transducer And Activator Of Transcription (STAT3), Transforming Growth Factor Beta 1 (TGF-β1), Tumor Necrosis Factor alpha 1 (TNF-α), S-adenosylhomocysteine (SAHH), Small Nucleolar RNA Host Gene 7 (SNHG7), Telomerase Reverse Transcriptase (TERT), TSC Complex Subunit 2 (TSC2), Twist Family BHLH Transcription Factor (TWIST), Vascular Endothelial Growth Factor (VEGF),YES1-associated transcriptional regulator (YAP).
2. Thyroid Cancer
Despite its good prognosis, around 10% of DTC cases progress to local and distant metastasis, lose the ability to capture radioactive iodine and no longer respond to conventional radioiodine therapy. Poorly undifferentiated TC and ATC are more aggressive, with reduced survival. Medullary thyroid cancer (MTC) derives from the parafollicular C-cells of the thyroid and is also aggressive and rare (1–2%) [10].
In human ATC and DTC cells, as well as in human thyroid primary cell cultures and rat follicular thyroid cells, metformin antimitogenic activity was correlated with induction of apoptosis and inhibition of cell growth and migration. Some variation was observed depending on the cell line [11][12][13][14][15][16][17][18][19][20][21]. The growth-inhibitory effect of metformin was also observed in thyroid cancer stem cells [11]. In Figure 1, wresearchers summarize the pathways regulated by metformin. Mechanistically, metformin increased p-AMPK, reduced mTOR phosphorylation, downregulated S6K1/S6 signaling and inhibited cyclin D1 and c-MYC through the mTOR pathway [11][12][21][22]. Besides that, metformin modulated the expression of epithelial–mesenchymal transition (EMT)-related markers E-cadherin, N-cadherin and SNAIL [21]. Knockdown of the mTOR inhibitor TSC2, or treatment with rapamycin (mTOR blockade), confirmed metformin suppression of proliferation, migration and EMT of TC cells [21]. The antiproliferative activity of metformin was also observed in doxorubicin-resistant ATC cell lines, and AMPK silencing in ATC cells partially recovered phosphorylation of mTOR and cell growth inhibition by metformin [11].
Metformin can target thyroid cancer growth through cell metabolism. Cancer cells, despite having oxygen, often switch from mitochondrial oxidative phosphorylation (OXPHOS) to glycolysis to generate ATP, a metabolic reprogramming known as the Warburg effect [23][24]. This effect is negatively regulated by AMPK and different compounds [25][26]. In PTC, the BRAF V600E mutation altered the HIF1α-MYC-PGC-1β axis, inhibiting mitochondrial respiration and enhancing aerobic glycolysis [27]. Metformin in high glucose (20 mM) inhibited cell proliferation and in low glucose (5 mM) induced cell death, autophagy and oncosis. Cell sensitivity to metformin increased after treatment with a glycolysis inhibitor, and metformin reduced expression of the glycolytic gene PKM2, upregulated in cancer cells [22], suggesting the relationship between glucose concentration with metformin response. High expression of the metformin transporter OCT1 and mitochondrial GAPDH (mGPDH), the key enzyme connecting glycolysis with OXPHOS, was observed in TC samples compared with nontumoral samples. In FTC133 and BCPAP cell lines, metformin reduced mGPDH expression and activity and inhibited OXPHOS. Moreover, when mGPDH was silenced, metformin-mediated growth inhibition and mitochondrial respiration were reduced, while mGPDH overexpression promoted TC cell growth and sensitized cells to metformin [28]. In parallel, in PTC cells, metformin reduced 18F-fluoro-2-deoxy-d-glucose (18F-FDG) uptake and reduced levels of hexokinase-2 (HK2) and glucose transporter-1 (GLUT1), important proteins for glycolysis, showing the ability of metformin to reduce glucose metabolism [29]. These data indicate that metformin activity in TC cells depends on glucose concentration. In line with the reprogramming of cellular metabolism, metformin reduced ATP levels and mitochondrial membrane potential in ATC and PTC cells [17].
In TPC1 cells, metformin reduced p-ERK, a member of the mitogen-activated protein kinase (MAPK) family associated with proliferation and cell survival. Metformin also induced p-AKT in TPC1 and FTC236 cells with PI3K/AKT signaling activated by RET/PTEC rearrangement and PTEN mutation, respectively [12]. In a H2O2-inducible oxidative stress model, metformin attenuated H2O2 p-ERK activation, enhanced H2O2 p-AMPK expression and blocked S6K1/S6 axis, attenuating prosurvival signals and potentiating the AMPK activation in TC cells under oxidative stress [12].
In TPC-1 cells, metformin also increased the expression of BIP, CHOP and caspase-12, markers of endoplasmic reticulum (ER) stress, another apoptotic mechanism [30]. Metformin ER-stress modulation was confirmed using thapsigargin, an ER-stress activator that enhanced apoptosis induced by metformin, while using the ER-stress inhibitor 4-phenylbutyrate decreased metformin-induced apoptosis [19]. The increase in metformin ER-stress was also observed in FTC 133 and BCPAP cells in low glucose medium but not in high-glucose medium [22].
Other mechanisms associated with metformin anticancer properties are the improvement in insulin resistance and reduction of serum insulin. In thyroid, the action of IGF-1/insulin is mainly by PI3K signaling promoting survival and proliferation. The PI3K pathway is the main regulator of the transcription factor FOXO1, a tumor suppressor downregulated in thyroid cancers, important for apoptosis, cell cycle, metabolism and proliferation [31]. In ATC cells, metformin reduced mRNA levels of AKT, PI3K and FOXO1 but did not modulate phosphorylation of PI3K, AKT and FOXO1, suggesting that this axis is not involved in metformin anticancer activity [18]. In turn, metformin antagonized the proliferative effect of insulin through the reduction of ERK phosphorylation [11].
Metformin, independent of AMPK, also reduced expression of the multiligand transmembrane receptor LRP2 and p-JNK, a member of the MAPK family associated with survival and proliferation. The overexpression of LRP2 suppressed metformin p-JNK inhibition, suggesting that metformin inhibits the JNK pathway through LRP2 [24].
In MTC cell lines (TT and MZ-CRC-1), metformin inhibited cell growth by cell cycle arrest but did not promote apoptosis, as shown by the reduced expression of cyclin D1 without cleavage of caspase 3 and PARP after treatment [32]. In TT cell lines, metformin also reduced migration and invasion [17][21]. Metformin inhibited downstream target proteins of mTOR S6K1, S6, 4EBP1, c-MYC and cyclin D1, reduced p-ERK but not p-AKT and induced AMPK activation in TT cells [21][32]. Inhibition or silencing of AMPK did not prevent metformin downregulation of p-PS6 and partially reduced metformin inhibition of cyclin D1; thus, in TT cells, loss of AMPK activity does not completely annul the inhibitory effects of metformin on mTOR signaling, suggesting metformin acts through other pathways [32].
Concern about the first studies of metformin action on TC [12][13][32] was the use of supraphysiological doses of metformin, 10–50 mM compared to 500 to 2500 mg/d dose for diabetics. However, treatment of PTC cells with reduced concentration of metformin (0, 0.5, 1.5 and 20 mM) also reduced cell viability, increased apoptosis and activated AMPK [15]. Likewise, metformin inhibited cell proliferation and colony formation at 0.03 mM, cell migration at 0.3 M, increased apoptosis at 0.1 mM and cell cycle arrest cycle at 0.3 M on DCT, ATC and thyroid noncancer (NThyOri) cell lines [33]. These results, together with the in vivo results, suggest antitumorigenic activity of the physiological dose of metformin.
It was shown that metformin inhibits the activation of cytokine-induced nuclear factor κB (NF-κB) via AMPK activation in vascular endothelial cells [34] and that metformin/AMPK activation inhibited NF-κB signaling, upregulating IκBα in hepatocarcinoma cells [35]. In thyroid, metformin reduced the secretion of CXCL8 induced by TNF in primary cultures of normal and papillary thyroid primary cell cultures but not in TPC-1 or BCPAP cell lines [16]. CXCL8 is one of the NF-κB downstream mediators. Besides its proinflammatory properties, its expression facilitates metastasis [36]. Thus, reduced CXCL8 secretion by metformin was considered an anticancer effect, at least in classical DTC [16].
Akt-mTOR activation is essential for the activation of innate immune cells and tumor-associated macrophages (TAM), important players in thyroid tumorigenesis [37][38]. It was hypothesized that metformin could modulate immune parameters associated with cancer. In a coculture model with monocytes from patients harboring PTEN mutation and thyroid cancer cells TPC1 (RET/PTC rearrangement) or FTC-133 (PTEN deficient), metformin did not alter the secretion of proinflammatory cytokines from TAM induced by thyroid cancer cells. In contrast, it promoted the reduction in the anti-inflammatory cytokines IL10 and IL1-Ra. On the other hand, blockage of the mTOR pathway with rapamycin reduced the production of proinflammatory cytokines, suggesting metformin may not be effective in modulating the PTEN-mTOR axis in TAM [39].
Metformin influences metastasis and the tumor microenvironment. Whole marrow cultures treated with conditioned medium (CM) from ATC cells (ATC-CM) significantly increased production of the osteoblastic RANKL mRNA and protein, inducing osteoclast differentiation through upregulation of TRACP5b and cathepsin K markers, which were blocked by metformin treatment. Similarly, ATC-CM induced osteoclast differentiation of bone-marrow-derived monocyte/macrophage, which was also blocked by metformin treatment. The ATC-CM contained high levels of IL-6/sIL-6R and induced osteoblast RANKL production through gp130/STAT3 signaling; however, this effect was blocked by metformin in a mechanism dependent on AMPK phosphorylation, suggesting that in the tumor microenvironments, p-AMPK inhibits STAT3 phosphorylation [40].
Iodide uptake is a crucial step for radioiodine therapy for thyroid cancer treatment. In rat, thyroid epithelial cells activation of AMPK reduced iodine uptake and the Na/iodide symporter (NIS) at protein and mRNA levels, while pharmacological blockage of AMPK signaling increased iodide uptake (data confirmed in an animal model) [41]. Similar results were observed in a follicular rat thyroid cell line, and AMPK modulation of NIS depended in part on the cAMP response element (CRE) present in the NIS promoter [42]. On the other hand, a recent study in ATC cells showed increased NIS mRNA and protein after metformin treatment and increased mRNA of thyroglobulin, TSHR and NKX2.1, as well as metformin acting as a demethylating agent [20]. Thus, more studies are necessary to understand the effect of metformin in iodide uptake in the thyroid cancer context.
Finally, the combination of drugs is a very interesting strategy for cancer treatment. Sorafenib is a multikinase inhibitor approved for radioiodine refractory thyroid cancer. One important issue is that it frequently promotes hard side effects, demanding a dose reduction; sorafenib combined with metformin showed a synergistic inhibition on cell growth and sphere formation in ATC cells. Additionally, metformin allowed a reduction of 25% of sorafenib dose for the same inhibitory effect [43]. In turn, the combined treatment of vemurafenib, a selective inhibitor of BRAFV600E mutant protein that constitutively activates MAPK signaling and is present in one-half of PTC and one-fourth of ATC with metformin and rapamycin, significantly reduced cell growth compared with the only one-drug treatment in 8505 (ATC) and BCPAP-vemurafenib resistant cells (PTC-BRAFV600E) [44]. In BCPAP, a similar reduction in cell growth was observed after combined treatment. Recently, synergistic effects leading to p-ERK reduction and p-AMPK increase were obtained in the combination of metformin and vemurafenib in T-238, BCPAP and HTH7 models [20].
Synergistic activity on cytotoxicity after combined treatment of metformin with gemigliptin (dipeptidyl peptidase-IV inhibitor) was observed in PTC cells through activation of AMPK and AKT. Gemigliptin increased metformin-mediated inhibition of proliferation and migration through MMP9, VCAM-1 and p-ERK reduction and p53 and p21 increase [17]. In ATC cells, the combination of metformin with pioglitazone decreased the expression of AKT3, DEPTOR, EIF4E, ILK, MTOR, PIK3C and PRKCA and increased expression of some tumor suppressor genes (e.g., EIF4EBP1, EIF4EBP2, PTEN) [45]. Pioglitazone is an insulin sensitizer for type 2 diabetes with therapeutic effects in mouse models of thyroid cancer due to the fusion protein (PAX8-PPARg) present in 30% of thyroid cancer [46].
Reproduced with permission from Rubio IGS, Biomolecules; published by MDPI, 2022.
References
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576.
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and Reduced Risk of Cancer in Diabetic Patients. Br. Med. J. 2005, 330, 1304–1305.
- Kamarudin, M.N.A.; Sarker, M.M.R.; Zhou, J.-R.; Parhar, I. Metformin in colorectal cancer: Molecular mechanism, preclinical and clinical aspects. J. Exp. Clin. Cancer Res. 2019, 38, 491.
- Jiao, Y.; Wang, X.; Luo, Z. Preventive and (Neo)Adjuvant Therapeutic Effects of Metformin on Cancer; IntechOpen: London, UK, 2020; pp. 1–22.
- Li, M.; Li, X.; Zhang, H.; Lu, Y. Molecular Mechanisms of Metformin for Diabetes and Cancer Treatment. Front. Physiol. 2018, 9, 1039.
- Dowling, R.J.O.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin Inhibits Mammalian Target of Rapamycin–Dependent Translation Initiation in Breast Cancer Cells. Cancer Res. 2007, 67, 10804–10812.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674.
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Fagin, J.A.; Wells, S.A. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067.
- Chen, G.; Xu, S.; Renko, K.; Derwahl, M. Metformin Inhibits Growth of Thyroid Carcinoma Cells, Suppresses Self-Renewal of Derived Cancer Stem Cells, and Potentiates the Effect of Chemotherapeutic Agents. J. Clin. Endocrinol. Metab. 2012, 97, 510–520.
- Klubo-Gwiezdzinska, J.; Costello, J.; Patel, A.; Bauer, A.; Jensen, K.; Mete, M.; Burman, K.D.; Wartofsky, L.; Vasko, V. Treatment With Metformin Is Associated With Higher Remission Rate in Diabetic Patients With Thyroid Cancer. J. Clin. Endocrinol. Metab. 2013, 98, 3269–3279.
- Moon, H.-S.; Mantzoros, C.S. Regulation of cell proliferation and malignant potential by irisin in endometrial, colon, thyroid and esophageal cancer cell lines. Metab. Clin. Exp. 2014, 63, 188–193.
- Kheder, S.; Hadad, S.; Sisley, K.; Balasubramanian, S. Investigation of the role of Metformin in thyroid cancer. Eur. J. Surg. Oncol. (EJSO) 2014, 40, S3–S4.
- Cho, S.W.; Yi, K.H.; Han, S.K.; Sun, H.J.; Kim, Y.A.; Oh, B.-C.; Park, Y.J.; Park, D.J. Therapeutic potential of metformin in papillary thyroid cancer in vitro and in vivo. Mol. Cell. Endocrinol. 2014, 393, 24–29.
- Rotondi, M.; Coperchini, F.; Pignatti, P.; Magri, F.; Chiovato, L. Metformin Reverts the Secretion of CXCL8 Induced by TNF-α in Primary Cultures of Human Thyroid Cells: An Additional Indirect Anti-Tumor Effect of the Drug. J. Clin. Endocrinol. Metab. 2015, 100, E427–E432.
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.-H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. Synergistic cytotoxicity of the dipeptidyl peptidase-IV inhibitor gemigliptin with metformin in thyroid carcinoma cells. Endocrine 2018, 59, 383–394.
- Nozhat, Z.; Mohammadi-Yeganeh, S.; Azizi, F.; Zarkesh, M.; Hedayati, M. Effects of metformin on the PI3K/AKT/FOXO1 pathway in anaplastic thyroid Cancer cell lines. DARU J. Pharm. Sci. 2018, 26, 93–103.
- Ye, J.; Qi, L.; Chen, K.; Li, R.; Song, S.; Zhou, C.; Zhai, W. Metformin induces TPC-1 cell apoptosis through endoplasmic reticulum stress-associated pathways in vitro and in vivo. Int. J. Oncol. 2019, 55, 331–339.
- Durai, L.; Ravindran, S.; Arvind, K.; Karunagaran, D.; Vijayalakshmi, R. Synergistic effect of metformin and vemurufenib (PLX4032) as a molecular targeted therapy in anaplastic thyroid cancer: An in vitro study. Mol. Biol. Rep. 2021, 48, 7443–7456.
- Han, B.; Cui, H.; Kang, L.; Zhang, X.; Jin, Z.; Lu, L.; Fan, Z. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the MTOR pathway. Tumor Biol. 2015, 36, 6295–6304.
- Bikas, A.; Jensen, K.; Patel, A.; Costello, J.; McDaniel, D.; Klubo-Gwiezdzinska, J.; Larin, O.; Hoperia, V.; Burman, K.D.; Boyle, L.; et al. Glucose-deprivation increases thyroid cancer cells sensitivity to metformin. Endocrine-Relat. Cancer 2015, 22, 919–932.
- Coelho, R.G.; Fortunato, R.S.; Carvalho, D.P. Metabolic Reprogramming in Thyroid Carcinoma. Front. Oncol. 2018, 8, 82.
- He, Y.; Cao, L.; Wang, L.; Liu, L.; Huang, Y.; Gong, X. Metformin Inhibits Proliferation of Human Thyroid Cancer TPC-1 Cells by Decreasing LRP2 to Suppress the JNK Pathway. OncoTargets Ther. 2020, 13, 45–50.
- Faubert, B.; Boily, G.; Izreig, S.; Griss, T.; Samborska, B.; Dong, Z.; Dupuy, F.; Chambers, C.; Fuerth, B.J.; Viollet, B.; et al. AMPK Is a Negative Regulator of the Warburg Effect and Suppresses Tumor Growth In Vivo. Cell Metab. 2013, 17, 113–124.
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype—concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398.
- Gao, Y.; Yang, F.; Yang, X.-A.; Zhang, L.; Yu, H.; Cheng, X.; Xu, S.; Pan, J.; Wang, K.; Li, P. Mitochondrial metabolism is inhibited by the HIF 1α- MYC-PGC-1β axis in BRAF V600E thyroid cancer. FEBS J. 2019, 286, 1420–1436.
- Thakur, S.; Daley, B.; Gaskins, K.; Vasko, V.V.; Boufraqech, M.; Patel, D.; Sourbier, C.; Reece, J.M.; Cheng, S.-Y.; Kebebew, E.; et al. Metformin Targets Mitochondrial Glycerophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin. Cancer Res. 2018, 24, 4030–4043.
- Shen, C.T.; Wei, W.J.; Qiu, Z.-L.; Song, H.-J.; Zhang, X.-Y.; Sun, Z.-K.; Luo, Q.-Y. Metformin reduces glycometabolism of papillary thyroid carcinoma in vitro and in vivo. J. Mol. Endocrinol. 2017, 58, 15–23.
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389.
- Zaballos, M.A.; Santisteban, P. FOXO1 Controls Thyroid Cell Proliferation in Response to TSH and IGF-I and Is Involved in Thyroid Tumorigenesis. Mol. Endocrinol. 2013, 27, 50–62.
- Klubo-Gwiezdzinska, J.; Jensen, K.; Costello, J.; Patel, A.; Hoperia, V.; Bauer, A.; Burman, K.D.; Wartofsky, L.; Vasko, V. Metformin inhibits growth and decreases resistance to anoikis in medullary thyroid cancer cells. Endocrine-Relat. Cancer 2012, 19, 447–456.
- Kheder, S.; Sisley, K.; Hadad, S.; Balasubramanian, S.P. Effects of prolonged exposure to low dose metformin in thyroid cancer cell lines. J. Cancer 2017, 8, 1053–1061.
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin Inhibits Cytokine-Induced Nuclear Factor κB Activation Via AMP-Activated Protein Kinase Activation in Vascular Endothelial Cells. Hypertension 2006, 47, 1183–1188.
- Zheng, L.; Yang, W.; Wu, F.; Wang, C.; Yu, L.; Tang, L.; Qiu, B.; Li, Y.; Guo, L.; Wu, M.; et al. Prognostic Significance of AMPK Activation and Therapeutic Effects of Metformin in Hepatocellular Carcinoma. Clin. Cancer Res. 2013, 19, 5372–5380.
- De Larco, J.E.; Wuertz, B.R.K.; Furcht, L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin. Cancer Res. 2004, 10, 4895–4900.
- Lv, J.; Feng, Z.P.; Chen, F.K.; Liu, C.; Jia, L.; Liu, P.J.; Yang, C.Z.; Hou, F.; Deng, Z.Y. M2-like tumor-associated macrophages-secreted Wnt1 and Wnt3a promotes dedifferentiation and metastasis via activating β-catenin pathway in thyroid cancer. Mol. Carcinog. 2021, 60, 25–37.
- Kabasawa, T.; Ohe, R.; Aung, N.Y.; Urano, Y.; Kitaoka, T.; Tamazawa, N.; Utsunomiya, A.; Yamakawa, M. Potential role of M2 TAMs around lymphatic vessels during lymphatic invasion in papillary thyroid carcinoma. Sci. Rep. 2021, 11, 1150.
- Sloot, Y.J.E.; Janssen, M.J.R.; van Herwaarden, A.E.; Peeters, R.P.; Netea-Maier, R.T.; Smit, J.W.A. The Influence of Energy Depletion by Metformin or Hypocaloric Diet on Thyroid Iodine Uptake in Healthy Volunteers: A Randomized Trial. Sci. Rep. 2019, 9, 5396.
- Shin, H.S.; Sun, H.J.; Whang, Y.M.; Park, Y.J.; Park, D.J.; Cho, S.W. Metformin Reduces Thyroid Cancer Tumor Growth in the Metastatic Niche of Bone by Inhibiting Osteoblastic RANKL Productions. Thyroid 2021, 31, 760–771.
- Andrade, B.M.; Araujo, R.L.; Perry, R.L.S.; Souza, E.C.L.; Cazarin, J.M.; Carvalho, D.P.; Ceddia, R.B. A novel role for AMP-kinase in the regulation of the Na+/I−-symporter and iodide uptake in the rat thyroid gland. Am. J. Physiol. Cell Physiol. 2011, 300, C1291–C1297.
- M.Abdulrahman, R.; Boon, M.R.; C.M.Sips, H.; Guigas, B.; Rensen, P.C.N.; Smit, J.W.A.; Hovens, G.C.J. Impact of Metformin and Compound C on NIS Expression and Iodine Uptake in Vitro and in Vivo: A Role for CRE in AMPK Modulation of Thyroid Function. Thyroid 2015, 24, 78–87.
- Chen, G.; Nicula, D.; Renko, K.; Derwahl, M. Synergistic anti-proliferative effect of metformin and sorafenib on growth of anaplastic thyroid cancer cells and their stem cells. Oncol. Rep. 2015, 33, 1994–2000.
- Hanly, E.K.; Bednarczyk, R.B.; Tuli, N.Y.; Moscatello, A.L.; Halicka, H.D.; Li, J.; Geliebter, J.; Darzynkiewicz, Z.; Tiwari, R.K. mTOR inhibitors sensitize thyroid cancer cells to cytotoxic effect of vemurafenib. Oncotarget 2015, 6, 39702–39713.
- Ozdemir Kutbay, N.; Biray Avci, C.; Sarer Yurekli, B.; Caliskan Kurt, C.; Shademan, B.; Gunduz, C.; Erdogan, M. Effects of metformin and pioglitazone combination on apoptosis and AMPK/MTOR signaling pathway in human anaplastic thyroid cancer cells. J. Biochem. Mol. Toxicol. 2020, 34, e22547.
- Dobson, M.E.; Diallo-Krou, E.; Grachtchouk, V.; Yu, J.; Colby, L.A.; Wilkinson, J.E.; Giordano, T.J.; Koenig, R.J. Pioglitazone Induces a Proadipogenic Antitumor Response in Mice with PAX8-PPARγ Fusion Protein Thyroid Carcinoma. Endocrinology 2011, 152, 4455–4465.
More
